Impact Factor
ISSN: 1837-9664
J Cancer 2016; 7(10):1328-1339. doi:10.7150/jca.15419 This issue Cite
Review
Pancreatic Cancer from Molecular Pathways to Treatment Opinion
1. 1ST Department of Surgery, University Hospital of Alexandroupolis, Democritus University of Thrace, Dragana, Alexandroupolis, 68100 Thrace, Greece.
2. Department of Medical Oncology, University Hospital of Alexandroupolis, Democritus University of Thrace, Dragana, Alexandroupolis, 68100 Thrace, Greece.
3. Research Laboratory and International Collaboration, Bon Secours Cancer Institute, VA, USA.
Received 2016-3-1; Accepted 2016-5-1; Published 2016-6-25
Abstract
Pancreatic cancer is considered one of the most lethal malignances. It has been observed that the five year survival rate is less than 5%. Early diagnosis, understanding the risk factors and investigation of the molecular pathways with targeted therapy are the keys for efficient treatment. Moreover; there are several local treatments for patients with unresectable pancreatic cancer. There are several combined therapies with chemotherapy and radiotherapy, however; a local therapy approach for many patients with poor performance status are in need. For those patients with good performance status new polychemotherapy regimens are used with success and increased survival improvement. Polychemotherapy has been observed to increase the rate of radical resections in some cases. Second line therapy is used for patients with good performance status and metastatic disease. Oxaliplatin-based regimens are mostly used, however; there are several other drugs that are being developed. Unfortunately, targeted therapy has not presented the expected efficiency. Moreover; immunotherapy; another treatment approach for several cancers types has again failed to present positive results for pancreatic cancer. In the current mini review, we will present information from the diagnosis to molecular pathways and targeted treatment.
Keywords: Chemotherapy, Irreversible electroporation, Pancreatic cancer, Radio-frequency, Stereotactic radiotherapy.
Introduction
Currently pancreatic ductal adenocarcinoma (PDAC) is considered the fourth leading cause of cancer death and is considered one the most lethal malignances. Surgery is considered the most effective treatment since the five-year survival rate is only 5%.[1] Upon diagnosis, only 20% of patients are fit for surgery based on disease staging however; it has been observed that disease recurrence is up to 80% of these patients.[2] Unfortunately, most commonly PDAC does not present signs for early diagnosis and therefore patients are diagnosed at advanced stage. Until now there are no clear guidelines regarding screening of patients even for those at high risk for pancreatic cancer such as those with a family history of pancreatic cancer and chronic pancreatitis). Investigation of pancreatic cancer (PC) has brought out four major driver genes: V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS), cyclin-dependent kinase Inhibitor 2A (CDKN2A), Tumor protein p53 (TP53), and SMAD family member n°4 (SMAD4). KRAS mutation and alterations in CDKN2A have been observed in pancreatic tumorigenesis. The most important tools for PC are endoscopic ultrasonography and endoscopic ultrasonography-guided fine-needle aspiration with 19 G, 21G and 22G which offer high diagnostic ability. One of the most common approaches for treatment after surgical resection is considered adjuvant chemotherapy with gemcitabine or S-1, an oral fluoropyrimidine derivative, or oxalipaltin. On the other hand FOLFIRINOX (fluorouracil, folinic acid [leucovorin], irinotecan, and oxaliplatin) and gemcitabine plus nanoparticle albumin-bound paclitaxel (nab-paclitaxel) are the treatments of choice for patients who are not surgical candidates but have good performance status. For now the most important improvement concerns to the conventional chemotherapy are represented by FOLFIRINOX and gemcitabine plus nab-paclitaxel regimens, but until now results in a modest outcome advantage.[3, 4] Regarding PC there has been no significant progress in the field of targeted therapy. A tyrosine kinase inhibitor (TKI) of epidermal growth factor receptor (EGFR), Eroltinib is the only biological agent approved for pancreatic cancer. However; its effect results in a very modest survival benefit in unselected patients.[5] In recent decades, several combinations of classic chemotherapy and novel biological agents have been studied, but they have not improved overall survival, and furthermore, those trials did not use biomarkers to select responder patients.[6] Moreover; several local therapies are being investigated for poor performance patients.[7, 8]
Current Management of Pancreatic Cancer
Surgical resection
Epidemiology data indicate that only 20% of patients who present with early disease are fit for radical surgical resection based on their staging. [9, 10] Adjuvant treatment is considered necessary with gemcitabine or 5-fluorouracil and/or chemoradiation as standard of care.[11] There are several randomized controlled trials[12, 13] demonstrating increased overall survival (OS) with postoperative therapy and therefore it is considered to be one of the most important advances in the treatment of pancreatic cancer.[10] Also, neoadjuvant chemotherapy is applicable in a number of patients in order to improve surgical margins of borderline resectable tumors.[14-17]
Locally advanced and metastatic disease
First line treatment has been established with gemcitabine or gemcitabine-based combination chemotherapy for advanced pancreatic cancer, however; it has been observed that the median survival rate is approximately 9 mo.[10, 18, 19] Further investigation with other agents has provided an advantage on the survival and quality of life with FOLFIRINOX (folinic acid, 5-fluorouracil, irinotecan, oxaliplatin) compared to gemcitabine alone. It was observed that this regimen significantly improved the OS, progression-free survival and objective response rate of patients with pancreatic cancer.[3] Approximately 10% of patients receiving these regimens have presented two year survival, which is a rare event in advanced disease.[4, 20] These two regimens have been associated with increased toxicities and therefore they can be offered to patients with good performance status.[3, 4] The care of patients with poor performance status or metastatic disease remains palliative, and gemcitabine based therapies have limited efficacy, however; local therapies can be used on a case by case choice.[7]
Targeted therapy
In the past decade the exploration of molecular pathways has provided targeted therapy which revolutionized cancer treatment for several cancer types and thus treatment for PC.[21] However; PC has a genetically heterogeneous nature[22] and therefore targeted therapies such as small molecule inhibitors and monoclonal antibodies have been sought to inhibit constitutively-active cell surface signaling molecules. Current results of phase I-III clinical trials (summarized by Seicean et al. [23]) are disappointing, increased treatment resistance is observed due to the high frequency of KRAS2 mutations and upregulation of alternate signaling pathways.[10, 24] (Table 1) Until now only erlotinib, a small molecule inhibitor of the epidermal growth factor receptor inhibitor, has been approved, in combination with gemcitabine, which offers a very modest but statistically significant increase in survival of two weeks.[5]
Imaging staging
Upon diagnosis staging is the next major step for optimal patient management. Pancreatic cancer is staged according to the TNM classification, which is based on the assessment of resectability by computed tomography (CT). A Consensus Statement of the Society of Abdominal Radiology and the American Pancreatic Association [25] has been recently presented with the international recommendations for the staging of PC. The standardization of imaging protocol was a necessity and it was long awaited to help overcome the difficulties encountered identifying the margins of the primary tumour and thereby in assessing its respectability. This imaging protocol provides improved decision-making process also allows easier communication and comparison among different institutions. While CT and magnetic resonance imaging (MRI)
perform similarly in the staging of PC [26, 27], CT remains for now the gold standard with access to MRI being limited in order to determine whether the patient can undergo radical resection.[25, 28] TNM staging includes: Tumor characteristics: size, appearance (hypo-, iso-, hyperattenuating compared with normal pancreatic parenchyma), location (uncinate process, head, body, tail) and biliary or pancreatic duct narrowing. Most cases of PC are slightly hypoattenuating but there are cases were the only sign of tumor is an abrupt interruption of the pancreatic or biliary duct. - Vascular evaluation: each of the peripancreatic vessels, superior mesenteric artery, (SMA), coeliac axis (CA), common hepatic artery (CHA) and main portal vein (PV) and superior mesenteric vein has to be carefully evaluated with regards to potential focal deformities or change in the vessel caliber and to the degree of tumor contact (more or less than 180_), including soft tissue contact and hazy attenuation zones surrounding the tumor. Evaluation of first SMA branches as well as the gastroduodenal artery is necessary as it can affect the surgical resection plan therefore it must be described. The assessment of arterial variants (especially right hepatic artery and arcuate ligament compression) is also mandatory. - Lymph node description is necessary as regional enlarged lymph nodes can either be metastatic or reactive, they must be described precisely (location and size in terms of short axis diameter). Moreover; pathological lymph nodes located in the retroperitoneum are considered metastatic. Extrapancreatic findings are considered direct extension to surrounding organs (transverse colon, stomach, spleen/) and focal hepatic lesions or peritoneal carcinomatosis.
