Impact Factor
ISSN: 1837-9664
J Cancer 2014; 5(9):765-773. doi:10.7150/jca.10471 This issue Cite
Review
Interleukin-7 and Interleukin-15 for Cancer
1. Pulmonary Department-Oncology Unit, ``G. Papanikolaou`` General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
2. Division of Pulmonary and Critical Care Medicine, Johns Hopkins University, Baltimore, U.S.A.
3. Surgery Department (NHS), University General Hospital of Alexandroupolis, Alexandroupolis, Greece.
4. II Medical Clinic, ``Coburg`` Hospital, University of Wuerzburg, Coburg, Germany.
5. Department of Respiratory Diseases, Changhai Hospital/First Affiliated Hospital of the Second Military Medical University, Shanghai, China.
6. Pathology Department, ``G. Papanikolaou`` General Hospital, Thessaloniki, Greece.
7. Oncology Department, ``BioMedicine`` Private Hospital, Thessaloniki, Greece.
8. Cardiothoracic Surgery Department, ``Saint Luke`` Private Hospital, Thessaloniki, Greece.
9. Oncology Department, ``G. Papageorgiou`` University Hospital, Thessaloniki, Greece.
10. Oncology Department, ``Sotiria`` Hospital, University of Athens, Athens, Greece.
Received 2014-9-2; Accepted 2014-10-6; Published 2014-10-22
Abstract
Interleukin 7 and 15 are considered powerful pro-inflammatory cytokines, they have the ability to destabilize chromosomes and induce tumorigenesis. Additionally, they can control malignancy proliferation by influencing the tumor microenvironment and immune system. Immunotherapy has been proposed as a treatment modality for malignancy for over a decade; the exact mechanisms of action and pathways are still under investigation. Interleukin 7 and 15 have been extensively investigated in hematological malignancies since their mode of action influences the stimulation of the immune system in a more direct way than other malignancies such as lung, melanoma, and breast, renal and colorectal cancer.
Keywords: IL-7, IL-15, Cancer, Imunomodulation.
Introduction
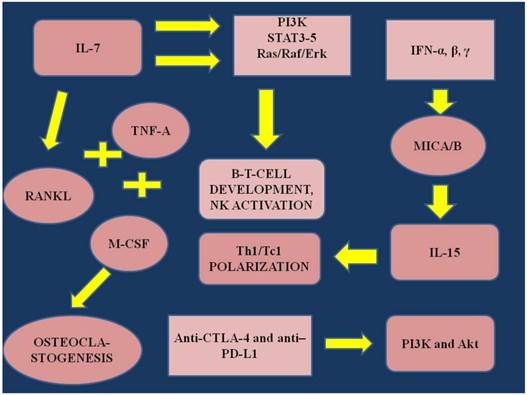
Interleukin-7 (IL-7) is an immunopotent regulatory protein produced by stromal cells and by several different inflammatory cells [1]. IL-7 assists in the development of lymphocytes and regulates peripheral T-cell populations [2]. Il-7 has also been found to be produced by solid tumors, however; the influence of the protein upon the tumor cell proliferation is unclear [3]. Additionally, IL-7 has been identified in lymphomas and leukemias [4, 5]. Gene therapy expressing IL-7 has been used as a treatment method through tumor environment immunomodulation for non-small cell lung cancer [6]. IL-7 levels have been associated with apoor prognosis in breast cancer [4]. Prostate IL-7 and Interleukin-15 (IL-15) levels have been increased in early stage prostate cancer [7]. IL-7 values have been associated with bone metastatic disease and hematological malignancies [8-10]. Different levels of IL-7 have been measured in renal [11], neuroblastoma [12], glioma [13], colorectal [14], central nervous system, and lung cancers [15], however; until now no clear conclusions had been drawn in regards to how these values influence tumor progression. It has also been observed that IL-7 is responsible for osteoclastogenesis through fusion with macrophage colony stimulating factor (M-CSF), binding with tumor necrosis factor-α (TNF-α) and receptor activator of nuclear factor-α (RANKL) [16-18]. However; again IL-7 has demonstrated a different behavior in different models, either as an activator or as an inhibitor for osteoclastogenesis. This observation could be attributed to other local microenvironment factors which influence IL-7 behavior [19, 20]. Furthermore, it has been previously observed that cytokines induce osteoclastogenesis, along with circulating tumor cells and TNF-α [21]. IL-7 or IL-7. Receptor deficiency blocks the B- and T-cell development [22, 23]. IL-7R is also a switch regulating the gene rearrangement at the Ig H chain locus [24-26]. The pathways that are activated with IL-7 are the phosphatidyl-inositol-3-kinase (PI3K)[23], the Ras/Raf signaling cascade and Janus kinase/STAT pathways (STAT3 and STAT 5) [27-29]. The role of the Ras/Raf/Erk pathway`s remain controversial. In the study by Crawley et. al. [30] it was demonstrated that IL-7 does not influence Ras/Raf signaling cascade, while in the study by Fleming et.al. [28] it was observed that IL-7 transiently induced the Erk cascade in pre-B cells. In the study by Goetz et. al. [31] it was presented that IL-7 through STAT5 regulates early B cell development. Interleukin-15 (IL-15) belongs to a four-α-helix-bundle protein family and its action is through the common receptor interleukin-2/interleukin-β [32, 33]. IL-2 and IL-15 have distinct mechanisms of action and often demonstrate competing roles. IL-2 