3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(3):354-362. doi:10.7150/jca.16720 This issue Cite
Research Paper
RSF1 regulates the proliferation and paclitaxel resistance via modulating NF-κB signaling pathway in nasopharyngeal carcinoma
1. Department of Otolaryngology Head and Neck Surgery, Xiangya Hospital, Central South University, Xiangya Road 87, Changsha 410008, Hunan, China.
2. Otolaryngology Major Disease Research Key Laboratory of Hunan Province, Changsha, 410008, Hunan, China.
Received 2016-7-4; Accepted 2016-10-15; Published 2017-2-9
Abstract
Purpose: Aberrant expression and dysfunction of RSF1 has been reported in diverse human malignancies. However, its exact role in nasopharyngeal carcinoma (NPC) remains unclear.
Methods: The expression of RSF1 mRNA and protein were assayed by qRT-PCR and western blotting, and their correlations with clinicopathological parameters of patients with NPC were further analysed. Lentivirus mediated RSF1 shRNA and RSF1 cDNA were used to knockdown and upregulate the expression of RSF1. CCK8 assays and flow cytometry were applied to monitor the changes of proliferation and paclitaxel sensitivity caused by RSF1 modulation, inhibition of NF-κB pathway by inhibitor Bay 11-7082 and Survivin knockdown. Western blotting was used to detect protein alterations in NF-κB signaling pathway.
Results: Our present study demonstrated that both mRNA and protein expressions of RSF1 were increased and correlated with advanced NPC clinical stage. Functional analyses revealed that RSF1 inhibition or overexpression induced changes in cell cycle, apoptosis, and then led to altered proliferation and paclitaxel sensitivity in diverse NPC cells in vitro. Further mechanism investigation hinted that RSF1 overexpression in NPC CNE-2 cells activated NF-κB pathway and promoted the expression NF-κB dependent genes involved in cell cycle and apoptosis including Survivin. Importantly, inhibition of NF-κB pathway by Bay 11-7082 and knockdown its downstream Survivin reversed the paclitaxel resistance caused by RSF1 overexpression.
Conclusions: Taken together, our data indicate that RSF1 regulates the proliferation and paclitaxel resistance via activating NF-κB signaling pathway and NF-κB-dependent Survivin upregulation, suggesting that RSF1 may be used as a potential therapeutic target in NPC.
Keywords: Nasopharyngeal carcinoma, RSF1, Proliferation, NF-κB, Survivin, Paclitaxel resistance.
Introduction
Nasopharyngeal carcinoma (NPC) is one kind of squamous cell carcinoma of the head and neck, which is originated from the nasopharynx and possesses a high incidence in Southern China and Southeast Asia [1]. Recent improvement of NPC management strategies including radiotherapy, chemotherapy and target therapy has greatly increased the therapeutic efficacy [2, 3]. However, patients with advanced clinical stages and resistance to therapy including chemotherapy still display frustrated survival status and prognosis [4]. Therefore, understanding of the dysfunctional mechanisms involved in these oncogenes or suppressors associated with malignant progression of cancer will benefit our future treatment for cancer patients.
RSF1, also known as hepatitis B X-antigen-associated protein (HBXAP), is a member of ATP-dependent chromatin remodeling factors [5]. RSF1 interacts with sucrose non-fermenting protein 2 homologous (hSNF2H) to form a RSF-1/hSNF2H complex [6, 7], which in turn functions in different biological and pathological processes including transcriptional regulation, DNA replication and cell cycle progression via regulating the nucleosome remodeling [8]. Recently, the abnormal expression and dysfunction of RSF1 has been discovered in a wide range of human solid malignancies including breast cancer [9], prostate carcinoma [10], ovarian carcinoma [5, 11] and colon cancer [12] etc. Elevated RSF1 expression is tightly correlated with the clinicopathological variables and is also found to be an independent prognostic factor in cancers [7, 9, 12-14]. Loss of function analyses have demonstrated that RSF1 knockdown can decrease the proliferation and paclitaxel resistance in ovarian cancer, indicating its potential role as a molecular therapy target in tumor [6, 7, 12]. However, the exact role of RSF1 in the development and progression of human cancers remains poorly studied.
A previous report based on archival tissues has revealed that RSF1 overexpression was common and was associated with an adverse prognosis in patients with NPC [15]. Therefore, in the present study, we aimed to investigate whether the aberrant expression of RSF1 was a driving force for the malignant behaviors including proliferation, invasion and chemotherapeutic response in NPC. Furthermore, we also explored the molecular mechanisms associated with these alterations that were caused by RSF1 overexpression.