Serum tumor markers
There are several serum biomarkers that provide early signs of pancreatic cancer. In specific the combination of serum carbohydrate antigen (CA) 19-9 and carcinoembryonic antigen (CEA)[29-31] has been reported to decrease sensitivity to 37%, but increase specificity to 84% compared with CA19-9 alone.[32] Recent evidence indicate that a serum protein panel biomarker consisting of CA125, CA19-9, and laminin γC (LAMC2) can significantly improve performance in detecting pancreatic cancer compared with CA19-9 alone under several conditions (ie, all pancreatic cancer and benign conditions, p<0・005; early-stage pancreatic cancer and benign conditions, p<0・05; and early-stage pancreatic cancer and chronic pancreatitis, p<0・05).[33] The serum biomarkers CA19-9 and CA125 have been observed to have the highest sensitivity for detecting preclinical pancreatic cancer because at a 95% specificity, CA19-9 has a sensitivity of 68% for up to 1 year and 53% for up to 2 years before diagnosis. It was observed that the combination of CA19-9 and CA125 improved sensitivity because the concentration of CA125 was raised in about 20% of CA19-9-negative cases.[34]
Gene Alteration and Molecular Pathology of Pancreatic Cancer
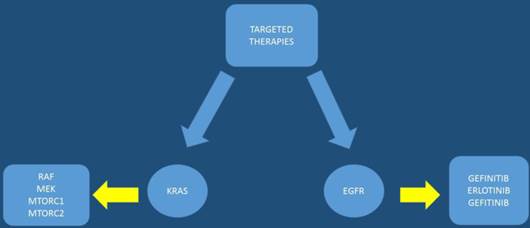
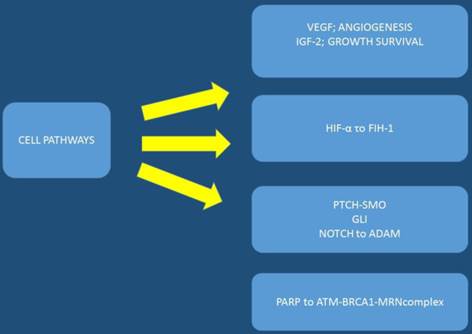
The pancreatic cancer genome has been investigated and 63 genetic alterations were found.[22] Therefore, it is necessary to try and combine the pathological features with the genetic alterations in order to develop an effective treatment. After molecular analysis in several pancreatic cancer specimens it was observed that known cancer genes and traditional cancer signaling pathways are involved in tumorigenesis and treatment effectiveness. The KRAS gene, which encodes a small GTPase that regulates the downstream signaling of growth factor receptors, is one of the most known mutated oncogenes found in most pancreatic cancers at advanced stages mostly in codon 12.[35, 36] Kras mutations are observed early in human pancreatic intraepithelial neoplasia (PanIN) progression.[37, 38] Other alterations in tumor suppressor genes have been observed with INK4 Tumor Suppressor Proteins in the CDK4/Rb Pathway (INK4A), Breast cancer 2 (BRCA2), and Serine/threonine kinase 11 (LKB1) in pancreatic cancer. It has been observed in a previous study that the tumor suppressor gene, P16/CDKN2A, which encodes a critical cell cycle regulator, is inactivated in > 90% of pancreatic cancer.[39] Moreover; mutation of the p53 gene is closely associated with cellular responses to cytotoxic stress by contributing to both cell cycle arrest and cell apoptosis.[40] [22] Alteration of SMAD4 which is a tumor suppressor gene that encodes the transforming growth factor beta (TGFβ) signaling pathway, has been found in approximately 55% of patients with pancreatic cancer.[41] Identifying early mutations in SMAD4 is important since it was found to be associated with a poorer prognosis and widespread metastases.[42] The mismatch repair gene, MutL homolog 1 (MLH1), and the cationic trypsinogen gene, Protease, serine, 1 (trypsin 1) (PRSS1), are also often found mutated in pancreatic cancer.[43] Based on the typical age of onset these mutations have been observed to b associated with the impact of malignant progression rather than cancer initiation. In some pancreatic cancers B-Raf proto-oncogene, serine/threonine kinase (BRAF) mutations have been observed rather than KRAS.[44] BRAF encodes RAF, a serine/threonine kinase belonging to a family of Mitogen-activated protein kinase kinase (MEK) (Figure 1). MEK activates extracellular-signal-regulated kinases (ERK), which forms the Mitogen-activated protein kinase (MAPK) signaling pathway. It has been found that mutations of KRAS and BRAF eventually result in triggering the MAPK signaling, which develop pancreatic cancer. Activation of the MAPK pathway has been observed in benign lesions, and late stage pancreatic cancer.[45] Overexpressed MAPK through the active form of RAF results in PanIN/pancreatic ductal adenocarcinoma formation;and the silencing of MAPK signaling inhibits tumor initiation.[46, 47] Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) signaling is another important pathway that has been studied in pancreatic cancer along with the MAPK pathway. The PI3K signaling mediates cell growth and survival via several downstream substrates such as protein kinase b (Akt) serine/threonine-specific protein kinase that plays a key role in multiple cellular processes, p70-S6K a serine/threonine kinase, and mammalian target of rapamycin (mTOR) (Figure 2). Constant activation of PI3K has been shown to be closely associated with the carcinogenesis of pancreatic cancer.[48] The PI3K downstream effector, Akt, is amplified in 10%-20% of pancreatic cancers[49] Several growth factor receptors, including vascular endothelial growth factor (VEGF) and insulin-like growth factor 1 receptor (IGF1R), are also expressed in pancreatic cancer.[50] (Figure 3,4)
MTORC1: mammalian target of rapamycin complex 1 or mechanistic target of rapamycin complex 1, MTORC2: mammalian target of rapamycin complex 1 or mechanistic target of rapamycin complex 1, KRAS: V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, RAF: proto-oncogen, MEK: Mitogen-activated protein kinase kinase, EGFR: epidermal growth factor receptor.