inhibits CD8+ and promotes regulatory T-cells, while IL-15 is required for initiation of T-cell activation, survival of memory cells and development of natural killer cells (NK) [34-39]. The cytokine-induced killer cells (CIK cells) are also expressing receptors such as NKG2D, which are responsible for cytotoxicity, triggering of granule exocytosis and feedback cytokine toxicity secretion. IL-15 is responsible for the survival, proliferation and synthesis of IFN-γ (through feedback), perforin and granzyme B in natural killer cells and CD8+ [37, 40-43]. The mode of action of IL-15 is through a ``trans-presentation``, where IL-15Rα (surface of macrophages, dendritic cells and epithelial cells) presents IL-15 in trans to responder cells (CD8+ and NK) which have IL-15Rβ/γ [44-46]. ΙL-15 is induced by interferons IFN-α, β, γ and promotes Th1/Tc1 polarization, which is fundamental for acquired and innate immunity. However; it has been observed that cancer cells use their defense mechanisms and obstruct NKG2D/NKG2D ligand by storing major histocompatibility complex class I-related chain A and B (MICA/B) intracellularly, or reduce/block/downregulate (inducing endocytosis, degradation of receptor) the surface of the ligands by shedding MICA/B in soluble forms [47, 48]. Increased levels of MICA have been associated with impaired activity of NK cells in pancreatic cancer [49]. In the current work we will present the pathways of action for Interleukin 7 and 15, refer to the regulatory mechanisms in several systems and finally present data to support using these cytokines as a possible anticancer treatment.
Research Strategies
We performed an electronic article search through PubMed, Google Scholar, Medscape, and Scopus databases, using combinations of the following keywords: interleukin-7 and cancer, interleukin 15 and cancer. All types of articles (randomized controlled trials, clinical observational cohort studies, review articles, case reports) were included. Selected references from identified articles were searched for further consideration, without language limitation.
IL-7 experimentation
Disease association
In the study by Mengus et. al. [7] a correlation was made for gene expression of IL-7 and IL-15 in tissue and serum values in patients with benign prostate hyperplasia (BPH) and early 1-2c stage prostate cancer (PCA). It was observed that both gene over-expression and serum values of IL-7 and IL-15 were increased in early PCA patients. Increased serum levels of IL-7 were associated with acute graft-versus-host disease (GVHD) and could be used as a prognostic factor for early treatment by modulation of IL-7 pathway [50]. The usefulness of recombinant IL-7 administration as an adjuvant treatment was observed in the study by Colombetti et. al. [51], however; an aspect of this therapy was elicited indicating that the efficiency depends on the expression of IL-7Rα at the surface of CD8+ T cells. This hypothesis was again verified with JQ1 inhibitor which belongs to the BET class of human bromodomain proteins, in B-cell acute lymphoblastic leukemia (ALL) [52]. The JQ1 was able to down-regulate IL-7R gene expression and additionally block STAT5 phosphorilation. However; the effectiveness of this therapeutic strategy depends on the expression of IL-7Rα at the surface of CD8+ T cells.
Immunisation
In the study by Silva et. al. [53] it was observed that IL-7 contributes to the stimulation of tumor microenvironment for T-ALL patients versus healthy patients. In the study by Otto et. al. [12] Fc IL-7 was administered in a mouse model of neuroblastoma malignancy and results suggest that this type of immunotherapy could be administered for this type of malignancy. In the study by Lu et. al [54] increased production of CD4+ T cells from IL-7 administration in lymphopenic baboons (irradiation and antithymocyte globulin) was observed in such a degree that death occurred in an animal. This data indicates that immunotherapy should be administered with caution and therefore evaluation of T-cell subpopulations should be made. In the study by Kim et. al. [55] mouse fibroblasts (H-2b) (Fb) expressing B7.1 and interleukin-7 (IL-7) were pulsed with an ovalbumin (OVA) epitope and tested for OVA-specific T cells in vivo. Additional fibroblasts lacking B7.1 or IL-7 were engineered as controls. Results indicated that B7.1, IL-7 and OVA induced a strong cytotoxic effect against EG7 tumor cells. CD8+ T cells were observed to have the major antitumor response. In addition, increased efficiency was observed when the mice were injected with EG7 tumor cells one week after immunization with Fb-B7.1- IL-7- OVA. This experiment suggests that genetically modified Fb to express B7.1- IL-7- OVA could be used as an antitumor vaccine therapy.
