Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(12):2231-2237. doi:10.7150/jca.18932 This issue Cite
Research Paper
Selective Gastric Cancer Patients with Peritoneal Seeding Benefit from Gastrectomy after Palliative Chemotherapy: A Propensity Score Matching Analysis
1. Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
2. Department of Gastric Surgery, the 6th Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
# These authors contributed equally to this study.
Received 2016-12-26; Accepted 2017-4-30; Published 2017-7-20
Abstract
Background: The present study aimed to explore whether gastric cancer patients with peritoneal seeding after receiving palliative chemotherapy could benefit from gastrectomy and to identify patients with peritoneal seeding who should be selected to receive gastrectomy.
Methods: A total of 201 gastric cancer patients were diagnosed with peritoneal seeding and received palliative chemotherapy. Propensity score matching (PSM) was performed to balance the selection bias.
Results: After PSM, compared with non-gastrectomy group, gastrectomy group had a longer median overall survival (OS) (23.60 vs. 13.80 moths; P=0.034). Patients with R0 resection had a median OS of 43.60 months compared with 11.27 months in patients who underwent R1/2 resection (P<0.001). The median OS times between the R1/2 resection and non-gastrectomy groups were not different (P=0.139). Subgroup analysis revealed that only patients receiving more than 4 periods of first-line chemotherapy benefited from gastrectomy (P=0.018), whereas patients receiving 1-4 periods of first-line chemotherapy did not (P=0.275). Multivariate analysis showed that gastrectomy (P=0.012) and the period of first-line chemotherapy (P<0.001) were independent prognostic factors. The overall postoperative morbidity was 3.03% (1/33) in the gastrectomy group, and no treatment-related death was observed.
Conclusions: The present study indicated that gastrectomy after palliative chemotherapy is a safe procedure and showed a survival benefit for gastric cancer patients with peritoneal seeding. Moreover, clinically curative R0 gastrectomy and more than 4 periods of palliative chemotherapy resulted in better clinical outcomes.
Keywords: gastrectomy, chemotherapy, gastric cancer, peritoneal seeding, survival
Introduction
Gastric cancer remains as the fourth most prevalent cancer and third leading cause of cancer-related death worldwide; there were an estimated 951600 new cases and 723100 deaths in 2012 [1].
With the screen of esophagogastroscopy, standardized D2 lymphadenectomy and adjuvant chemotherapy [2-4], the overall survival (OS) time of gastric cancer patients after curative surgery is increasing. Unfortunately, approximately 20-30% of gastric cancer patients in China are diagnosed with advanced gastric cancer at the first clinic visit. Among the patterns of metastasis, peritoneal seeding is the most common pattern and cause of death in patients with gastric cancer [5]. Various therapeutic strategies, including combination chemotherapy regimens, hyperthermic intraperitoneal chemotherapy, and peritonectomy, have been demonstrated to improve the prognosis of gastric cancer patients with peritoneal seeding [6-8]. However, the overall survival time remains dismal. Although palliative gastrectomy can theoretically reduce cancer-related symptoms and improve the prognosis of advanced gastric cancer [9-11], the final analysis of the REGATTA trial demonstrated that gastrectomy followed by chemotherapy did not show any survival benefit compared with chemotherapy alone in advanced gastric cancer [12].
Recently, T. Kanda et al. reported that secondary gastrectomy after preoperative chemotherapy is a safe and effective treatment for advanced gastric cancer [13]. However, the result may be attributable to careful patient selection and study was a non-comparative design. Therefore, the result was inconclusive, whether gastric cancer patients with peritoneal seeding could benefit from gastrectomy after chemotherapy remains debatable [13-19]. Using the propensity score matching method to balance the selected bias, the present study aimed to explore whether gastric cancer patients with peritoneal seeding after receiving palliative chemotherapy could benefit from gastrectomy and to identify patients with peritoneal seeding who should be selected to receive gastrectomy.