VEGF: vascular endothelial growth factor, IGF-2: insulin like growth factor-2, HIF-α: Hypoxia-inducible factor 1-alpha, asparaginyl hydroxylase enzyme, PARP: Poly ADP ribose polymerase, PTCH-SMO: a cell-surface transmembrane protein called Patched (PTCH) acts to prevent high expression and activity of a 7 membrane spanning receptor called Smoothened (SMO), NOTCH: Notch homolog, BRCA1: breast cancer gene 1, ADAM: a disintegrin and metalloproteinase.
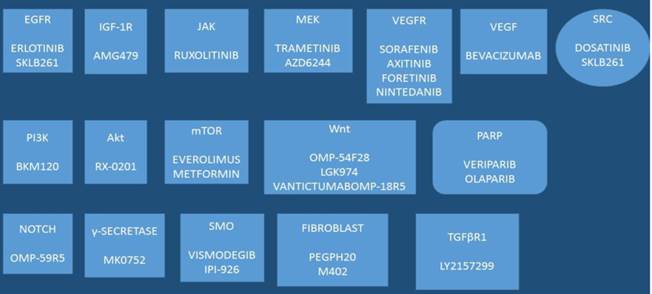
Drugs under development: SKLB261, AMG479, AZD6244, BKM120, RX-0201, OMP-54F28, LGK974, OMP-59R5, LY2157299, IPI-926, ML0752, M402, VANTICTUMABOMPP-18R5, PEGPH20; Akt: Protein kinase B (PKB), TGFβR1: Tumor growth factor-β-receptor-1, VEGF: Vascular endothelial growth factor, VEGFR: vascular endothelial growth factor receptor, IGF-1R: Insulin growth factor-1 receptor, NOTCH: Notch homolog, PI3K: Phosphatidylinositol-4,5-bisphosphate 3-kinase, mTOR: mammalian target of rapamycin, JAK: janus kinase, Wnt: Wnt signaling pathway, a complex protein network, SRC: SRC proto-oncogene, non-receptor tyrosine kinase, SMO: 7 membrane spanning receptor called Smoothened, PARP: Poly ADP ribose polymerase.
EGFR: epidermal growth factor receptor, VEGFR: vascular endothelial growth factor, IGF-1R: insulin like growth factor 1-receptor, TGFβR1-2: Tumor growth factor-β-receptor-1 and 2, NOTCH: Notch homolog, PTCH-SMO: a cell-surface transmembrane protein called Patched (PTCH) acts to prevent high expression and activity of a 7 membrane spanning receptor called Smoothened (SMO).
EGFR pathway inhibitors
Epidermal growth factor receptor (EGFR) is a transmembrane receptor member of the ErbB family with a tyrosine kinase domain that is activated by many ligands including epidermal growth factor (EGF), tumor growth factor-α (TGF-α), heparin-binding epidermal growth factor (EGF), amphiregulin, epiregulin, betacellulin and neuregulin. EGFR is involved in cell cycle regulation, adhesion, cell survival and differentiation through activation of the Ras/MAP kinase, phosphatidylinositol 3'-kinase (PI3K)/Akt, Janus kinase/Stat and phospholipase C/protein kinase C pathways. Previous studies have presented data that EGFR is overexpressed in up to 90% of pancreatic cancer samples. Therefore, tyrosine kinase inhibitors targeting EGFR have been approved with gemcitabine.[51] Eroltinib is a tyrosine kinase inhibitor (TKI) molecule that competes with ATP for binding to the kinase domain, thereby blocking downstream signal transduction. In a previous phase III trial, which enrolled 569 chemotherapy naïve patients with locally advanced or metastatic pancreatic adenocarcinoma patients were randomized to receive gemcitabine plus placebo or gemcitabine plus erlotinib 100-150 mg daily. The median overall survival (mOS) and progression free survival (PFS) were modestly, but statistically significantly, improved in the combination arm, 6.24 mo vs 5.91 mo (P = 0.038) and 3.75 mo vs 3.55 mo (P = 0.004), respectively.[5] In this study EGFR status and KRAS status were analyzed in the subgroup of patients treated with erlotinib and neither was observed to be predictive of a survival benefit in patients receiving the combination schedule.[52] Moreover; cetuximab, a monoclonal antibody binding the extracellular domain of EGFR after encouraging results in a phase I trial, failed to demonstrate any survival benefit.[53, 54] A phase II study has evaluated the possible therapeutic role of gefitinib, however; results were not as promising as those of erlotinib.[55] Another ErbB family of transmembrane tyrosine kinase receptors is erb-b2 receptor tyrosine kinase 2 (HER-2), which is overexpressed in 11% of pancreatic adenocarcinoma cases. HER2-positive status was observed to be associated with shorter survival.[56] In another study trastuzumab plus gemcitabine was administered in 34 metastatic pancreatic cancer patients with HER-2 overexpression as determined by immunohistochemistry, and partial responses were observed in 6% of cases.[57] Harder et al. [58] in a multicentre phase II study, investigated the efficacy and toxicity of the HER2 antibody, trastuzumab, plus capecitabine in patients with pancreatic cancer and HER2 overexpression, however; results were poorer compared with standard chemotherapy. Recently FDA approved lapatinib and clinical trials have been initiated to test the effect of this HER-2 inhibitor combined with chemotherapy in pancreatic carcinoma. Lapatinib was tested in combination with capecitabine as a second-line treatment in advanced pancreatic cancer with promising preliminary results, however; further studies are needed to evaluate the ffectiveness and role of this molecule in the treatment of PADC.[59] Furthermore, nimotuzumab, another anti-EGFR monoclonal antibody, showed promising results.[60] Strumberg et al. [61] showed PFS after 1 year of 10.3% and median overall survival of 18.1 wk with a tolerable toxicity profile with nimotuzumab. Finally afatinib which is another TKI inhibitor of EGFR, HER2 and HER4, is under evaluation in an ongoing phase II trial.[62]
The KRAS pathway and downstream signalling cascade inhibitors
KRAS activating mutations have been found in 70% to 90% of cases of pancreatic cancer. K-Ras is a GTPase protein belonging to the Ras protein family, which has oncogenic activity, activates proliferation and inhibits apoptosis through the RAF/MEK/ERK and PIK3/AKT pathways. K-Ras is very difficult to target, and no inhibitors are actually available to use in clinical practice.[63] In a preclinical study it was observed that farnesylation is an important post-translational modification required for Ras activation, allowing the protein to be attached to the plasma membrane for signal transduction.[64] Tipifarnib a farnesyl-transferase inhibitors failed to improve overall survival either as a single agent or in combination with gemcitabine in a phase III trial.[65, 66] Since it is difficult to target Ras directly, another solution would be to block targets downstream of KRAS, such as the protein kinase MEK. Selumetinib is an oral small molecule that inhibits MEK1/2 and n a phase II trial, patients were randomized to receive single-agent for advanced pancreatic cancer. The selumetinib arm showed a median overall survival of 5.4 mo vs 5.0 mo in the capecitabine arm, however results were not statistically significant.[67] In another study MEK1/2 inhibitor, trametinib, was tested in pancreatic cancer in combination with gemcitabine against a regimen of gemcitabine plus placebo in a phase II randomized multicentre study. However; no significant overall survival or PFS were observed.[68] Rigosertib, a first-in-class Ras mimetic and small molecule inhibitor of multiple signalling pathways, including polo-like kinase 1 and phosphoinositide 3-kinase (PI3K), was assessed in combination with gemcitabine in patients with treatment-naïve metastatic pancreatic adenocarcinoma in a phase II/III randomized study, however; combination regimen did not improve survival or response, as recently presented at the 2015 ASCO Annual Meeting.[69]
IGFR pathway inhibitors
Insulin like growth factor 1 receptor (IGF-1R), is highly expressed in pancreatic cells, and upon ligand binding activates several pathways involved in cell proliferation and cell survival such as the PIK3/AKT pathway.[70] Monoclonal antibodies against IGFR (cixutumumab, ganitumab) were evaluated in PDAC treatment, but unfortunately, however; they failed to show a statically significant survival benefit.[71] In the phase III trial assessing ganitumab in combination with gemcitabine due to futility analysis the study closed early. The median overall arm vs 7.2 mo in the placebo arm (HR, 0.97, P = 0.397).[72]
Angiogenesis pathway inhibitors