Methods
NPC tissues collection
A total of 46 cases of NPC tissues and 11 cases of the noncancerous epithelial tissues (NCET) were biopsied from June 2011 to Mar 2012 at the Outpatient Department of the Head and Neck Surgery, Xiangya hospital of Central South University, Changsha, Hunan, China. All patients were diagnosed as NPC via histopathological examination, which had no previous malignancies and no therapy history. Metastases were confirmed by clinical examination, imaging evaluations of CT and MRI. Clinical stages were defined according to the 2008 NPC staging system of China [16]. The clinical characteristics of all patients were summarized in detail in Table 1. All specimens were chosen to be big enough to be spliced into two parts. One was snap-frozen immediately and stored in liquid nitrogen for total RNA extraction and the remaining part was prepared for total protein extraction. The study was approved by the Research Ethics Committee of the Xiangya hospital of Central South University, Changsha, China. Informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
Clinicopathological features of the 46 patients with NPC.
| Parameters | Numbers | Percentage (%) |
|---|---|---|
| Age | ||
| ≤45 | 24 | 52.2 |
| >45 | 22 | 47.8 |
| Sex | ||
| Female | 10 | 21.7 |
| Male | 36 | 78.3 |
| Tumor size | ||
| T1 | 6 | 13.0 |
| T2 | 13 | 28.3 |
| T3 | 16 | 34.8 |
| T4 | 11 | 23.9 |
| Clinic stage | ||
| I | 6 | 13.0 |
| II | 12 | 26.1 |
| III | 16 | 34.8 |
| IV | 12 | 26.1 |
| Lymph node metastasis | ||
| N0 | 20 | 43.5 |
| N+ | 26 | 56.5 |
RNA isolation, cDNA transcription and quantitative real time PCR
Total RNAs of NPC tissues and cells in different groups were extracted with TRIzol® Reagent (Invitrogen) according to the manufacture's recommendation. 2 µg qualified RNA was used for reverse transcription to synthesize cDNA (High Capacity RNA-to-cDNA Kit, Applied Biosystems). 25 µl reaction system was established and quantitative real time PCR assays were performed via the SYBR® Green PCR Master Mix (Applied Biosystems) on the Applied Biosystems 7500 Real-Time PCR System. The expression data were normalized to housekeeping gene GAPDH using the 2-∆CT/[2-(CT of target genes - CT of GAPDH)] method, then the expressions of RSF1 in NPC were presented as mean ± SD and compared with NCET [17]. The primer sequences used are listed in Table S1. All PCR experiments were performed in triplicate.
Protein extraction and Western blotting
Whole cell, nuclear and cytosolic proteins were extracted from NPC cell lines via Nuclear/Cytosolic Fraction Kit according to the manufacturer's manual (Cell Biolabs, Inc.). All the following western blot analyses were performed as we previously described [18-20]. In brief, 30-50 μg proteins were separated by 8-12% SDS-PAGE and then transferred onto PVDF membrane (Millipore). The blotted membranes were then incubated with the antibodies listed in Table S1. GAPDH or β—actin were used as internal controls.
Cell cultures and transfection
Human NPC CNE-2, 5-8F and 6-10B cell lines were provided by the Cell Center of Central South University, Changsha, China. All these NPC cell lines were cultured in RPMI-1640 (GIBCO) medium with 10% fetal bovine serum (FBS), 100 μg/ml penicillin and 100 μg/ml streptomycin and maintained at 37 °C with 5% CO2. Cells in the exponential phase were used in the following experiments.
Lentivirus mediated RSF1 shRNA Plasmids and control plasmids were purchased from Santa Cruz Biotechnology (sc-72261-SH), which generally consist of a pool of 3 to 5 lentiviral vector plasmids each encoding target-specific 19-25 nt (plus hairpin) shRNAs designed to knockdown RSF1 expression. The RSF1 cDNA was inserted into the pLenti-C-mGFP vector (NM_016578.3, Origene). The lentivirus plasmid transfection was performed as we previously reported [19].
For Survivin siRNA transfection, 5 μl siRNA was used to knockdown the expression of Survivin according to the protocol provided by Santa Cruz (sc-29499). Gene and protein knockdown efficiencies were examined by qPCR and Western blotting.
Cells treated with chemicals
NPC cells (3000-5000/well) were seeded into 96-well plate. Then CCK-8 assay kits (Sigma-Aldrich) were used to assay the survival cell at different time points as we did previously [19, 21]. For paclitaxel cytotoxicity experiments, 5000 cells were seeded into 96-well plate and diverse concentrations of paclitaxel with or without NF-κB inhibitor (5 μM Bay 11-7082) were added to different groups of NPC cells for 48 hours and CCK-8 assays were performed. The optical densities were measured at 450 nm. These experiments were performed three times.
Cell apoptosis and cell cycle analysis by Flow cytometry
Cell apoptosis and cell cycle analyses by flow cytometry were performed similarly as we did previously [19].
Transwell invasion assay
The transwell invasion assay was performed according to our previous publications [19, 21].
Statistical analysis
All the statistical tests were performed using IBM SPSS 20.0 software. Quantitative data was presented as the means ± SD. Student's t-test (two-tailed) or a one-way ANOVA test was employed to compare statistical differences. P values < 0.05 were defined as statistically significant. * P < 0.05; ** P < 0.01; *** P < 0.001.