Patients and Methods
Patients
Between January 2000 and June 2014, patients were eligible if they were histologically proven diagnoses of gastric adenocarcinoma with peritoneal seeding received palliative chemotherapy at Sun Yat-sen University Cancer Center and The Sixth Affiliated Hospital of Sun Yat-sen University. The present study was approved by institutional review board of our centers. Among 201 patients, 33 patients underwent gastrectomy, and the other 168 did not. We reviewed the clinicopathologic characteristics before initial chemotherapy and clinical outcomes of all patients. The characteristics included the age, sex, Eastern Cooperative Oncology Group performance score (ECOG PS), tumor location, tumor size, baseline serum carcinoembryonic antigen (CEA), pathological pattern, ascites, degree of peritoneal seeding, multisite distant metastasis, period of chemotherapy, response to chemotherapy, curative intent and gastrectomy type. The degree of peritoneal metastasis is classified according to the first English edition of the Japanese classification of gastric carcinoma [20]. The tumor response was objectively assessed after each treatment course according to the Response Evaluation Criteria in Solid Tumors (RECIST). Surgical resection was classified as curative (R0, complete resection with no residual tumor) or non-curative (R1 or R2, microscopic or gross residual tumor) gastrectomy.
Propensity Score Matching Analysis
The propensity score, representing the conditional probability of receiving a therapy given a vector of covariates, is commonly built in observational studies to adjust for selection bias [21, 22]. In this study, the 1:1 nearest neighbor matching were chosen for the propensity score. Propensity score matching was performed using Stata13.0 (StataCorp LP, College Station, TX, USA).
Statistical Analysis
The categorical variables were presented as the numbers and percentages, and Chi-squared tests were used to compare categorical variables. The overall survival (OS) was calculated from the diagnosis of peritoneal seeding to death from any cause. Unadjusted Kaplan-Meier survival curves with log-rank testing were generated to compare the survival benefits. Prognostic factors were analyzed by searching the clinicopathological factors in univariate analysis. Variables with a P value < 0.05 in the univariate analysis were entered into multivariate analysis using Cox proportional hazard regression models. The forward selection method was used for multivariate Cox proportional analysis. The hazard ratio (HR) and 95% confidence interval (CI) were used to estimate the survival predictor. P values were two sided. All of the above statistical analyses were performed using the SPSS software (version 17.0; SPSS, Chicago, IL, USA).
All regular follow-up assessments after 1:1 propensity score matching were completed by June 2016, and the median follow-up time was 14.91 months (range, 1.20-82.00 months).
Results
Patient Characteristics
In this study, a total of 201 patients were included, with 33 patients in the gastrectomy group and 168 patients in the non-gastrectomy group. Table 1 shows the baseline characteristics of the 201 patients. As shown in Table 1, compared with the non-gastrectomy group, patients in the gastrectomy group had less ascites (P=0.012) and peritoneal seeding (P=0.007), more periods of first-line chemotherapy (P<0.001) and better disease control (P<0.001). Therefore, the covariates for propensity score matching were ascites, peritoneal metastasis classification, disease control and the period of first-line chemotherapy. After 1:1 propensity score matching, the covariates were balanced (Table 1). For the gastrectomy group, twenty patients (20/30) underwent R0 resection, and the mean number of preoperative chemotherapy periods was 3.41 (range: 1-12).
Survival
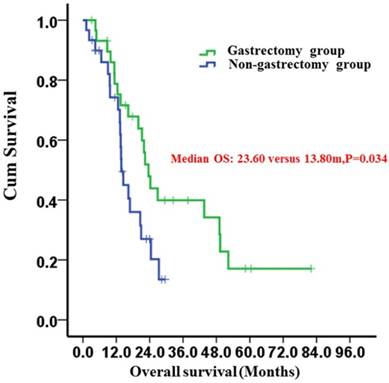
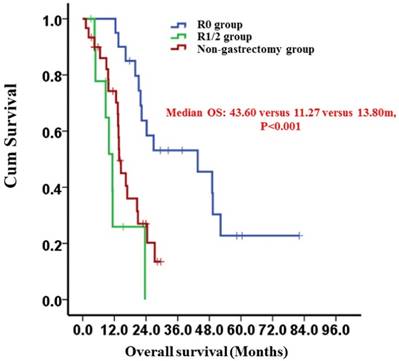
After propensity score matching, the 2-year survival rates of the gastrectomy and non-gastrectomy groups were 43.9% (95%CI: 24.9-62.9%) and 20.2% (95%CI: 2.4%-38.0%), respectively. The median OS was 23.60 (95%CI: 19.93-27.28) months in the gastrectomy group and 13.80 (95%CI: 12.37-15.23) months in the non-gastrectomy group (P=0.034) (Fig. 1). The median OS of patients who underwent curative gastrectomy with R0 resection was significantly longer than those who underwent non-curative gastrectomy with R1/2 resection; the survival times were 43.60 (95%CI: 14.92-72.28) months and 11.27 (95%CI: 7.91-14.63) months, respectively (P<0.001) (Fig. 2). However, the median OS times between the non-curative gastrectomy and non-gastrectomy groups were not different (P=0.139) (Fig. 2).