Neo-angiogenesis is known to be essential for tumor progression and metastatization mechanisms. Vascular endothelial growth factor (VEGF) stimulates the proliferation of endothelial cells and is overexpressed in human pancreatic cancer. However; anti VEGFR inhibitors, failed to improve overall survival in combination with gemcitabine in advanced pancreatic cancer, after encouraging results, phase III trials that tested the efficacy of bevacizumab in association with gemcitabine alone, or gemcitabine plus erlotinib.[73, 74] Aflibercept, a new recombinant fusion protein with extracellular portions of VEGFR-1 and VEGFR-2, which binds VEGF-A, VEGF-B and placental growth factors 1 and 2 thereby inhibiting VEGF-ligand-dependent signalling processes, was observed to suppresse tumour growth in pancreatic cell lines and xenografts. In another phase III study aiming to investigate OS in metastatic pancreatic cancer patients receiving standard gemcitabine and either aflibercept or placebo demonstrated that adding aflibercept to gemcitabine did not improve OS in metastatic pancreatic cancer patients.[75] Moreover; sorafenib, an oral multikinase inhibitor of Raf-kinase, VEGF-R2/-R3 and PDGFR-β, tested alone or in combination with gemcitabine in small phase I and II trials, and axitinib, an anti-angiogenesis agent assessed in combination with gemcitabine, showed no statistically significant efficacy in a phase III trial in advanced PDAC.[76-78] Novel phase II studies combining chemotherapy with new anti-angiogenic molecular agents, such as TL-118, a nonsteroidal anti-inflammatory oral medication, or necuparanib, are currently investigated.[79, 80]
Embryonic pathway inhibitors
The embryonic signaling pathway hedgehog is known to have a critical role in cell proliferation and survival during embryonic development. Normally this pathway is silenced in pancreatic cells, however; in PDAC it has been observed that it is pathologically activated. It is known that Hedgehog binds to the extracellular receptor Patched, which, in the absence of Hedgehog, suppresses activation of the G-protein-coupled receptor Smoothened and upregulates glioma associated oncogene homolog1 (GL1) transcriptional activity. Bailey et al. [81] presented data in regard to Sonic hedgehog (SHH) and other proteins downstream of the Hedgehog pathway, in PDAC primary tumour samples, which assist in the formation of the desmoplastic reaction, which limits the effective delivery of anticancer agents to pancreatic cancer cells. In order to overtake this obstacle genetically engineered mouse models demonstrated a depletion of tumour matrix from SHH pathway inhibition.[82] Vismodegib (GDC-0449), an oral small-molecule inhibitor targeting Smoothened [83], is under assessment in open phase II trials in combination with gemcitabine in advanced cancer, in combination with gemcitabine and nab-paclitaxel in metastatic settings with promising preliminary data.[84] Moreover; it is under investigation as a single agent in neoadjuvant settings followed by surgery.[85-87] The Smoothened inhibitor saridegib (IPI-926) administered in association with gemcitabine against gemcitabine plus placebo in a randomized, double-blind, placebo-controlled phase II trial enrolling patients with metastatic disease, however; this study was closed ahead of time due to poor effectiveness in the saridegib arm.[88] Hedgehog signaling pathway is under extensive investigation and clinical trials are ongoing in order to identify the proper inhibitor.[89] Notch signaling is another embryonic pathway crucial for pancreatic organogenesis, however; again this pathway is inactive after pancreas development. It has been observed that this pathway is upregulated in PDAC and promotes tumourigenesis. Binding of Notch ligand to its receptor promotes a cascade of proteolytic cleavages, mediated by γ-secretase (presenilin). The activated form ICN (intra cellular notch) forms part of a transcription complex which interacts with other pathways such as Hedgehog, KRAS and nuclear factor κB (NF-κB) signalling.[90, 91] RO4929097 which is a selective inhibitor of the γ-secretase enzyme is being investigated for its anti-tumour activity in preclinical studies.[92] In a recent phase II single-arm trial the possible role of RO4929097 was evaluated by enrolling 18 previously treated advanced PDAC patients. It was observed that the treatment was well tolerated; the median survival was 4.1 mo, and the median progression-free survival was 1.5 mo.[93] Encouraging clinical results were observed testing demcizumab, an anti- Delta-like ligand 4 antibody, plus gemcitabine and nab-paclitaxel in advanced PDAC in a phase I b trial, however; a larger clinical trial is necessary in order to confirm these findings.[94]
PARP inhibitors
It has been previously studied that mutations affecting breast cancer protein (BRCA) pathway components, especially the tumour suppressor gene breast cancer protein-2 (BRCA2), which is associated with hereditary predisposition to breast, ovarian and pancreatic cancer, promote tumorigenesis.[95] It has been observed that patients with defects in the homologous DNA recombination pathway may benefit from the use of Poly ADP ribose polymerase (PARP) inhibitors as monotherapy or in combination with radiation or other chemotherapeutic agents. These agents are currently under development.[96-98]
mTOR and PI3K/Akt pathway inhibitors
PI3K is phosphorylated after Ras activation, which activates Akt, a serine/threonine kinase. Signal transduction by activated PI3K/Akt plays a role in tumour cell proliferation, survival and metabolism, through several downstream targets, such as; mammalian target of rapamycin (mTOR).[43] Several trials with PI3K/AKT inhibitors are currently being performed in advanced pancreatic cancer patients after encouraging preclinical model results.[99] In these trials the following PI3K/AKT inhibitors are being investigated: BKM120, a PI3K in combination with
the mFOLFOX-6 schedule; RX-0201, an Akt antisense oligonucleotide tested in a phase II study plus gemcitabine; and BEZ235, a combined inhibitor of PI3K and mTOR were assessed in combination with the MEK inhibitor MEK.[100-102] In the study by Wolpin et al. [103] everolimus, an oral mTOR inhibitor, was administered as monotherapy in 33 gemcitabine-refractory pancreatic cancer patients. The PFS and OS were 1.8 and 4.5 mo, respectively. In a recent study, the results of a single arm phase II study with everolimus in combination with capecitabine were published. The median OS was 8.9 mo and PFS was 3.6 mo.[104] Moreover; the results of a phase I/II study testing everolimus in combination with gemcitabine in advanced settings and the results of a phase II trial testing temsirolimus, another mTOR inhibitor, in locally advanced or metastatic settings are anticipated.[105, 106]
Tumour stroma inhibitors
A very part of pancreatic cancer is the stroma which is involved in tumor formation, progression and the metastasis process. Therefore, targeting the matrix stroma could be a novel therapeutic strategy in addition to previously described trials evaluating with Hedgehog signalling inhibitors.[107] A novel agent PEGPH20, a pegylated formulation of recombinant hyaluronidase, a phase II trial[79] is currently in the recruitment phase after promissing results in a preclinical trial. In the current study untreated patients with metastatic disease to receive a combination of PEGPH20, nab-naclitaxel and gemcitabine or a combination of nabpaclitaxel and gemcitabine.[108] Another treatment approach would be inhibition of Platelet-derived growth factor receptors (PDGFR), a receptor expressed in stromal cells with a critical role in recruiting pericytes and in interstitial fluid pressure in the tumour stroma. It was observed in preclinical studies using an orthotopic pancreatic tumour mouse model that blocking this pathway induces apoptosis in pancreatic cancer.[109] Moreover; TKI258, a PDGFR inhibitor, is under evaluation in a phase I dose assessment for advanced pancreatic cancer patients.[110] Matrix metalloproteinase inhibitors such as marimastat have been investigated. Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes responsible for the degradation of connective tissue proteins, and aberrant MMP expression is observed in PDAC.