Results
RSF1 expression is increased in NPC tissues
RSF1 has been reported to be upregulated in numerous human malignancies. Its aberrant expression is tightly with diverse progressive cancer phenotypes [5, 14, 22, 23]. Therefore, we initially examined the expression of mRNA and protein of RSF1 in 46 tissue samples collected from the biopsies from patients with NPC. Compared to 11 cases of the noncancerous epithelial tissues (NCET) from nasopharynx, the relative expression of RSF1 mRNA was obviously increased in NPC (P < 0.01; Fig.1A). Consistent with the qRT-PCR results, western blotting data revealed that the expression of RSF1 protein was also upregulated in NPC tissues (P < 0.01; Fig.1D and Supplementary Fig.1A). However, no significant correlation between mRNA and protein expressions was observed (P > 0.05, Supplementary Fig.1B). Next, we analyzed the associations between RSF1 and the clinicopathological variables in patients with NPC. Our data demonstrated that elevated expressions of both RSF1 mRNA (Fig.1B) and protein (Fig.1E) were tightly correlated with NPC clinical stages (I+II v.s III+IV). However, positive connections between RSF1 and metastasis existed only at mRNA level (Fig.1C) but not at protein level (Fig.1F). In addition, mRNA and protein expression of RSF1 showed no significant connections with other clinical parameters such as age and gender (All P > 0.05). Collectively, these data indicate that RSF1 displays a higher expression in NPC specimens, which promotes us to further investigate the potential functional alterations caused by its overexpression.
RSF1 regulates the proliferation of NPC cells in vitro
In order to observe the effects of RSF1 on the malignant behaviors of NPC cells, its expression was initially assayed in 6 NPC cell lines (Fig.2A). And then lentivirus mediated shRNA was used to inhibit RSF1 expression in 6-10B and 5-8F cell lines, which showed a relatively higher expression of RSF1 protein. Western blot assays indicated RSF1 protein was efficiently blocked in both NPC 6-10B and 5-8F cell lines (Fig.2B). As displayed in Fig.2C and D, RSF1 knockdown successfully led to decreased growth ability of NPC cells in vitro at day 3 and following days. Flow cytometry analysis using propidium iodide DNA staining further confirmed that the growth inhibition was caused by RSF1-mediated cell cycle arrest at G1/S checkpoint (Fig.2E) and synchronously increased apoptosis (Fig.2F) in both 6-10B and 5-8F cells. Correspondingly, CNE-2 cells with RSF1 overexpression displayed enhanced growth capacity (Supplementary Fig.2A and 2B), increased cells in phases of S and G2/M (Supplementary Fig.2C), and decreased apoptotic rate (Supplementary Fig.2D). Additionally, no significant influence of RSF1 on NPC invasion was found in these two NPC cell lines via the Transwell invasion assays in vitro (data not shown). Together, these data indicate that RSF1 functions as an oncogene that regulates the growth of NPC cells via influencing cell cycle and apoptosis.
RSF1 participates in the paclitaxel resistance in NPC cells in vitro
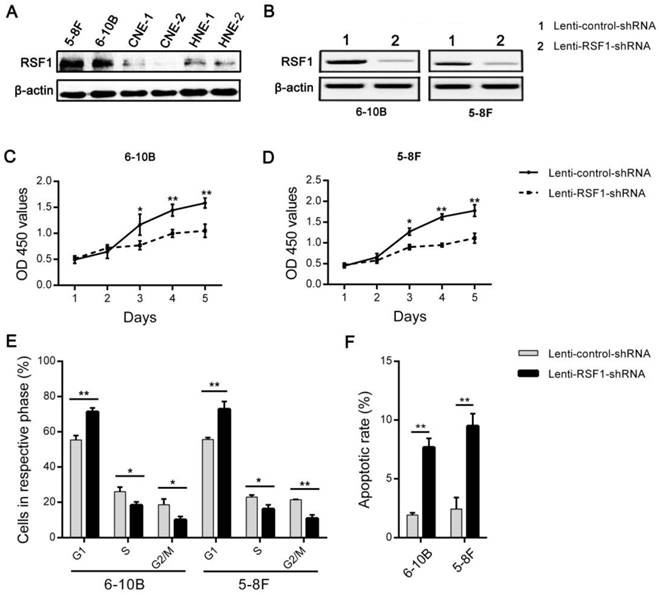
Paclitaxel, one member of the taxane family of chemotherapy agents, is widely used in the management of numerous human cancers including NPC [24, 25]. However, the emergence of chemoresistance limits the efficacy of its clinical application [24, 26]. Therefore, we upregulated the expression of RSF1 in NPC CNE-2 cells (Fig.3B), which showed the lowest RSF1 protein expression in these 6 NPC cell lines (Fig.2A). Consequently, RSF1 overexpression rendered CNE-2 cells to be more resistant to paclitaxel at the concentrations of IC50 and IC70 (Fig.3A and C). Conversely, RSF1 inhibition in 5-8F cells led to enhanced sensitivity to paclitaxel in vitro at the concentrations of IC30 and IC50 (Fig.3D, E and F). Together, these results confirm that RSF1 inhibition potentiates NPC cells to be sensitive to paclitaxel.