Clinicopathological characteristics of gastric cancer patients with peritoneal seeding before and after propensity score matching
| Characteristics | Before propensity score matching | P-value | After propensity score matching | P-value | ||
|---|---|---|---|---|---|---|
| Gastrectomy group | Non-gastrectomy group | Gastrectomy group | Non-gastrectomy group | |||
| No. of patients | 33 | 168 | 30 | 30 | ||
| Age | 0.098 | 0.150 | ||||
| ≤70 years | 33 (100.0) | 155 (92.3) | 30 (100) | 28 (93.3) | ||
| >70 years | 0 (0) | 13 (7.7) | 0 (0) | 2 (6.7) | ||
| Sex, n | 0.628 | 1.000 | ||||
| Male | 14 (42.4) | 79 (47.0) | 14 (46.7) | 14 (46.7) | ||
| Female | 19 (57.6) | 89 (53.0) | 16 (53.3) | 16 (53.3) | ||
| PS, n | 0.462 | 0.371 | ||||
| <2 | 24 (72.7) | 132 (78.6) | 21 (70.0) | 24 (80.0) | ||
| ≥2 | 9 (27.3) | 36 (21.4) | 9 (30.0) | 6 (20.0) | ||
| Tumor location | 0.901 | 0.982 | ||||
| Cardia | 9 (28.1) | 39 (24.5) | 9 (31.1) | 10 (33.3) | ||
| Middle | 13 (40.6) | 70 (44.0) | 11 (37.9) | 11 (36.7) | ||
| Antrum | 10 (31.3) | 50 (31.5) | 9 (31.0) | 9 (30.0) | ||
| Size | 0.094 | 0.445 | ||||
| ≥10 cm | 11 (33.3) | 25 (19.7) | 9 (30.0) | 5 (20.8) | ||
| <10 cm | 22 (66.7) | 102 (80.3) | 21 (70.0) | 19 (79.2) | ||
| CEA (ng/ml, mean, range) | 21.7 (0.363-571) | 18.3 (0.25-918) | 0.586 | 23.1 (0.363-571) | 7.31 (0.295-97) | 0.792 |
| SRCC | 0.863 | 1.000 | ||||
| Yes | 12 (36.4) | 63 (38.0) | 12 (40.0) | 12 (40.0) | ||
| No | 21 (63.6) | 103 (62.0) | 18 (60.0) | 18 (60.0) | ||
| Ascites | 0.012 | 1.000 | ||||
| Yes | 15 (45.5) | 115 (68.5) | 12 (40.0) | 12 (40.0) | ||
| No | 18 (54.5) | 53 (31.5) | 18 (60.0) | 18 (60.0) | ||
| Peritoneal seeding | 0.007 | 1.000 | ||||
| P1/2 | 22 (66.7) | 69 (41.1) | 19 (63.3) | 19 (63.3) | ||
| P3 | 11 (33.3) | 99 (58.9) | 11 (36.7) | 11 (36.7) | ||
| Multisite distant metastasis | 0.220 | 0.426 | ||||
| Yes | 11 (33.3) | 75 (44.9) | 10 (33.3) | 13 (43.3) | ||
| No | 22 (66.7) | 92 (55.1) | 20 (66.7) | 17 (56.7) | ||
| Period of first-line chemotherapy | <0.001 | 1.000 | ||||
| 1-4 | 5 (15.2) | 101 (60.1) | 5 (16.7) | 5 (16.7) | ||
| ≥5 | 28 (84.8) | 67 (39.9) | 25 (83.3) | 25 (83.3) | ||
| DC (CR+PR+SD) | <0.001 | 1.000 | ||||
| Yes | 26 (78.8) | 54 (32.1) | 23 (76.7) | 23 (76.7) | ||