Malignancy treatment
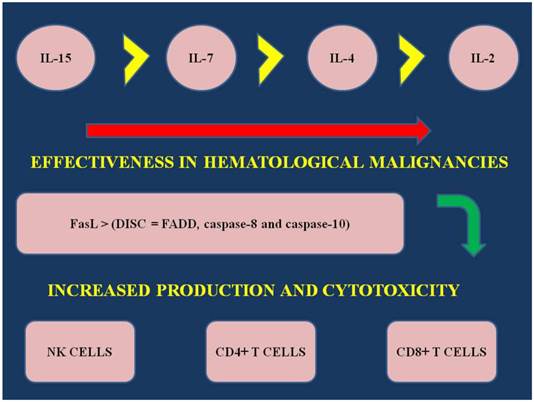
In the study by Fritzell et. al. [13] in a rat glioma model dual immunotherapy was administered with interferon-γ and IL-7 and increased survival was observed via increased T-cell proliferation. In another model of dual immunotherapy it was observed that combination of IL-7 and IL-2 has the highest rate of effectiveness, when compared to IL-7 alone or IL-2. Additionally, when comparing the following interleukin 2,4 and 7 the effectiveness of each one alone as a immunotherapy is demonstrated as follows: IL-7>IL-2>IL-4 [56]. (Figure 1) Furthermore, in the study by Touw et. al. [57] recombinant IL-7 was administered in ALL cell lines and patients with ALL. 128I-IL-7 binding experiments demonstrated that there are two types of receptors: high affinity and low affinity. The authors concluded again that IL-7 plays a major role in the regulation of ALL cell proliferation. Gene therapy expressing IL-7 with transduced bone marrow cells was also observed to be effective in in vivo of allogenic bone marrow transplantation [58]. In the study by Consolini et. al. [59] (37 childhood patients) IL-4, IL-7 stem cell factor (SCF) and insulin like growth factor-1 (IGF-1) were used in Nalm 1 and Nalm 6. These are two acute lymphoblastic leukemia cell lines (ALL) derived from pre-B cell lines. Il-7 co-administration with hyperthermia has been also used as a combination to immunotherapy in a melanoma in vivo model [60]. Il-7 administration has been also found effective in renal carcinoma [61].
IL; interleukin, NK; natural killer cells, CD4-8+ T cells; subfamily of T cells, FasL; Fas ligand, a transmembrane protein part of the Tumor Necrosis Family. The interaction between Fas and FasL results in the formation of the death-inducing signaling complex (DISC), which contains the FADD, caspase-8 and caspase-10.
IL; interleukin, RANKL; receptor activator of nuclear factor-α, TNF-α; tumor necrosis factor, PI3K; phosphatidyl-inositol-3-kinase, STAT3-5; mammalian family members 3-5, Ras/Raf/Erk: Ras/Raf/Mitogen-activated protein kinase/ERK kinase (MEK)/extracellular-signal-regulated kinase (ERK) cascade, MICA/B; major histocompatibility complex class I-related chain A and B, NK; natural killer cells, IFN-α,β,γ; Interferons α,β,γ, ΤΗ1-2; effector T-cells. Akt; also known as Protein Kinase B (PKB), is a serine/threonine-specific protein kinase, Anti-CTLA-4; Cytotoxic T-Lymphocyte Antigen 4, PD-L1; Programmed cell death 1 ligand 1.
Negative effects
IL-4 did not present any promoting activity and in two patients showed evidence ofinhibition instead. IL-7 demonstrated heterogenous effects, with minor proliferation and clonal growth activity observed. SCF, although it is known to synergize with IL-7 in primitive stages of normal B-cell development, in the study by Consolini et. al. [59] did not enhance the IL-7 response to B-cell precursor ALL. Moreover; IGF-1 failed to influence proliferation and clonal growth, either alone or in combination with IL-7. In another study by Markley et. al. [62] the activities of IL-7, IL-21, IL-2 and IL-15 were evaluated in obese diabetic/severe combined immunodeficient (NOD/SCID)/γcnull mice. It was observed that IL-7 and IL-21 transduced T cells efficiently in vivo although their effector function was not as effective as that of IL-2 and IL-15 transduced T cells. Il-7 was observed to preserve in vitro T-cell accumulation under repeated antigenic stimulation, however; it did not promote in vivo long term T-cell persistence. Regarding Il-15 and IL-21 long-term T-cell over-expression was observed in mice, however; after 100 days different phenotypes of memory T-cells were observed. This study supportsimmunotherapy as an anticancer treatment and established that there is more than one human memory T-cell phenotype.
IL-15 experimentation
Disease association
In the study by Roberti et. al. [63] cetuximab was co-administered with IL-2 or IL-15 in triple-negative breast cancer (TNBC) patients. IL-2 and IL-15 stimulated NK cells to perform antibody-dependent cellular cytotoxicity (ADCC) which was triggered by Cetuximab. ADCC is reduced in TNBC; it has been observed that in advanced disease ADCC is diminished, compared to healthy volunteers. The combination therapy can restore the ADCC and enhance the therapeutic activity of Cetuximab.
Immunisation
In the study by Decot et. al. [64] the impact of three cytokines; IL-2, IL-7 and IL-15 was evaluated on NK-cell amplification for adoptive leukemia. The amplification was evaluated for three receptors; NKG1D, KIR2DL1 and KIR2DL2. All three receptors were significantly up-regulated, however; IL-2 and IL-15 were the two cytokines observed to have the highest impact. Combination of IL-2 and IL-15 did not demonstrate any additional effects. In the study by Anguille et. al. [65] IL-15 induced monocyte dendritic cells demonstrated their cytotoxic effect in K562 human tumor cells. Their cytotoxic effect is mediate by perforin and tumor necrosis factor-α-(TNF-α) related apoptosis-inducing ligand (TRAIL). It is however idependent of perforin, Fas ligand and TNF-α. IL-15 can be used to transform monocyte DCs to IL-15 DC killer cells, making them a future immunotherapy treatment for cancer.