Elevated expression of RSF1 mRNA and protein correlates with clinicopathological parameters. (A and D) Expression of RSF1 mRNA and protein was assayed by qRT-PCR and western blot assay in 46 cases of NPC tissues and 11 cases of NCET tissues. (B, C, E and F) Expression levels of RSF1 mRNA and protein were correlated with the clinicopathological variables including clinical stages and metastasis in patients with NPC. Protein expression levels were quantified by FluorChem FC2 (San Leandro, CA).
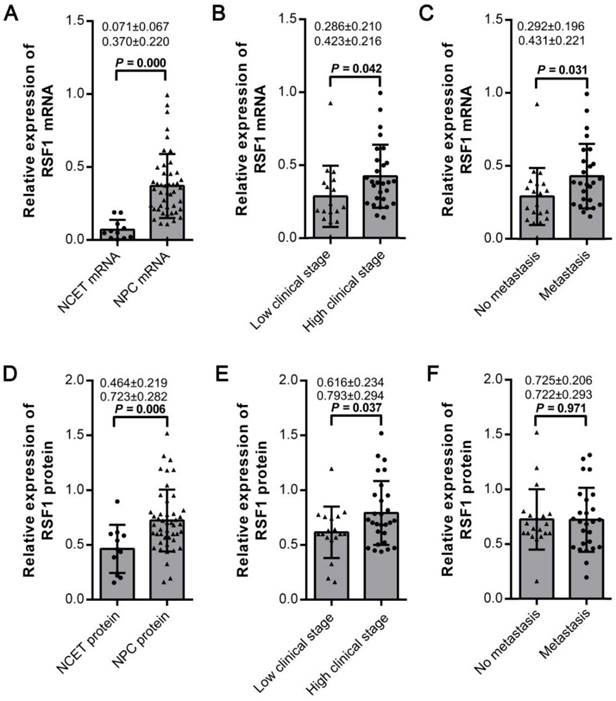
RSF1 regulates the proliferation of NPC cells. (A) RSF1 protein was assayed by western blotting in 6 NPC cell lines. (B) Lentivirus mediated RSF1 shRNA and control shRNA were used to transfect NPC 6-10B and 5-8F cells. 72 hours after transfection, RSF1 knockdown efficiency was examined by western blotting. (C and D) The effects of RSF1 knockdown on the proliferation of 6-10B and 5-8F cells were investigated at day 1 to 5 via CCK8 assays. (E and F) Alterations of cell cycle and apoptosis in 6-10B and 5-8F cells were examined by flow cytometry 72 hours after RSF1 knockdown. * P < 0.05, **P < 0.01.
RSF1 activates NF-κB pathway and enhances Survivin expression
To elucidate how RSF1 modulates paclitaxel resistance in NPC, we further strived to clarify its downstream signaling and key effector molecules. NF-κB has been involved in multiple processes associated with cancer such as malignant proliferation and drug resistance [27, 28]. The positive connection between RSF1 and NF-κB has also been reported elsewhere [7, 29]. We ectopically overexpressed RSF1 in NPC CNE-2 cells, which correspondingly led to increased phosphorylation of inhibitor IκBα, and meantime decreased phospho-p65 (p-p65) in cytoplasm and elevated p-p65 level in nucleus (Fig.4A). These results indicate that RSF1 promotes activation of NF-ĸB p65 and its nucleus translocation. Then, we evaluated the mRNA and protein expression of NF-κB-dependent genes, which associated with pro-proliferation and anti-apoptosis [28]. Accompanied with NF-κB activation, RSF1 activation simultaneously increased mRNA and protein expression of Cyclin D1, which was required for cell cycle during G1/S transition (Fig.4A and 4B). In addition, anti-apoptotic molecules including Bcl-2, Bcl-xL were also upregulated (Fig.4A and 4B). Interestingly, Survivin gene has been previously reported to have a potential role in NPC chemo- and radio-resistance by our group [30], which was also correspondingly increased as a result of RSF1 overexpression (Fig.4A and 4B). Taken together, these results highlight the critical role of RSF1 in the activation of NF-κB signaling pathway and NF-κB-dependent genes expression that regulate cell cycle and apoptosis.