| No | 7 (21.2) | 114 (67.9) | 7 (23.3) | 7 (23.3) | ||
| Curative | ||||||
| R0 | 22 | 20 | ||||
| R1/2 | 11 | 10 | ||||
| Gastrectomy type | ||||||
| Subtotal | 16 | 14 | ||||
| Total | 17 | 16 | ||||
Data are expressed as n (%) unless otherwise stated
PS performance status, SRCC signet ring cell carcinoma, CEA baseline carcinoembryonic antigen, DC disease control, CR complete response, PR partial response, and SD stable disease
Kaplan-Meier survival curves of the gastrectomy and non-gastrectomy groups for gastric cancer patients with peritoneal seeding (P =0.034). P-values were calculated using the log-rank test
Kaplan-Meier survival curves of the R0 and R1/2 resection groups and the non-gastrectomy group (P < 0.001). P-values were calculated using the log-rank test
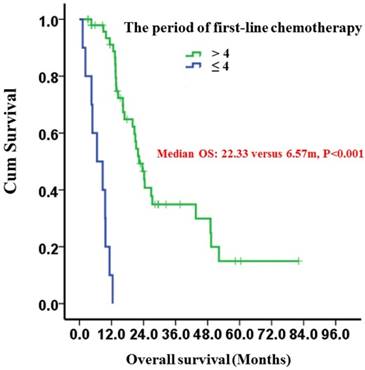
Patients receiving more than 4 periods of first-line chemotherapy had a significantly longer median OS of 22.33 (95%CI: 18.54-26.12) months compared to 6.57 (95%CI: 0.58-12.56) months in patients who received only 1 to 4 periods (P<0.001) (Fig. 3).
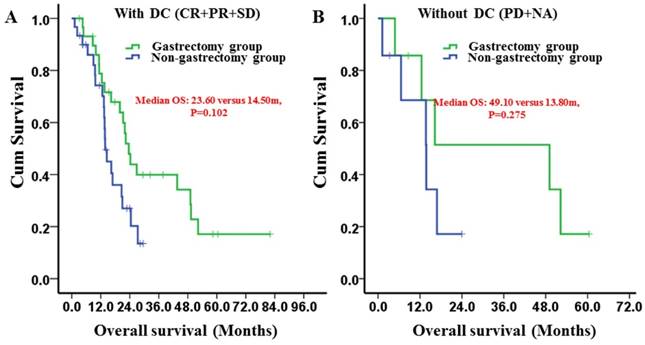
In the subgroup analysis, the patients who gained disease control after chemotherapy had a longer median OS in the gastrectomy group than did patients in the non-gastrectomy group (23.60 [95% CI: 20.38-26.82] months versus 14.50 [95% CI: 10.69-18.31] months, respectively) (Fig. 4A). However, the differences were not significant (P=0.102). For patients who did not gain disease control, the median OS times were not different between the groups (P=0.275) (Fig. 4B).
Kaplan-Meier survival curves of gastric cancer patients with peritoneal seeding according to the period of first-line chemotherapy (P < 0.001). P-values were calculated using the log-rank test
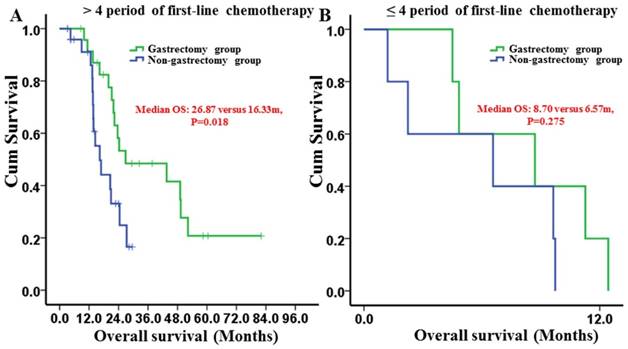
For patients who received more than 4 periods of first-line chemotherapy, gastrectomy group had a longer median OS than non-gastrectomy group (26.87 [95% CI: 1.14-52.59] months versus 16.33 [95% CI: 11.55-21.11] months, respectively) (P=0.018) (Fig. 5A). However, for patients receiving 1-4 periods of first-line chemotherapy, the median OS times between the groups were not different (8.70 [95% CI: 0.49-17.00] months versus 6.57 [95% CI: 0-15.87] months, respectively) (P=0.275) (Fig. 5B).