However; results of a phase III trial did not provide positive results of marimastat with gemcitabine in patients with advanced pancreatic cancer. [111] Table 1.
Phase II and III clinical trials.
| II |
| Cetuximab EGFR signaling GEM plus cisplatin plus cetuximab M/LA vs GEM plus cisplatin |
| Gefitinib EGFR signaling GEM plus gefitinib M/LA (single arm) |
| Trastuzumab EGFR signaling GEM plus trastuzumab M/LA (single arm) 2+/3+ HER-2 expression |
| Trastuzumab EGFR signaling Capecitabine plus trastuzumab M/LA (single arm) 3+ HER-2 expression or gene amplification |
| Nimotuzumab EGFR signaling GEM plus nimotuzumab M/LA (single arm) |
| Nimotuzumab EGFR signaling Nimotuzumab monotherapy Refractory to first line standard chemotherapy M/LA(single arm) |
| Selumetinib KRAS/MEK pathway Capecitabine plus selumetinib Refractory to first line standard chemotherapy M/LA vs Capecitabine |
| Trametinib KRAS/MEK pathway GEM plus trametinib M/LA vs GEM plus P Sorafenib Angiogenesis GEM plus sorafenib M/LA (single arm) |
| RO4929097 Hedgehog signaling RO4929097 monotherapy Refractory to first line standard chemotherapy M (single arm) |
| Everolimus mTOR pathway Everolimus plus capecitabine M/LA(single arm) |
| III |
| Erlotinib EGFR signaling GEM plus erlotinib M/LA vs GEM plus P |
| Cetuximab EGFR signaling GEM plus cetuximab M/LA vs GEM |
| Tipifarnib KRAS pathway GEM plus tipifarnib vs GEM |
| Ganitumab IGFR pathway GEM plus ganitumab M vs GEM plus P |
| Bevacizumab Angiogenesis GEM plus bevacizumab vs GEM plus P |
| Aflibercept Angiogenesis GEM plus aflibercept vs GEM plus P |
| Axitinib Angiogenesis GEM plus axitinib vs GEM plus P |
| Marimastat Tumor stroma GEM plus marimastat vs GEM |
GEM: gemcitabine, IGFR: insulin like growth factor receptor, EGFR: epidermal growth factor receptor, KRAS: V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, M: metastatic cancer, LA: locally advanced cancer, P: placebo.
Discussion
It has been observed that, embryonic signaling pathways like tumor growth factor-β (TGF-β), Wnt-β-catenin, and Hedgehog alone are not sufficient for the initiation of PC.[112] Until now it was observed active Kras mutation is the most important drive for pancreatic cancer progression. Acinar cells present the highest degree of cellular plasticity and can adopt an undifferentiated progenitor state upon inflammatory or oncogenic stimuli. Pancreatic cancer also represents an extremely complex disease morphologically. There are no clear subtypes for pancreatic cancer until now as in other solid tumors such as breast cancer. The cell of origin or precursor type leading to pancreatic cancer has a significant impact towards the prognosis of PC patients. This suggests that not only the genetic motif, but also the primordial lineage plays an important role pancreatic cancer development and prognosis. Our knowledge is mostly based on mouse models of pancreatic cancer, however; due to the complexity of the disease a human model system is required. In the study by Kim et al. an elegant induced-pluripotency approach to reprogram human PDA cells in order to recapitulate human disease progression was investigated.[113] In the study by Boj et al. a three-dimensional matrix was developed from surgery specimens or endoscopic biopsy material which could be a useful method to address this complexity.[114] In this mode, this model system harbors the opportunity to test targeted therapies for pancreatic cancer patients. Mouse models until now were very useful contributing to our understanding of PC biology. Treatment approach until now is mostly based on non-specific chemotherapy agents in pancreatic cancer treatment and not molecular defined strategies.[3,4] Unfortunately the genetic heterogeneity of PC and distinct oncogenic susceptibilities with a complex matrix within the gland make this disease difficult to handle with local therapies. Moreover; tailored therapies have presented favorable results taking the mutational landscape of the respective tumor into account, however; better understanding of the regulating factors is in need. Major flaw which was observed in many studies with molecular or chemotherapeutic agents in pancreatic cancer was that they enrolled an unselected population of patients to treat. In the past three years, approximately 116 trials specific for PC systemic therapy were registered of which only about the 8% applied criteria to select a patient subset based upon molecular biomarkers.[115] In order to stratify patients, the Australian Pancreatic Cancer Genome Initiative has started a pilot study to evaluate pancreatic cancer through predefined actionable molecular phenotypes. Patients are enrolled in this trial, called IMPaCT (Individualised Molecular Pancreatic Cancer Therapy), after a preliminary phenotype screening. This is done in order to compare the use of gemcitabine in an unselected population based on a stratified approach.[116] Moreover; novel molecules such as biological agents have found application in cancer treatment by tumour-targeted delivery of cytotoxic drugs. In the study by Ahn et al. [117] an antibody fragment-installed polymeric micelles via maleimide-thiol conjugation for selective delivery of platinum drugs to pancreatic tumors. This antibody-drug conjugate significantly suppressed the growth of pancreatic tumour xenografts. This novel approach with the help of biotechnology, with activity in vitro and in a mouse model, is a future strategy for pancreatic cancer therapy.[117] Moreover; since the incidence of pancreatic cancer in the elderly has increased, further investigation of this group of patients is necessary. The special features of this patient population, and the poor information available from clinical trials regarding the management of older patients has resulted remains a challenge for these patients. Regarding advanced pancreatic cancer local treatment options should be considered. Currently several studies have demonstrated efficiency with local cryo ablation, microwave/RF ablation, gene therapy and radioactive coils.[7] It remains to the treating physician and center experience for the best local treatment application. It is the belief of the authors that age should not be the determining factor in decisions regarding the best approach. An integral evaluation of the patient in accordance with appropriate tools should be conducted in combination with the mutation status and performance status.