NF-κB activation and Survivin are essential for RSF1 mediated paclitaxel resistance in NPC
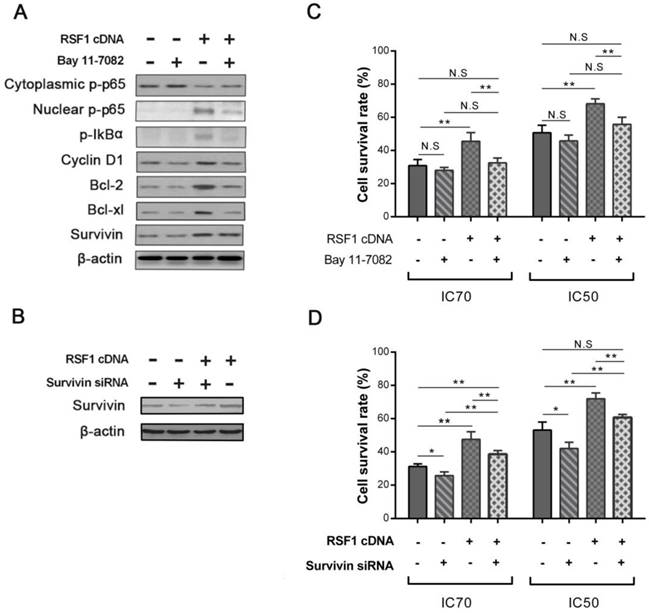
To directly investigate the function of NF-κB activation in RSF1 mediated paclitaxel resistance, we used NF-κB signaling inhibitor Bay 11-7082 to see whether NF-κB inactivation can reverse the effects caused by RSF1 overexpression. As an irreversible molecule to inhibit IκBα phosphorylation, 5 μM Bay 11-7082 treatment successfully decreased p-IκBα and p-p65 and impeded the nuclear translocation of p65 in RSF1-overexpressing CNE-2 cells, which accordingly led to decreased levels of Bcl-2, Bcl-xL and Survivin (Fig.5A). As expected in RSF1 overexpressing CNE-2 cells, Bay 11-7082 significantly recovered the sensitivity of paclitaxel at the concentrations of IC50 and IC70 (Fig.5C). Furthermore, we also focused on the function of Survivin, the expression of which was augmented by RSF1 overexpression and activation of NF-κB signaling pathway in this study. Survivin siRNA was used to hinder its expression in RSF1 overexpressing CNE-2 cells (Fig.5B), we found that paclitaxel resistance caused by RSF1 overexpression was correspondingly declined following the inhibition of Survivin (Fig.5D). These data clearly reveal that RSF1 activates NF-κB pathway and downstream Survivin, which together regulates the process of paclitaxel resistance in NPC.
Discussion
Increased expression of RSF1 has been reported in a variety of human malignancies and correlated with multiple malignant behaviors of cancer cells [14]. Our present investigation has found that protein and mRNA of RSF1 is increased in NPC tissues. Moreover, RSF1 overexpression is tightly associated with malignant proliferation and paclitaxel resistance of NPC cells in vitro. Further mechanism exploration reveals that NF-κB activation by RSF1, together with NF-κB-dependent pro-proliferative and anti-apoptotic molecules including Survivin, are implicated in RSF1-mediated paclitaxel resistance in NPC.
RSF1 participates in the paclitaxel resistance in NPC cells. (A and D) Exposed to increased concentrations of paclitaxel, inhibitory rates analyzed by SPSS 20.0 indicated the concentrations of IC30, IC50 and IC70 in CNE-2 and 5-8F cells. (B and C) CNE-2 cells were transfected with Lenti-RSF1-cDNA and vector, then survival rates were assayed with CCK8 48 hours after exposure to paclitaxel at the concentrations of IC50 and IC70. (E and F) 5-8F cells were transfected with lenti-RSF1-shRNA and control shRNA, survival rates were examined 48 hours after exposure to paclitaxel at the concentrations of IC30 and IC50. * P < 0.05, **P < 0.01.
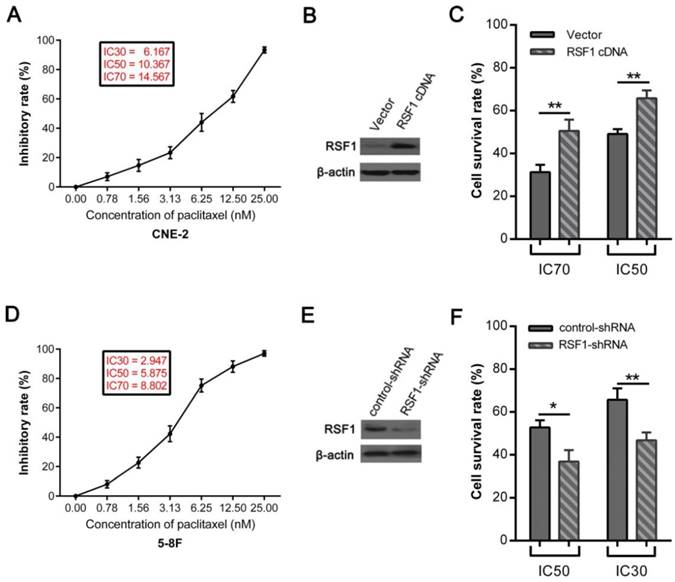
RSF1 activates NF-κB pathway and enhances Survivin expression. RSF1 was overexpressed in NPC CNE-2 cells, and proteins of NK-κB signaling pathway and its dependent genes were assayed via western blotting (A) and qPCR (B). **P < 0.01, ***P < 0.001.