Univariate and Multivariate Analysis for the Overall Survival
In the univariate analysis, gastrectomy (P=0.038) and the period of first-line chemotherapy (P<0.001) were significant predictors of overall survival. Multivariate analysis demonstrated that gastrectomy (P=0.012) and the period of first-line chemotherapy (P<0.001) remained as prognostic factors (Table 2).
Morbidity and mortality
The overall postoperative morbidity was 3.03% (1/33) in the gastrectomy group. Anastomotic bleeding was a complication of gastrectomy, and the patient with this complication was cured after conservative treatment. No treatment-related death was observed in the gastrectomy group.
Kaplan-Meier survival curves of the gastrectomy and non-gastrectomy groups for gastric cancer patients with peritoneal seeding stratified by the chemotherapy response. A, versus the disease control group (DC) (P = 0.102); B, versus the disease control group (P = 0.275). P-values were calculated using the log-rank test
Kaplan-Meier survival curves of the gastrectomy and non-gastrectomy groups for gastric cancer patients with peritoneal seeding stratified by the period of first-line chemotherapy. A, > 4 periods of first-line chemotherapy group (P = 0.018); B, 1-4 periods of first-line chemotherapy group (P = 0.275). P-values were calculated using the log-rank test
Discussion
Gastric cancer patients with peritoneal seeding usually have a very poor prognosis. To date, systemic chemotherapy has been the standard treatment for these patients. Combination chemotherapy regimens are demonstrated to improve anticancer responses and patient survival [6, 7]. However, because of the heterogeneity of gastric cancer, tumors can acquire drug resistance after several periods of chemotherapy. In the SPIRIT trial, the time median period before tumor progression after treatment of S-1 plus cisplatin was 6.0 months [6]. Various treatment strategies were explored to improve the survival of gastric cancer patients with peritoneal seeding [8, 23, 24], including gastrectomy after palliative chemotherapy [16, 19, 25].
Gastric cancer patients with peritoneal seeding appear to benefit from gastrectomy after palliative chemotherapy: to reduce the cancer-related symptoms, such as bleeding, obstruction, and perforation; to improve the metabolism and immunity of the patients [26]; to improve the efficacy of chemotherapy by minimizing the chemotherapy-resistance cancer cells. H. Okabe et al. demonstrated that limited peritoneal seeding of gastric origin is highly sensitive to induction chemotherapy [19]. Moreover, resection after induction chemotherapy could cure selective patients. Other investigators also reported similar results [14-17, 25]. However, previous studies had obvious selection bias, such as a less severe degree of peritoneal metastasis in the gastrectomy group, which confounded the results. Moreover, the previous studies did not compare gastrectomy after palliative chemotherapy with chemotherapy alone directly. To minimize the selection bias, we used propensity score matching. After propensity score matching, our results showed that patients in the gastrectomy group had a longer median OS than did patients in the non-gastrectomy group, thus demonstrating the benefit of gastrectomy after palliative chemotherapy, which is in agreement with other studies [14-17, 19, 25].
Univariate and multivariate of analyses of the overall survival in gastric cancer patients with peritoneal dissemination after propensity score matching
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 0.586 | |||
| ≤70 years | 1 | |||
| >70 years | 0.58 (0.08-4.22) | |||
| Sex, n | 0.945 | |||
| Male | 1 | |||
| Female | 1.02 (0.55-1.19) | |||
| PS, n | 0.954 | |||
| <2 | 1 | |||
| ≥2 | 0.98 (0.47-2.02) | |||
| Tumor location | 0.398 | |||
| Cardia | 1 | |||
| Middle | 1.72 (0.79-3.74) | 0.175 | ||
| Antrum | 1.41 (0.61-3.26) | 0.427 | ||
| Size | 0.203 | |||
| <10 cm | 1 | |||
| ≥10 cm | 1.62 (0.77-3.39) | |||
| CEA | 0.597 | |||
| <5 ng/ml | 1 | |||
| ≥5 ng/ml | 0.81 (0.36-1.79) | |||
| SRCC | 0.161 | |||
| No | 1 | |||
| Yes | 1.56 (0.84-2.91) | |||
| Ascites | 0.128 | |||
| No | 1 | |||
| Yes | 1.65 (0.87-3.12) | |||
| Peritoneal seeding | 0.319 | |||
| P1/2 | 1 | |||