Malignancy Treatment
In the study by Marrero et. al. [66] electroporation (EP) was used as a method to deliver phIL-15 plasmid directly to tumors to evaluate the immune-antitumor effect. The experiment was conducted in mice (C57BL/6 with B16.F10 melanoma tumor model) and phIL-15 was delivered three times per week. Increased expression of IL-15 was observed within the tumor within 12-18 hours of the first administration. Mild long term effect was observed after the second and third administration. Tumor regression, prolonged survival and protection against tumor recurrence (additional melanoma cell inoculation vs. placebo plasmid) were observed. Further elucidation of the underlying gene expression of EP is necessary in regards to stability of this methodology of gene delivery. In another gene therapy model by Ochoa et. al. [67] apolipoprotein A-I (Apo A-I) was used as a vehicle for the plasmid encoding pApo-hIL-15. The bioactivity of Apo A-I (pApo-hIL-15) was longer than non-stabilized IL-15 and it was observed that the fusion protein APO-IL-15 was partially incorporated in circulating high density lipoprotein (HDL). A number of NK cells and CD8+ cells were increased in the blood circulation. Additionally populations of NK and memory T-cell in a deficiency mouse model IL-15Rα-/- were increased. Tumor control was observed in a MC38 colon carcinoma model with this methodology. In the study by Perna et. al. [68] the effect of IL-15 was assessed on the adoptively transferred antigen-specific cytotoxic T lymphocytes (CTLs) in the presence of regulatory T cells (T-regs). Epstein-Barr (EBV) was used as it capable for adoptive transfer. It was observed that IL-15 selectively favored the proliferation, survival and effector function of antigen-specific (CTLs) in the presence of Tregs. The administration of IL-15 could be also effective for patients with EBV-associated malignancies where EBV-CTLs are infused.
Negative effects
In the study by Mishra et. al. [69] it was demonstrated that in vitro exposure of wild type (WT) LGL to IL-15 results in Myc-mediated up regulation of aurora kinases, centrosome aberrancies, and aneuploidy. Moreover; IL-15 represses miR-29b via induction of Myc/NF-κBp65/Hdac-1, resulting in Dnmt3b overexpression and DNA hypermethylation. All this is validated in human LGL leukemia. Adoptive transfer of WT LGL cultured with IL-15 leads to malignant transformation in vivo; therefore drug targeting which reverses miR-29b repression, cures otherwise fatal LGL leukemia.
In the study by Zhao et. al. [70] combination of gene therapy with plasmid expressing IL-15 and everolimus was administered in a breast cancer mouse model. Mice inoculated with 4T1 mouse breast cancer cells were monitored until tumor and metastasis were observed and the administration of the combined treatment began. Immunohistochemistry was used to detect CD8+, CD4+ and NKG2D+cells and to evaluate the expression of Ki-67 in tumor tissue. IL-15 gene therapy increased the CD4+ T cells and NK cells, but had no effect on CD8+ T cells. On the contrary, everolimus had no effect on CD4+ T cells and NK cells, but its administration decreased CD4+ T cells. IL-15 and everolimus, both decreased Ki67 and increased apoptosis. The combination of the two treatments did not demonstrate any synergistic effect.
Discussion
In the study by Li et. al. [71] data were presented in favor of using GM-CSF as an adjuvant immunotherapy due to the significant prolongation of the survival of tumor-bearing mice. An antitumor-effect was observed due to the activation of dendritic cells (DC) and T-cells. The combination of IL-7 with GM-CSF increased the number of activated effector T-cells in the tumor microenvironment. Granulocyte -macrophage colony stimulating factor (GM-CSF) could be used as an adjuvant therapy, enhancing anti-tumor effect in the tumor`s microenvironment [71]. Cytokines have rapid blood clearance rates and they lack tumor cell specific connections [72]. Therefore high doses are necessary for effective anti-tumor effects to be observed; however, these high doses induce toxic adverse effects [73]. Development of an active transportation system (antigen- antibody) for cytokine administration is the next wise concept in drug design [74-76]. Interleukin-2 (IL-2) has been tested in clinical trials as an anticancer immunotherapy for renal cancer and melanoma [77, 78], however; adverse effects were observed. On the contrary, IL-15 although it has a similar structure with IL-2, demonstrated safety and efficiency [79, 80]. The mechanism of immunostimulation is through activation of cytokine-induced killer cells (CIK) as these cells are known to be effective in hematological and solid malignancies. CIK cells belong to a polyclonal T cell population which has the phenotype of NK cells and also has functional properties of the NK cells [81]. Recombinant IL-15 was first administered in rhesus macaques and increased circulating population of NK cells and CD8+ cells were observed. Therefore IL-15 is