Conflict of Interest
None to declare.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5-29 doi:10.3322/caac.21254
2. Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA. et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2000;4:567-79
3. Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364:1817-25 doi:10.1056/NEJMoa1011923
4. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine. 2013;369:1691-703 doi:10.1056/NEJMoa1304369
5. Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S. et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:1960-6 doi:10.1200/JCO.2006.07.9525
6. Di Marco M, Di Cicilia R, Macchini M, Nobili E, Vecchiarelli S, Brandi G. et al. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? (Review). Oncology reports. 2010;23:1183-92
7. Zarogoulidis P, Pavlioglou P, Pivert PL, Machairiotis N, Katsikogiannis N, Kougioumtzi I. et al. Current and future intratumoral targeted treatment for pancreatic cancer. Therapeutic delivery. 2014;5:913-26 doi:10.4155/tde.14.44
8. Matthaios D, Zarogoulidis P, Balgouranidou I, Chatzaki E, Kakolyris S. Molecular pathogenesis of pancreatic cancer and clinical perspectives. Oncology. 2011;81:259-72 doi:10.1159/000334449
9. Shaib Y, Davila J, Naumann C, El-Serag H. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. Population-based study. The American journal of gastroenterology. 2007;102:1377-82 doi:10.1111/j.1572-0241.2007.01202.x
10. Hidalgo M. Pancreatic cancer. The New England journal of medicine. 2010;362:1605-17 doi:10.1056/NEJMra0901557
11. Rossi ML, Rehman AA, Gondi CS. Therapeutic options for the management of pancreatic cancer. World journal of gastroenterology. 2014;20:11142-59 doi:10.3748/wjg.v20.i32.11142
12. Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Archives of surgery. 1985;120:899-903
13. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H. et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. The New England journal of medicine. 2004;350:1200-10 doi:10.1056/NEJMoa032295
14. Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS medicine. 2010;7:e1000267. doi:10.1371/journal.pmed.1000267
15. Lemmens VE, Bosscha K, van der Schelling G, Brenninkmeijer S, Coebergh JW, de Hingh IH. Improving outcome for patients with pancreatic cancer through centralization. The British journal of surgery. 2011;98:1455-62 doi:10.1002/bjs.7581
16. Takahashi H, Ohigashi H, Gotoh K, Marubashi S, Yamada T, Murata M. et al. Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Annals of surgery. 2013;258:1040-50 doi:10.1097/SLA.0b013e31829b3ce4
17. Nanda RH, El-Rayes B, Maithel SK, Landry J. Neoadjuvant modified FOLFIRINOX and chemoradiation therapy for locally advanced pancreatic cancer improves resectability. Journal of surgical oncology. 2015;111:1028-34 doi:10.1002/jso.23921
18. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR. et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1997;15:2403-13
19. Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC cancer. 2008;8:82. doi:10.1186/1471-2407-8-82
20. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. The New England journal of medicine. 2014;371:2140-1 doi:10.1056/NEJMc1412266
21. Gerber DE. Targeted therapies: a new generation of cancer treatments. American family physician. 2008;77:311-9
22. Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P. et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-6 doi:10.1126/science.1164368
23. Seicean A, Petrusel L, Seicean R. New targeted therapies in pancreatic cancer. World journal of gastroenterology. 2015;21:6127-45 doi:10.3748/wjg.v21.i20.6127
24. Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nature medicine. 2013;19:1389-400 doi:10.1038/nm.3388
25. Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS. et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology. 2014;146:291-304
26. Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goere D. et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2015;26(Suppl 5):v56-68 doi:10.1093/annonc/mdv295
27. Pietryga JA, Morgan DE. Imaging preoperatively for pancreatic adenocarcinoma. Journal of gastrointestinal oncology. 2015;6:343-57 doi:10.3978/j.issn.2078-6891.2015.024
28. Murakami Y, Satoi S, Sho M, Motoi F, Matsumoto I, Kawai M. et al. National Comprehensive Cancer Network Resectability Status for Pancreatic Carcinoma Predicts Overall Survival. World journal of surgery. 2015;39:2306-14 doi:10.1007/s00268-015-3096-3
29. Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y. et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2005;31:164-9 doi:10.1016/j.ejso.2004.09.007
30. Safi F, Schlosser W, Kolb G, Beger HG. Diagnostic value of CA 19-9 in patients with pancreatic cancer and nonspecific gastrointestinal symptoms. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 1997;1:106-12
31. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2007;33:266-70 doi:10.1016/j.ejso.2006.10.004
32. Gattani AM, Mandeli J, Bruckner HW. Tumor markers in patients with pancreatic carcinoma. Cancer. 1996;78:57-62 doi:10.1002/(SICI)1097-0142(19960701)78:1<57::AID-CNCR10>3.0.CO;2-6
33. Chan A, Prassas I, Dimitromanolakis A, Brand RE, Serra S, Diamandis EP. et al. Validation of biomarkers that complement CA19.9 in detecting early pancreatic cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20:5787-95 doi:10.1158/1078-0432.CCR-14-0289
34. O'Brien DP, Sandanayake NS, Jenkinson C, Gentry-Maharaj A, Apostolidou S, Fourkala EO. et al. Serum CA19-9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: implications for early disease detection. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:622-31 doi:10.1158/1078-0432.CCR-14-0365
35. Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M. et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer research. 1997;57:1731-4
36. Yashiro M, Carethers JM, Laghi L, Saito K, Slezak P, Jaramillo E. et al. Genetic pathways in the evolution of morphologically distinct colorectal neoplasms. Cancer research. 2001;61:2676-83
37. Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer research. 1997;57:2140-3
38. Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN. et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. The American journal of pathology. 1993;143:545-54
39. Schutte M, Hruban RH, Geradts J, Maynard R, Hilgers W, Rabindran SK. et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer research. 1997;57:3126-30
40. Muller PA, Vousden KH. p53 mutations in cancer. Nature cell biology. 2013;15:2-8 doi:10.1038/ncb2641
41. Iacobuzio-Donahue CA, Song J, Parmiagiani G, Yeo CJ, Hruban RH, Kern SE. Missense mutations of MADH4: characterization of the mutational hot spot and functional consequences in human tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:1597-604
42. Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, Zhang X. et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:4674-9 doi:10.1158/1078-0432.CCR-09-0227
43. Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes & development. 2006;20:1218-49 doi:10.1101/gad.1415606
44. Calhoun ES, Jones JB, Ashfaq R, Adsay V, Baker SJ, Valentine V. et al. BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) mutations in distinct subsets of pancreatic cancer: potential therapeutic targets. The American journal of pathology. 2003;163:1255-60 doi:10.1016/S0002-9440(10)63485-2
45. Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA. et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer cell. 2003;4:437-50
46. Ardito CM, Gruner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK. et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer cell. 2012;22:304-17 doi:10.1016/j.ccr.2012.07.024
47. Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, Galban CJ. et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. The Journal of clinical investigation. 2012;122:639-53 doi:10.1172/JCI59227
48. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655-7 doi:10.1126/science.296.5573.1655
49. Altomare DA, Tanno S, De Rienzo A, Klein-Szanto AJ, Tanno S, Skele KL. et al. Frequent activation of AKT2 kinase in human pancreatic carcinomas. Journal of cellular biochemistry. 2002;87:470-6
50. Hirakawa T, Yashiro M, Murata A, Hirata K, Kimura K, Amano R. et al. IGF-1 receptor and IGF binding protein-3 might predict prognosis of patients with resectable pancreatic cancer. BMC cancer. 2013;13:392. doi:10.1186/1471-2407-13-392
51. Tobita K, Kijima H, Dowaki S, Kashiwagi H, Ohtani Y, Oida Y. et al. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. International journal of molecular medicine. 2003;11:305-9
52. da Cunha Santos G, Dhani N, Tu D, Chin K, Ludkovski O, Kamel-Reid S. et al. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer. 2010;116:5599-607 doi:10.1002/cncr.25393
53. Cascinu S, Berardi R, Labianca R, Siena S, Falcone A, Aitini E. et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multicentre, phase II trial. The Lancet Oncology. 2008;9:39-44 doi:10.1016/S1470-2045(07)70383-2
54. Philip PA, Benedetti J, Corless CL, Wong R, O'Reilly EM, Flynn PJ. et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:3605-10 doi:10.1200/JCO.2009.25.7550
55. Fountzilas G, Bobos M, Kalogera-Fountzila A, Xiros N, Murray S, Linardou H. et al. Gemcitabine combined with gefitinib in patients with inoperable or metastatic pancreatic cancer: a phase II Study of the Hellenic Cooperative Oncology Group with biomarker evaluation. Cancer investigation. 2008;26:784-93 doi:10.1080/07357900801918611
56. Kimura K, Sawada T, Komatsu M, Inoue M, Muguruma K, Nishihara T. et al. Antitumor effect of trastuzumab for pancreatic cancer with high HER-2 expression and enhancement of effect by combined therapy with gemcitabine. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:4925-32 doi:10.1158/1078-0432.CCR-06-0544
57. Safran H, Iannitti D, Ramanathan R, Schwartz JD, Steinhoff M, Nauman C. et al. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer investigation. 2004;22:706-12
58. Harder J, Ihorst G, Heinemann V, Hofheinz R, Moehler M, Buechler P. et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. British journal of cancer. 2012;106:1033-8 doi:10.1038/bjc.2012.18
59. Safran H, Miner T, Resnick M, Dipetrillo T, McNulty B, Evans D. et al. Lapatinib/gemcitabine and lapatinib/gemcitabine/oxaliplatin: a phase I study for advanced pancreaticobiliary cancer. American journal of clinical oncology. 2008;31:140-4 doi:10.1097/COC.0b013e318145b9a5
60. Su D, Jiao SC, Wang LJ, Shi WW, Long YY, Li J. et al. Efficacy of nimotuzumab plus gemcitabine usage as first-line treatment in patients with advanced pancreatic cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:2313-8 doi:10.1007/s13277-013-1306-x
61. Strumberg D, Schultheis B, Scheulen ME, Hilger RA, Krauss J, Marschner N. et al. Phase II study of nimotuzumab, a humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody, in patients with locally advanced or metastatic pancreatic cancer. Investigational new drugs. 2012;30:1138-43 doi:10.1007/s10637-010-9619-8
62. Ioannou N, Dalgleish AG, Seddon AM, Mackintosh D, Guertler U, Solca F. et al. Anti-tumour activity of afatinib, an irreversible ErbB family blocker, in human pancreatic tumour cells. British journal of cancer. 2011;105:1554-62 doi:10.1038/bjc.2011.396
63. di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220-9 doi:10.1053/j.gastro.2013.01.071
64. Takashima A, Faller DV. Targeting the RAS oncogene. Expert opinion on therapeutic targets. 2013;17:507-31 doi:10.1517/14728222.2013.764990
65. Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nature reviews Cancer. 2011;11:761-74 doi:10.1038/nrc3106
66. Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A. et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:1430-8 doi:10.1200/JCO.2004.10.112
67. Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Pover G. et al. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Investigational new drugs. 2012;30:1216-23 doi:10.1007/s10637-011-9687-4
68. Infante JR, Somer BG, Park JO, Li CP, Scheulen ME, Kasubhai SM. et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. European journal of cancer. 2014;50:2072-81 doi:10.1016/j.ejca.2014.04.024
69. O'Neil BH, Scott AJ, Ma WW, Cohen SJ, Leichman L, Aisner DL. et al. A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2015;26:2505. doi:10.1093/annonc/mdv477
70. Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Molecular cancer therapeutics. 2007;6:1-12 doi:10.1158/1535-7163.MCT-06-0080
71. Philip PA, Goldman B, Ramanathan RK, Lenz HJ, Lowy AM, Whitehead RP. et al. Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer. 2014;120:2980-5 doi:10.1002/cncr.28744
72. Fuchs CS, Azevedo S, Okusaka T, Van Laethem JL, Lipton LR, Riess H. et al. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2015;26:921-7 doi:10.1093/annonc/mdv027
73. Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H. et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:3617-22 doi:10.1200/JCO.2010.28.1386
74. Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL. et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:2231-7 doi:10.1200/JCO.2008.20.0238
75. Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C. et al. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. European journal of cancer. 2013;49:2633-42 doi:10.1016/j.ejca.2013.04.002
76. Chiorean EG, Schneider BP, Akisik FM, Perkins SM, Anderson S, Johnson CS. et al. Phase 1 pharmacogenetic and pharmacodynamic study of sorafenib with concurrent radiation therapy and gemcitabine in locally advanced unresectable pancreatic cancer. International journal of radiation oncology, biology, physics. 2014;89:284-91 doi:10.1016/j.ijrobp.2014.02.024
77. Kindler HL, Wroblewski K, Wallace JA, Hall MJ, Locker G, Nattam S. et al. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Investigational new drugs. 2012;30:382-6 doi:10.1007/s10637-010-9526-z
78. Kindler HL, Ioka T, Richel DJ, Bennouna J, Letourneau R, Okusaka T. et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. The Lancet Oncology. 2011;12:256-62 doi:10.1016/S1470-2045(11)70004-3
79. Tiltan Pharma Ltd. IsIPISfPCPICgIBMNLoM.
80. Momenta Pharmaceuticals I. M402 in Combination With Nab- Paclitaxel and Gemcitabine in Pancreatic Cancer. ClinicalTrials. gov [Internet] Bethesda (MD): National Library of Medicine (US)
81. Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T. et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:5995-6004 doi:10.1158/1078-0432.CCR-08-0291
82. Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-61 doi:10.1126/science.1171362
83. Singh BN, Fu J, Srivastava RK, Shankar S. Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms. PloS one. 2011;6:e27306. doi:10.1371/journal.pone.0027306
84. Kaye SB, Fehrenbacher L, Holloway R, Amit A, Karlan B, Slomovitz B. et al. A phase II, randomized, placebo-controlled study of vismodegib as maintenance therapy in patients with ovarian cancer in second or third complete remission. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:6509-18 doi:10.1158/1078-0432.CCR-12-1796
85. NCI. Gemcitabine Hydrochloride With or Without Vismodegib in Treating Patients With Recurrent or Metastatic Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
86. SKCC. Pancreas, Hedgehog Inhibitors for Metastatic Adenocarcinoma of the Pancreas. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
87. Bax L. Hedgehog Inhibition for Pancreatic Ductal Adenocarcinoma (PDAC) in the Preoperative Setting (HIPPoS). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
88. Infinity Reports Update from Phase 2 Study of Saridegib Plus Gemcitabine in Patients with Metastatic Pancreatic Cancer.