NF-κB activation and Survivin are essential for RSF1 mediated paclitaxel resistance. (A) Inhibitor Bay 11-7082 was used to inhibit the activation of NF-κB pathway caused by RSF1 overexpression. (C) Bay 11-7082 restored the sensitivity of paclitaxel caused by RSF1 overexpression in NPC CNE-2 cells. (B and D) Survivin inhibition recovered the sensitivity of paclitaxel caused by RSF1 overexpression in NPC CNE-2 cells. * P < 0.05, **P < 0.01. N.S indicates non-significant.
The connections between RSF1 expression and clinicopathological parameters and prognosis has been reported in numerous human solid cancers including breast cancer [9], hepatocellular carcinoma [31], prostate cancer [10], ovarian cancer [5], gastric adenocarcinoma [13], bladder urothelial carcinoma [32] and colon cancer [12] etc. These abundant publications display a consistently elevated expression of RSF1 in cancer tissues compared to paracancerous or normal tissues. Several studies above also indicate the role of RSF1 as a prognostic biomarker in human cancers [10, 12, 13]. NPC RSF1 overexpression in our study is similar to previous reports, especially Tai HC group also confirms that RSF1 is an independent adverse prognosticator for the survival of patients with NPC [15]. The positive evidence of RSF1 in clinical samples promotes us to investigate the exact function of RSF1 in cancer malignant behaviors.
Previous reports show that RSF1 enables assembly of centromeric core nucleosomes and functions in the process of mitosis [8]. Meantime, RSF1 facilitates the process of DNA repair via accelerating the assembly of CENP-S and CENP-X at DNA damage sites [33-35]. RSF1 depletion also causes aberrant mitotic progression and chromosome misalignment [8, 36]. These reports strongly hint a potential role of RSF1 in cell cycle and apoptosis. As expected, in our present study, RSF1 knockdown inhibits cell growth ability and paclitaxel resistance, which is correlated with cell cycle arrest at G1/S phase and increased apoptosis. RSF1 overexpression correspondingly regulates the expression of proteins associated with cell cycle progression and apoptosis, which include Cyclin D1, Bcl-2 protein family (Bcl-2 and Bcl-xL) and Survivin. Cyclin D1 and Cyclin E1, as members of cyclin protein family that are involved in cell cycle progression, are also reported to be regulated by or directly interact with RSF1 by other groups [37, 38].
One major discovering of our study is that NF-κB signaling pathway and Survivin expression are critical to paclitaxel resistance in RSF1 overexpressing NPC cells. NF-κB signaling family contains 5 members including p65 (RelA), RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2), which can form hetero- or homodimers and interact with DNA to regulate numerous target genes after activation [39]. Inactivated NF-κB complex is usually located in the cytoplasm by the combination with inhibitor kappaBs (IκBs). Once IκBs are phosphorylated and degraded by upstream kinases such as IKK, the following translocation of p65 into the nucleus can activate the transcription of target genes [39]. In numerous human malignancies, aberrant activation of NF-κB and its target genes are involved in diverse cancer behaviors including chemoresistance [27, 39, 40]. In our study, RSF1 overexpression leads to activation of NF-κB signaling pathway, which is indicated by the phosphorylation of its inhibitor IκBα and the activation and translocation of p65 from cytoplasm to nucleus. Consequently, the nuclear p65 regulates the expression of target genes, such as Cyclin, Bcl-2 family and Survivin. These findings are consistent with the reports in ovarian cancers, in which RSF1 functions as a coactivator for NF-κB to regulate NF-κB-dependent genes [6, 7]. We have to note that RSF1 is not a kinase for phosphorylation, therefore the phosphorylation of IκBα caused by RSF1 may be explained by changes of other potential genes that can increase the phosphorylation of IκBα. Further studies are required to clarify what other genes are involved in the RSF1 mediated phosphorylation of IκBα. Finally, both NF-κB inactivation by molecule inhibitor and downstream Survinin knockdown can reverse paclitaxel resistance caused by RSF1 overexpression. Collectively, these data clearly indicate that RSF1 regulates the proliferation and paclitaxel sensitivity of NPC via the NF-kB signaling pathway and NF-kB dependent genes.
In summary, our current study demonstrates that RSF1, as an oncogene, has been implicated in the malignant proliferation and paclitaxel resistance in NPC, which is tightly with the activation of NF-κB signaling and NF-κB-dependent genes expression such as Survivin. Further mechanisms and in vivo studies are required to put it into clinical application as a molecular biotarget in future.
Supplementary Material
Additional File 1Supplementary Figures.
Supplementary Table S1.