| P3 | 1.40 (0.72-2.74) | |||
| Multisite distant metastasis | 0.345 | |||
| No | 1 | |||
| Yes | 1.36 (0.72-2.57) | |||
| Period of first-line chemotherapy | <0.001 | <0.001 | ||
| 1-4 | 1 | 1 | ||
| ≥5 | 0.03 (0.01-0.11) | 0.03 (0.01-0.10) | ||
| DC (CR+PR+SD) | 0.765 | |||
| Yes | 1 | |||
| No | 0.90 (0.43-1.86) | |||
| Treatment | 0.038 | 0.012 | ||
| Non-gastrectomy group | 1 | 1 | ||
| Gastrectomy group | 0.49 (0.25-0.96) | 0.42 (0.21-0.83) | ||
HR hazard ratio, CI confidence interval, PS performance status, SRCC signet ring cell carcinoma, CEA baseline carcinoembryonic antigen, DC disease control, CR complete response, PR partial response, and SD stable disease
Kim at el. found that both clinically curative conversion surgery and non-curative gastrectomy can improve the survival of gastric cancer patients with peritoneal seeding [16]. Nevertheless, in the present study, the median OS of patients who underwent R1/2 resection with non-curative intent was not different from that of patients who underwent chemotherapy alone (11.27 versus. 13.80; P=0.139), indicating that non-curative gastrectomy after chemotherapy did not show any survival benefit. A study by Ishigami et al revealed that in a multivariate analysis, R0 resection was the only independent prognostic factor for stage IV gastric cancer patients whose distant lesions showed complete response after chemotherapy [14]. Moreover, in our study, the overall postoperative morbidity was low (1/33), and no treatment-related death was observed in patients who underwent gastrectomy. Previous studies reported that the morbidity and mortality of palliative gastric resection ranged from 12 to 65% and 0 to 27%, respectively [27, 28]. Therefore, we considered that gastrectomy after palliative chemotherapy was a safe procedure for patients with peritoneal seeding, which is in accordance in other studies [15, 19].
The optimal timing for gastrectomy after palliative chemotherapy and the ideal period of palliative chemotherapy are debatable. For resectable gastric cancer, the periods of neoadjuvant chemotherapy usually range from 2 to 4 [29, 30]. However, in our study, the period of the chemotherapy varied (range: 1-12) because of the variable tumor response, which is in agreement with other studies [14, 15, 19]. Our subgroup analysis showed that patients achieving disease control after chemotherapy had a longer median OS in the gastrectomy group than in the non-gastrectomy group, although the difference was not significant because of the low power. Moreover, our study revealed that only patients receiving more than 4 periods of first-line chemotherapy benefited from gastrectomy, whereas patients receiving 1-4 periods of first-line chemotherapy did not benefit. Therefore, undergoing more than 4 periods of palliative chemotherapy to gain tumor control may be reasonable before gastrectomy, which can select for the good biological behavior of gastric cancer patients with peritoneal seeding and increase the success of R0 resection.
There are also some limitations in our study. First, this study is predominantly a retrospective study. Second, the number of gastric cancer patients with peritoneal seeding who underwent gastrectomy after chemotherapy was small, and the study time span was over 14 years. However, the data in our study were collected from two high-volume institutions. Additionally, we used propensity score matching and multivariate analysis to balance the selection bias and explore the value of gastrectomy after palliative chemotherapy in patients with peritoneal seeding. In the future, large-scale and well-designed randomized controlled trials are required.
Therefore, the present study indicated that gastrectomy after palliative chemotherapy was a safe procedure with survival benefit for gastric cancer patients who have peritoneal seeding. Moreover, clinically curative R0 gastrectomy and more than 4 periods of palliative chemotherapy resulted in better clinical outcomes.
Acknowledgements
This work was supported in part by a grant from National Natural Science Foundation of China (81302144) and the Guangdong Science and Technology Department (No2012B061700087).
Competing Interests
All authors declare that they have no conflicts of interest.