89. Center. SKCC. Gemcitabine + Nab-paclitaxel With LDE-225 (Hedgehog Inhibitor) as Neoadjuvant Therapy for Pancreatic Adenocarcinoma. ClinicalTrials. gov [Internet] Bethesda (MD)National Library of Medicine (US)
90. Sjolund J, Manetopoulos C, Stockhausen MT, Axelson H. The Notch pathway in cancer: differentiation gone awry. European journal of cancer. 2005;41:2620-9 doi:10.1016/j.ejca.2005.06.025
91. Ristorcelli E, Lombardo D. Targeting Notch signaling in pancreatic cancer. Expert opinion on therapeutic targets. 2010;14:541-52 doi:10.1517/14728221003769895
92. Plentz R, Park JS, Rhim AD, Abravanel D, Hezel AF, Sharma SV. et al. Inhibition of gamma-secretase activity inhibits tumor progression in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2009;136:1741-9 e6. doi:10.1053/j.gastro.2009.01.008
93. De Jesus-Acosta A, Laheru D, Maitra A, Arcaroli J, Rudek MA, Dasari A. et al. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Investigational new drugs. 2014;32:739-45 doi:10.1007/s10637-014-0083-8
94. Smith DC, Eisenberg PD, Manikhas G, Chugh R, Gubens MA, Stagg RJ. et al. A phase I dose escalation and expansion study of the anticancer stem cell agent demcizumab (anti-DLL4) in patients with previously treated solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20:6295-303 doi:10.1158/1078-0432.CCR-14-1373
95. Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495-501 doi:10.1038/nature14169
96. Tangutoori S, Baldwin P, Sridhar S. PARP inhibitors: A new era of targeted therapy. Maturitas. 2015;81:5-9 doi:10.1016/j.maturitas.2015.01.015
97. AstraZeneca. Study to Assess the Safety & Tolerability of a PARP Inhibitor in Combination With Gemcitabine in Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
98. NCI. Gemcitabine Hydrochloride and Cisplatin With or Without Veliparib or Veliparib Alone in Patients With Locally Advanced or Metastatic Pancreatic Cancer.
99. Ito D, Fujimoto K, Mori T, Kami K, Koizumi M, Toyoda E. et al. In vivo antitumor effect of the mTOR inhibitor CCI-779 and gemcitabine in xenograft models of human pancreatic cancer. International journal of cancer Journal international du cancer. 2006;118:2337-43 doi:10.1002/ijc.21532
100. Center. ULCC. BKM120 + mFOLFOX6 in Advanced Solid Tumors With Expansion Cohort Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
101. Rexahn Pharmaceuticals I. A Safety and Efficacy Study of RX-0201 Plus Gemcitabine in Metastatic Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
102. Pharmaceuticals N. Safety, Pharmacokinetics and Pharmacodynamics of BEZ235 Plus MEK162 in Selected Advanced Solid Tumor Patients. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
103. Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA. et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:193-8 doi:10.1200/JCO.2008.18.9514
104. Kordes S, Klumpen HJ, Weterman MJ, Schellens JH, Richel DJ, Wilmink JW. Phase II study of capecitabine and the oral mTOR inhibitor everolimus in patients with advanced pancreatic cancer. Cancer chemotherapy and pharmacology. 2015;75:1135-41 doi:10.1007/s00280-015-2730-y
105. Pharmaceuticals N. Treatment of Patients Suffering From a Progressive Pancreas Carcinoma With Everolimus (RAD001) and Gemcitabine. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
106. NCI. CCI-779 in Treating Patients With Locally Advanced or Metastatic Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
107. Heinemann V, Reni M, Ychou M, Richel DJ, Macarulla T, Ducreux M. Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies. Cancer treatment reviews. 2014;40:118-28 doi:10.1016/j.ctrv.2013.04.004
108. Therapeutics H. PEGPH20 Plus Nab-Paclitaxel Plus Gemcitabine Compared With Nab-Paclitaxel Plus Gemcitabine in Subjects With Stage IV Untreated Pancreatic Cancer (HALO-109-202). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
109. Hwang RF, Yokoi K, Bucana CD, Tsan R, Killion JJ, Evans DB. et al. Inhibition of platelet-derived growth factor receptor phosphorylation by STI571 (Gleevec) reduces growth and metastasis of human pancreatic carcinoma in an orthotopic nude mouse model. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:6534-44
110. Institute RPC. Dovitinib Lactate, Gemcitabine Hydrochloride, and Capecitabine in Treating Patients With Advanced or Metastatic Solid Tumors, Pancreatic Cancer and Biliary Cancers. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
111. Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. British journal of cancer. 2002;87:161-7 doi:10.1038/sj.bjc.6600446
112. Morris JPt, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nature reviews Cancer. 2010;10:683-95 doi:10.1038/nrc2899
113. Kim J, Hoffman JP, Alpaugh RK, Rhim AD, Reichert M, Stanger BZ. et al. An iPSC line from human pancreatic ductal adenocarcinoma undergoes early to invasive stages of pancreatic cancer progression. Cell reports. 2013;3:2088-99 doi:10.1016/j.celrep.2013.05.036
114. Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324-38 doi:10.1016/j.cell.2014.12.021
115. ClinicalTrials.gov. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
116. Chantrill LA, Nagrial AM, Watson C, Johns AL, Martyn-Smith M, Simpson S. et al. Precision Medicine for Advanced Pancreas Cancer: The Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) Trial. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:2029-37 doi:10.1158/1078-0432.CCR-15-0426
117. Ahn J, Miura Y, Yamada N, Chida T, Liu X, Kim A. et al. Antibody fragment-conjugated polymeric micelles incorporating platinum drugs for targeted therapy of pancreatic cancer. Biomaterials. 2015;39:23-30 doi:10.1016/j.biomaterials.2014.10.069
Author contact
![]() Corresponding author: Michael Karanikas, University Surgery Department, University General Hospital of Alexandroupolis, Democritus University of Thrace, Alexandroupolis, Greece. Mobile: 6974107504 Email: mkaranikascom.
Corresponding author: Michael Karanikas, University Surgery Department, University General Hospital of Alexandroupolis, Democritus University of Thrace, Alexandroupolis, Greece. Mobile: 6974107504 Email: mkaranikascom.

 Global reach, higher impact
Global reach, higher impact