Acknowledgements
This study was funded by grants from the National Natural Science Foundation of China (Nos. 81202128 and 30901663), the Natural Science Foundation of Hunan Province (No. 2015JJ3137), the Research Fund for the Doctoral Program of Higher Education of China (No. 20120162120049) and the Freedom Explore Program of Central South University (No. 2012QNZT099).
Conflicts of interest
The authors declare no conflict of interest.
References
1. Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30:114-9
2. Hong JS, Tian J, Han QF, Ni QY. Quality of life of nasopharyngeal cancer survivors in China. Curr Oncol. 2015;22:e142-7
3. Rottey S, Madani I, Deron P, Van Belle S. Modern treatment for nasopharyngeal carcinoma: current status and prospects. Curr Opin Oncol. 2011;23:254-8
4. Tan P, Liu Y, Yu C, Su Z, Li G, Zhou X. et al. EphA2 silencing in nasopharyngeal carcinoma leads to decreased proliferation, invasion and increased sensitization to paclitaxel. Oncol Lett. 2012;4:429-34
5. Shih IM, Sheu JJ, Santillan A, Nakayama K, Yen MJ, Bristow RE. et al. Amplification of a chromatin remodeling gene, Rsf-1/HBXAP, in ovarian carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14004-9
6. Choi JH, Sheu JJ, Guan B, Jinawath N, Markowski P, Wang TL. et al. Functional analysis of 11q13.5 amplicon identifies Rsf-1 (HBXAP) as a gene involved in paclitaxel resistance in ovarian cancer. Cancer research. 2009;69:1407-15
7. Yang YI, Ahn JH, Lee KT, Shih IM, Choi JH. RSF1 is a positive regulator of NF-κB-induced gene expression required for ovarian cancer chemoresistance. Cancer research. 2014;74:2258-69
8. Perpelescu M, Nozaki N, Obuse C, Yang H, Yoda K. Active establishment of centromeric CENP-A chromatin by RSF complex. J Cell Biol. 2009;185:397-407
9. Ren J, Chen QC, Jin F, Wu HZ, He M, Zhao L. et al. Overexpression of Rsf-1 correlates with pathological type, p53 status and survival in primary breast cancer. Int J Clin Exp Pathol. 2014;7:5595-608
10. Li H, Zhang Y, Zhang Y, Bai X, Peng Y, He P. Rsf-1 overexpression in human prostate cancer, implication as a prognostic marker. Tumour Biol. 2014;35:5771-6
11. Hennessy BT, Nanjundan M, Cheng KW, Nolden L, Mills GB. Identification of remodeling and spacing factor 1 (rsf-1, HBXAP) at chromosome 11q13 as a putative oncogene in ovarian cancer. European journal of human genetics: EJHG. 2006;14:381-3
12. Liu S, Dong Q, Wang E. Rsf-1 overexpression correlates with poor prognosis and cell proliferation in colon cancer. Tumour Biol. 2012;33:1485-91
13. Hu BS, Yu HF, Zhao G, Zha TZ. High RSF-1 expression correlates with poor prognosis in patients with gastric adenocarcinoma. Int J Clin Exp Pathol. 2012;5:668-73
14. Wu J, Hu L, Wu F, He T. Prognostic value of rsf-1/hbxap in human solid tumors: a meta-analysis of cohort studies. International journal of clinical and experimental medicine. 2015;8:1944-55
15. Tai HC, Huang HY, Lee SW, Lin CY, Sheu MJ, Chang SL. et al. Associations of Rsf-1 overexpression with poor therapeutic response and worse survival in patients with nasopharyngeal carcinoma. Journal of clinical pathology. 2012;65:248-53
16. CCSNPC. Report on revision of the Chinese 1992 staging system for nasopharyngeal carcinoma. J Radiat Oncol. 2013;2:233-40
17. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101-8
18. Li G, Liu Y, Su Z, Ren S, Zhu G, Tian Y. et al. MicroRNA-324-3p regulates nasopharyngeal carcinoma radioresistance by directly targeting WNT2B. Eur J Cancer. 2013;49:2596-607
19. Liu Y, Yu C, Qiu Y, Huang D, Zhou X, Zhang X. et al. Downregulation of EphA2 expression suppresses the growth and metastasis in squamous-cell carcinoma of the head and neck in vitro and in vivo. J Cancer Res Clin Oncol. 2012;138:195-202
20. Liu Y, Xie C, Zhang X, Huang D, Zhou X, Tan P. et al. Elevated expression of HMGB1 in squamous-cell carcinoma of the head and neck and its clinical significance. Eur J Cancer. 2010;46:3007-15
21. Yu C, Liu Y, Tan H, Li G, Su Z, Ren S. et al. Metadherin regulates metastasis of squamous cell carcinoma of the head and neck via AKT signalling pathway-mediated epithelial-mesenchymal transition. Cancer Lett. 2014;343:258-67
22. Chen TJ, Huang SC, Huang HY, Wei YC, Li CF. Rsf-1/HBXAP overexpression is associated with disease-specific survival of patients with gallbladder carcinoma. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2011;119:808-14