currently being tested as immunotherapy in clinical trials [32]. In the case of acute myeloid leukemia (AML) immunodeficiency attributed to functional deficiency of NK cells and cytotoxic T-cells could be a possible target for immune therapy. However, previous experimentation with IL-2 and histone deacetylase (HDAC) administered separately have indicated poor results, therefore sequential administration of both has been proposed [82, 83]. Immune therapy as a vaccine modality is a future perspective for malignancies [55]. Levels of serum IL-7 and IL-15 and tissue gene expression in biopsies can be used for diagnosis and prognosis [7]. The site of IL-7 production has not been clearly identified, however; intravital imaging techniques have been used as a method of IL-7 observation [84]. Administration of recombinant IL-7 (rhIL-7) in clinical trials resulted in an increase in CD4+ and CD8+ T cells populations in peripheral blood and an enhanced immune response with limited naïve T cells due to age, human immunodeficiency and patients receiving chemotherapy [85]. Therefore in solid tumors or malignancies other than hematological rhIL-7 administration could be used as an adjuvant treatment while in hematological malignancies it is used as a primary therapy. Moreover; it has been observed that IL-7 production occurs in intestinal epithelial cells (IECs) from bacterial stimuli. Flagellin (a globular protein) is the principal substituent of bacterial flagellum down-regulated the production of IL-7 via the Toll-like receptor pathway. This mode of action provides evidence for a future concept of local immunotherapy therapy via an independent gene down-regulation mechanism [86]. Future drug design requires novel molecules such as in the study by Vincent et. al. [87] where immunotherapy can be achieved along with cytotoxicty. The molecules of immunocytokines can be designed to have a bifunctional nature, both cytokine and antibody. The mechanism where inflammation induces cancer is unclear. IL-15 is a pro-inflammatory cytokine elevated in human large granular lymphocyte (LGL) leukemia. Mice over-expressing IL-15 have been fund to develop LGL leukemia.
In conclusion, excessive IL-15 initiates' cancer and therefore administration of an agent inhibiting chromosome instability is welcomed. On the other hand if malignancy has already occurred then IL-15 good be used as immunotherapy. Reactive oxygen species (ROS) have been also found to become upregulated by IL-7 and consequently activate PI3K/Akt/mTOR pathway. Survival of T-ALL cells is mediated from the interaction of these three parameters [88]. Il-7 administration could be also used as an adjuvant treatment for fast reconstitution of T-cells after intensive chemotherapy [89]. We have no data regarding the levels of IL-7 and IL-15 in advanced disease or as a prognostic factor as we have with IL-6 [90]. The data demonstrated in this work indicates that these pro-inflammatory cytokines play a major role in hematologic malignancies [61, 91], although they have been observed to play an important role in the modulation of the tumor microenvironment in other malignancies such; renal carcinoma [61], melanoma [60] and lung carcinoma [92]. Immunotherapy has been efficiently used in lung cancer [93, 94], however; it seems that this treatment modality has a special role as a blocker for early chromosome instability and afterwards as therapy. Cytokines could be also used as biomarkers for survival [95]. Cytokines could also be used as adjuvant or switch therapy in cancer patients [96]. We would like to have in the future targeted therapy for IL-7 and IL-15 as in the case of IL-6, however; the time of administration is crucial and therefore more trials are needed to elicit this parameter.
Interleukin experimentation.
| Author | Design/cells | Evaluation | Result | Ref |
|---|---|---|---|---|
| Kim et. Al. | In vitro/ invivo | Construction, OVA preparation, bioassay, cytotoxicity evaluation, immunofluorescent staining, cytofluometry, immunoprotection | Effective immunization | 55 |
| Mengus et. al. | Clinical trial | ELISA assays, quantitative Real-Time PCR | Increased levels of serum IL-7 and IL-15 correlated with increased tissue over-expression in early stage PCA | 7 |
| Collombetti et. al. | In vivo | Construction of lentivector, vaccination, flow cytometry, BrdU administration, in vivo IL-7 | IL-7 effective as an adjuvant long term treatment enhancing CD8+ T-cell response | 51 |
| Ott et. al. | In vivo B-all | Flow cytometry, Expression analysis, ChIP, Immunoblotting, gene expression arrays, cell viability, proliferation, caspace activity assays | BET inhibitor as a possible treatment | 52 |
| Silva et.al. | In vitro | Intracellular staining, in vivo bioluminescence imaging, blood and organ analysis, immunoblotting, quantitative reverse transcriptase PCR | Indication for IL-7R targeted treatment | 53 |
| Otto et. al. | In vitro, In vivo | Cytotoxic assays, isolation and purity of γδ T cells, animal survival | Future concept for neuroblastoma immune treatment | 12 |