References
1. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
2. Bang YJ, Kim YW, Yang HK. et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321
3. Sasako M, Sakuramoto S, Katai H. et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393
4. Songun I, Putter H, Kranenbarg EM. et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449
5. Hioki M, Gotohda N, Konishi M. et al. Predictive factors improving survival after gastrectomy in gastric cancer patients with peritoneal carcinomatosis. World J Surg. 2010;34:555-562
6. Koizumi W, Narahara H, Hara T. et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. The Lancet Oncology. 2008;9:215-221
7. Bang YJ, Van Cutsem E, Feyereislova A. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697
8. Wu X, Li Z, Li Z. et al. Hyperthermic intraperitoneal chemotherapy plus simultaneous versus staged cytoreductive surgery for gastric cancer with occult peritoneal metastasis. J Surg Oncol. 2015;111:840-847
9. Nie RC, Chen S, Yuan SQ. et al. Significant Role of Palliative Gastrectomy in Selective Gastric Cancer Patients with Peritoneal Dissemination: A Propensity Score Matching Analysis. Ann Surg Oncol. 2016
10. Yang K, Liu K, Zhang WH. et al. The Value of Palliative Gastrectomy for Gastric Cancer Patients With Intraoperatively Proven Peritoneal Seeding. Medicine (Baltimore). 2015;94:e1051
11. Chang YR, Han DS, Kong SH. et al. The value of palliative gastrectomy in gastric cancer with distant metastasis. Ann Surg Oncol. 2012;19:1231-1239
12. Fujitani K, Yang HK, Mizusawa J. et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309-318
13. Yamamoto M, Sakaguchi Y, Matsuyama A. et al. Surgery after preoperative chemotherapy for patients with unresectable advanced gastric cancer. Oncology. 2013;85:241-247
14. Ishigami S, Natsugoe S, Nakajo A. et al. Salvage gastrectomy following a combination of biweekly paclitaxel and S-1 for stage IV gastric cancer. J Gastrointest Surg. 2008;12:1370-1375
15. Kanda T, Yajima K, Kosugi S. et al. Gastrectomy as a secondary surgery for stage IV gastric cancer patients who underwent S-1-based chemotherapy: a multi-institute retrospective study. Gastric Cancer. 2012;15:235-244
16. Kim SW. The result of conversion surgery in gastric cancer patients with peritoneal seeding. J Gastric Cancer. 2014;14:266-270
17. Fukuchi M, Ishiguro T, Ogata K. et al. Prognostic Role of Conversion Surgery for Unresectable Gastric Cancer. Ann Surg Oncol. 2015;22:3618-3624
18. Satoh S, Okabe H, Teramukai S. et al. Phase II trial of combined treatment consisting of preoperative S-1 plus cisplatin followed by gastrectomy and postoperative S-1 for stage IV gastric cancer. Gastric Cancer. 2012;15:61-69
19. Okabe H, Ueda S, Obama K. et al. Induction chemotherapy with S-1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol. 2009;16:3227-3236
20. Japanese Research Society for Gastric Cancer. Japanese Classification of Gastric Carcinoma. 1st English ed. Tokyo: Kanehara & Co, Ltd. 1995
21. Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327-333
22. Garrido MM, Kelley AS, Paris J. et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49:1701-1720
23. Yang XJ, Huang CQ, Suo T. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575-1581
24. Ko KJ, Shim JH, Yoo HM. et al. The clinical value of non-curative resection followed by chemotherapy for incurable gastric cancer. World J Surg. 2012;36:1800-1805
25. Iwahashi M, Nakamori M, Tani M. et al. Complete response of highly advanced gastric cancer with peritoneal dissemination after new combined chemotherapy of S-1 and low-dose cisplatin: report of a case. Oncology. 2001;61:16-22
26. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. The Lancet Oncology. 2013;14:e218-e228
27. Hartgrink HH, Putter H, Klein Kranenbarg E. et al. Value of palliative resection in gastric cancer. Br J Surg. 2002;89:1438-1443
28. Medina-Franco H, Contreras-Saldivar A, Ramos-De La Medina A. et al. Surgery for stage IV gastric cancer. Am J Surg. 2004;187:543-546
29. Cunningham D, Allum WH, Stenning SP. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20
30. Ychou M, Boige V, Pignon JP. et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721
Author contact
![]() Corresponding author: Ying-Bo Chen, MD: Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, 651 E Dongfeng Road, Guangzhou, Guangdong, 510060, China. Tel: +86-020-87343625; E-mail: chenyborg.cn
Corresponding author: Ying-Bo Chen, MD: Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, 651 E Dongfeng Road, Guangzhou, Guangdong, 510060, China. Tel: +86-020-87343625; E-mail: chenyborg.cn

 Global reach, higher impact
Global reach, higher impact