23. Fang FM, Li CF, Huang HY, Lai MT, Chen CM, Chiu IW. et al. Overexpression of a chromatin remodeling factor, RSF-1/HBXAP, correlates with aggressive oral squamous cell carcinoma. The American journal of pathology. 2011;178:2407-15
24. Nehate C, Jain S, Saneja A, Khare V, Alam N, Dubey RD. et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11:666-86
25. Chen C, Wang FH, An X, Luo HY, Wang ZQ, Liang Y. et al. Triplet combination with paclitaxel, cisplatin and 5-FU is effective in metastatic and/or recurrent nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 2013;71:371-8
26. Zhang X, Li W, Li H, Ma Y, He G, Tan G. Genomic methylation profiling combined with gene expression microarray reveals the aberrant methylation mechanism involved in nasopharyngeal carcinoma taxol resistance. Anticancer Drugs. 2012;23:856-64
27. Yang L, Zhou Y, Li Y, Zhou J, Wu Y, Cui Y. et al. Mutations of p53 and KRAS activate NF-kappaB to promote chemoresistance and tumorigenesis via dysregulation of cell cycle and suppression of apoptosis in lung cancer cells. Cancer Lett. 2015;357:520-6
28. Erstad DJ, Cusack JC Jr. Targeting the NF-kappaB pathway in cancer therapy. Surg Oncol Clin N Am. 2013;22:705-46
29. Huang JY, Shen BJ, Tsai WH, Lee SC. Functional interaction between nuclear matrix-associated HBXAP and NF-kappaB. Experimental cell research. 2004;298:133-43
30. Zhang S, Xiao J, Zhao S, Qiu Y, Liu Y, Wang C. et al. [Expression and significance of Survivin mRNA in xenotransplanted nasopharyngeal carcinoma treated by paclitaxel combined with radiotherapy]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;23:796-9
31. Xie C, Fu L, Xie L, Liu N, Li Q. Rsf-1 overexpression serves as a prognostic marker in human hepatocellular carcinoma. Tumour Biol. 2014;35:7595-601
32. Liang PI, Wu LC, Sheu JJ, Wu TF, Shen KH, Wang YH. et al. Rsf-1/HBXAP overexpression is independent of gene amplification and is associated with poor outcome in patients with urinary bladder urothelial carcinoma. Journal of clinical pathology. 2012;65:802-7
33. Helfricht A, Wiegant WW, Thijssen PE, Vertegaal AC, Luijsterburg MS, van Attikum H. Remodeling and spacing factor 1 (RSF1) deposits centromere proteins at DNA double-strand breaks to promote non-homologous end-joining. Cell cycle. 2013;12:3070-82
34. Helfricht A, van Attikum H. Remodeling and spacing factor 1 (RSF1): a rising star in DNA repair. Epigenomics. 2014;6:261-5
35. Pessina F, Lowndes NF. The RSF1 histone-remodelling factor facilitates DNA double-strand break repair by recruiting centromeric and Fanconi Anaemia proteins. PLoS biology. 2014;12:e1001856
36. Lee HS, Park YY, Cho MY, Chae S, Yoo YS, Kwon MH. et al. The chromatin remodeller RSF1 is essential for PLK1 deposition and function at mitotic kinetochores. Nature communications. 2015;6:7904
37. Sehdev AS, Kurman RJ, Kuhn E, Shih IM. Serous tubal intraepithelial carcinoma upregulates markers associated with high-grade serous carcinomas including Rsf-1 (HBXAP), cyclin E and fatty acid synthase. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2010;23:844-55
38. Keilty D, Buchanan M, Ntapolias K, Aleynikova O, Tu D, Li X. et al. RSF1 and not cyclin D1 gene amplification may predict lack of benefit from adjuvant tamoxifen in high-risk pre-menopausal women in the MA.12 randomized clinical trial. PloS one. 2013;8:e81740
39. Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344-62
40. Gilmore T, Gapuzan ME, Kalaitzidis D, Starczynowski D. Rel/NF-kappa B/I kappa B signal transduction in the generation and treatment of human cancer. Cancer Lett. 2002;181:1-9
Author contact
![]() Corresponding author: Shuai Zhang, Department of Otolaryngology Head and Neck Surgery, Xiangya Hospital, Central South University, No. 87 Xiangya Road, Changsha 410008, Hunan, China. Tel.: +86073184327469; Fax: +86073184327469; E-mail: 414570540com (Zhang Shuai).
Corresponding author: Shuai Zhang, Department of Otolaryngology Head and Neck Surgery, Xiangya Hospital, Central South University, No. 87 Xiangya Road, Changsha 410008, Hunan, China. Tel.: +86073184327469; Fax: +86073184327469; E-mail: 414570540com (Zhang Shuai).

 Global reach, higher impact
Global reach, higher impact