| Dean et. al. | Clinical trial | Flow cytometry, serum IL-7 enzyme-linked immunosorbent assay | IL-7 serum values as a prognostic factor | 50 |
| Fritzell et. al. | In vivo | Cell surface molecule staining, survival study, blood analysis | Effective dual (IFN-γ and IL-7) immunotherapy | 13 |
| Lu et. al. | In vivo | Enumeration of blood mononuclear cell subsets, CMV serology, TREC, assay, histology, CT, IL-7 Nabs, detection of CMV by PCR, Intracellular cytokine staining for detection of CMV-specific CD4+ T cells | Flagellin as a future local immunotherapy | 54 |
| Li et. al. | In vitro, In vivo | Generation of adenovirus vector and IL-7 gene transduced bone MSC, Allo-BMT | MSC-IL-7 gene therapy is effective in BMT | 58 |
| Lynch et. al. | In vitro, In vivo | Adoptive immunotherapy of tumors in vivo, cytokines, tumor immunization and generation of cytotoxic lymphocytes | IL-7 more effective than IL-2 or IL-4 alone, however; combination of IL-7 and IL-2 has the highest rate of efficiency | 56 |
| Consolini et. al. | In vitro, clinical trial | Cell separation, immunophenotype, cytogenetic analysis, southern blot analysis, proliferation assay, leukemic colony assays, cell cultures | IL-4 and IGF-1 did not induce proliferation. IL-7 induced minor proliferative results. SCF did not enhance IL-7 acitivity | 59 |
| Markley et. al. | In vitro, In vivo | PBL collection and retroviral transduction, flow cytometry, mouse tumor model and quantitative bioluminescence, in vitro T-cell assays | More than 1 T-cell memory phenotype | 62 |
| Decot et. al. | In vitro | NK-cell enrichment, Expansion of NK cells, Cytotoxicity assay, HLA typing, flow cytometry | 10ng/mL IL-2 or 50ng/mL administration concentrations are the optimal dosage for enhanced cytotoxicity and modification of NK-cell receptor expression pattern | 64 |
| Touw et. al. | In vitro, clinical trial | FACS, In vitro culture, recombinant growth factors, radioiodination of IL-7 and biding experiments | Two IL-7 receptors high affinity (kd 29-51 pmol/L) and low affinity (kd 2.3 to 76 nmol/L) | 57 |
| Anguille et. al. | In vitro | Flow cytometry immunophenotyping, CD56 expression kinetics, granzyme B secretion, Allogenic mixed lymphocyte reaction (allo-MLR), antigen presentation assay, cytotoxicity assays, cytotoxicity blocking studies | IL-15 DCs future immunotherapy | 65 |
| Ochoa et. al. | In vitro, In vivo | Apolipoprotein A-I and Interleukin 15 gene fusion designs, hydrodynamic injections and ELISA, IL-15 bioactivity assay, antibodies and flow cytometry, electrophoresis and Apo A-I immunoblotting, CFSE labeling of cells, Adoptive transfer and BrdU assessment of proliferation in vivo | Efficient as a future immunotherapy | 67 |
| Roberti et. al. | Clinical trial | PBMC isolation, lymphocytes isolation from mammary tissue, flow cytometry, degranulation assay, lysis and ADCC experiments, Co-culture experiments, tumor transplantation and Ab therapy, IHC | Combination immunotherapy with IL-2 and IL-15 | 63 |
| Perna et. al. | In vivo | Isolation of Tregs from healthy donors, Isolation of Tregs from Hodgkin lymphoma samples, Activation of CD4+CD25bright cells, single-cell cloning Tregs, generation of EBV-CTLs, immunophenotyping, evaluation of apoptosis, ELISA, evaluation of antitumor activity, CFSE | IL-15 influences proliferation of CTLs, and EBV-CTLs | 68 |
| Mishra et. al. | In vitro, In vivo | Generation of transgenic mice, in vitro culture of LGL cells, antibody staining and flow cytometry, enrichment of LGL, total RNA and DNA isolation, first strand synthesis for RT-PCR and quantitative Taqman PCR, confocal, immune fluorescence, ChIP and quantitative ChIP PCR, transfection of primary murine, in vitro transformation assay | IL-15 a possible target for malignancy inhibition at early stage | 69 |
| Zhao et. al. | In vitro, In vivo | Cell transfection, ELISA, IHC and histological, TUNEL | IL-15 gene therapy a future application for metastatic breast cancer | 70 |
| Wu et. al. | In vitro, In vivo | histological, tumor growth | Effective combination treatment with hyperthermia | 60 |
ELISA; Enzyme-Linked Immunosorbent Assay, IHC; immunohistochemistry, TUNEL; Terminal deoxynucleotidyl transferase mediated dUTP Nick End Labeling assay, RT-PCR; Reverse transcription polymerase chain reaction, CFSE; IL-2,4,7,15; interleukin-2,4,7,15, PCA; prostate cancer, OVA; ovalbumin, IL-7R; interleukin-7receptor, IFN-γ; interferon-γ, CMV; Cytalomegalovirus, Nabs; neutralizing antibodies, TREC; T cell receptor excision circle assay, CT; computed tomography, BMT; bone marrow transplantation, MSC; bone marrow stromal cells, IGF-1; insulin like growth factor-1, SCF; stem cell factor, NK cells; natural killer cells, HLA; human leukocyte antigen, FACS; immunofluorescence and purification of ALL cells by fluorescence-activated cell sorting, ALL; acute lymphoblastic leukemia, EBV; Epstein bar, CTLs; cytotoxic T lymphocytes, LGL; large granular lymphocyte.
Conflict of Interest
None to declare.
References
1. Appasamy PM. Biological and clinical implications of interleukin-7 and lymphopoiesis. Cytokines Cell Mol Ther. 1999;5:25-39
2. Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564-71 doi:S1471-4906(01)02028-2 [pii]
3. Al-Rawi MA, Mansel RE, Jiang WG. Interleukin-7 (IL-7) and IL-7 receptor (IL-7R) signalling complex in human solid tumours. Histol Histopathol. 2003;18:911-23
4. Eder M, Ottmann OG, Hansen-Hagge TE, Bartram CR, Falk S, Gillis S. et al. In vitro culture of common acute lymphoblastic leukemia blasts: effects of interleukin-3, interleukin-7, and accessory cells. Blood. 1992;79:3274-84
5. Foss HD, Hummel M, Gottstein S, Ziemann K, Falini B, Herbst H. et al. Frequent expression of IL-7 gene transcripts in tumor cells of classical Hodgkin's disease. Am J Pathol. 1995;146:33-9
6. Sharma S, Wang J, Huang M, Paul RW, Lee P, McBride WH. et al. Interleukin-7 gene transfer in non-small-cell lung cancer decreases tumor proliferation, modifies cell surface molecule expression, and enhances antitumor reactivity. Cancer Gene Ther. 1996;3:302-13
7. Mengus C, Le Magnen C, Trella E, Yousef K, Bubendorf L, Provenzano M. et al. Elevated levels of circulating IL-7 and IL-15 in patients with early stage prostate cancer. J Transl Med. 2011;9:162. doi:10.1186/1479-5876-9-162 1479-5876-9-162 [pii]
8. Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, La Monica S. et al. Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood. 2002;100:4615-21 doi:10.1182/blood-2002-04-1121 2002-04-1121 [pii]
9. Okopien B, Krysiak R, Kowalski J, Madej A, Belowski D, Zielinski M. et al. Monocyte release of tumor necrosis factor-alpha and interleukin-1beta in primary type IIa and IIb dyslipidemic patients treated with statins or fibrates. J Cardiovasc Pharmacol. 2005;46:377-86 doi:00005344-200509000-00020 [pii]
10. Roato I, Brunetti G, Gorassini E, Grano M, Colucci S, Bonello L. et al. IL-7 up-regulates TNF-alpha-dependent osteoclastogenesis in patients affected by solid tumor. PLoS One. 2006;1:e124. doi:10.1371/journal.pone.0000124
11. Trinder P, Seitzer U, Gerdes J, Seliger B, Maeurer M. Constitutive and IFN-gamma regulated expression of IL-7 and IL-15 in human renal cell cancer. Int J Oncol. 1999;14:23-31
12. Otto M, Barfield RC, Martin WJ, Iyengar R, Leung W, Leimig T. et al. Combination immunotherapy with clinical-scale enriched human gammadelta T cells, hu14.18 antibody, and the immunocytokine Fc-IL7 in disseminated neuroblastoma. Clin Cancer Res. 2005;11:8486-91 doi:11/23/8486 [pii] 10.1158/1078-0432.CCR-05-1184
13. Fritzell S, Eberstal S, Sanden E, Visse E, Darabi A, Siesjo P. IFNgamma in combination with IL-7 enhances immunotherapy in two rat glioma models. J Neuroimmunol. 2013;258:91-5 doi:10.1016/j.jneuroim.2013.02.017 S0165-5728(13)00055-6 [pii]
14. Maeurer MJ, Walter W, Martin D, Zitvogel L, Elder E, Storkus W. et al. Interleukin-7 (IL-7) in colorectal cancer: IL-7 is produced by tissues from colorectal cancer and promotes preferential expansion of tumour infiltrating lymphocytes. Scand J Immunol. 1997;45:182-92
15. Cosenza L, Gorgun G, Urbano A, Foss F. Interleukin-7 receptor expression and activation in nonhaematopoietic neoplastic cell lines. Cell Signal. 2002;14:317-25 doi:S0898656801002455 [pii]
16. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337-42 doi:10.1038/nature01658 nature01658 [pii]
17. Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858-64
18. Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood. 2000;96:1873-8
19. Lee SK, Kalinowski JF, Jastrzebski SL, Puddington L, Lorenzo JA. Interleukin-7 is a direct inhibitor of in vitro osteoclastogenesis. Endocrinology. 2003;144:3524-31
20. Miyaura C, Onoe Y, Inada M, Maki K, Ikuta K, Ito M. et al. Increased B-lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: similarity to estrogen deficiency. Proc Natl Acad Sci U S A. 1997;94:9360-5
21. Roato I, Grano M, Brunetti G, Colucci S, Mussa A, Bertetto O. et al. Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. FASEB J. 2005;19:228-30 doi:04-1823fje [pii] 10.1096/fj.04-1823fje
22. Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC. et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955-60
23. von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519-26
24. Corcoran AE, Riddell A, Krooshoop D, Venkitaraman AR. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature. 1998;391:904-7 doi:10.1038/36122
25. Chowdhury D, Sen R. Stepwise activation of the immunoglobulin mu heavy chain gene locus. EMBO J. 2001;20:6394-403 doi:10.1093/emboj/20.22.6394
26. Hesslein DG, Pflugh DL, Chowdhury D, Bothwell AL, Sen R, Schatz DG. Pax5 is required for recombination of transcribed, acetylated, 5' IgH V gene segments. Genes Dev. 2003;17:37-42 doi:10.1101/gad.1031403
27. Venkitaraman AR, Cowling RJ. Interleukin-7 induces the association of phosphatidylinositol 3-kinase with the alpha chain of the interleukin-7 receptor. Eur J Immunol. 1994;24:2168-74 doi:10.1002/eji.1830240935
28. Fleming HE, Paige CJ. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity. 2001;15:521-31 doi:S1074-7613(01)00216-3 [pii]
29. Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L. et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331-9
30. Crawley JB, Willcocks J, Foxwell BM. Interleukin-7 induces T cell proliferation in the absence of Erk/MAP kinase activity. Eur J Immunol. 1996;26:2717-23 doi:10.1002/eji.1830261125
31. Goetz CA, Harmon IR, O'Neil JJ, Burchill MA, Farrar MA. STAT5 activation underlies IL7 receptor-dependent B cell development. J Immunol. 2004;172:4770-8
32. Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33:35-41 doi:10.1016/j.tips.2011.09.004 S0165-6147(11)00173-8 [pii]
33. Giri JG, Anderson DM, Kumaki S, Park LS, Grabstein KH, Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J Leukoc Biol. 1995;57:763-6
34. Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L. et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:11445-50 doi:10.1073/pnas.200363097 200363097 [pii]
35. Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105-10 doi:S1074-7613(01)00093-0 [pii]
36. Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC. et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417-26 doi:10.1182/blood-2008-12-189266 blood-2008-12-189266 [pii]
37. Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771-80
38. Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J Exp Med. 2001;194:1187-94
39. Li XC, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek TR. et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114-8 doi:10.1038/83253
40. Agostini C, Siviero M, Facco M, Carollo D, Binotto G, Tosoni A. et al. Antiapoptotic effects of IL-15 on pulmonary Tc1 cells of patients with human immunodeficiency virus infection. Am J Respir Crit Care Med. 2001;163:484-9 doi:10.1164/ajrccm.163.2.2006028
41. Liu K, Catalfamo M, Li Y, Henkart PA, Weng NP. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc Natl Acad Sci U S A. 2002;99:6192-7 doi:10.1073/pnas.092675799 092675799 [pii]
42. Yajima T, Nishimura H, Ishimitsu R, Watase T, Busch DH, Pamer EG. et al. Overexpression of IL-15 in vivo increases antigen-driven memory CD8+ T cells following a microbe exposure. J Immunol. 2002;168:1198-203
43. Kokaji AI, Hockley DL, Kane KP. IL-15 transpresentation augments CD8+ T cell activation and is required for optimal recall responses by central memory CD8+ T cells. J Immunol. 2008;180:4391-401 doi:180/7/4391 [pii]
44. Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537-47 doi:S1074761302004296 [pii]
45. Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825-34 doi:10.1084/jem.20041389 jem.20041389 [pii]
46. Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B. et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811-22 doi:10.1016/j.immuni.2009.09.017 S1074-7613(09)00457-9 [pii]
47. Poggi A, Catellani S, Garuti A, Pierri I, Gobbi M, Zocchi MR. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia. 2009;23:641-8 doi:10.1038/leu.2008.354 leu2008354 [pii]
48. Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG. et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389-96 doi:10.1182/blood-2003-01-0019 2003-01-0019 [pii]
49. Duan X, Deng L, Chen X, Lu Y, Zhang Q, Zhang K. et al. Clinical significance of the immunostimulatory MHC class I chain-related molecule A and NKG2D receptor on NK cells in pancreatic cancer. Med Oncol. 2011;28:466-74 doi:10.1007/s12032-010-9480-9
50. Dean RM, Fry T, Mackall C, Steinberg SM, Hakim F, Fowler D. et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol. 2008;26:5735-41 doi:10.1200/JCO.2008.17.1314 JCO.2008.17.1314 [pii]
51. Colombetti S, Levy F, Chapatte L. IL-7 adjuvant treatment enhances long-term tumor-antigen-specific CD8+ T-cell responses after immunization with recombinant lentivector. Blood. 2009;113:6629-37 doi:10.1182/blood-2008-05-155309 blood-2008-05-155309 [pii]
52. Ott CJ, Kopp N, Bird L, Paranal RM, Qi J, Bowman T. et al. BET bromodomain inhibition targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia. Blood. 2012;120:2843-52 doi:10.1182/blood-2012-02-413021 blood-2012-02-413021 [pii]
53. Silva A, Laranjeira AB, Martins LR, Cardoso BA, Demengeot J, Yunes JA. et al. IL-7 contributes to the progression of human T-cell acute lymphoblastic leukemias. Cancer Res. 2011;71:4780-9 doi:10.1158/0008-5472.CAN-10-3606 0008-5472.CAN-10-3606 [pii]
54. Lu H, Zhao Z, Kalina T, Gillespy T 3rd, Liggitt D, Andrews RG. et al. Interleukin-7 improves reconstitution of antiviral CD4 T cells. Clin Immunol. 2005;114:30-41 doi:S1521-6616(04)00250-5 [pii] 10.1016/j.clim.2004.08.008
55. Kim TS, Chung SW, Hwang SY. Augmentation of antitumor immunity by genetically engineered fibroblast cells to express both B7.1 and interleukin-7. Vaccine. 2000;18:2886-94 doi:S0264-410X(00)00061-X [pii]
56. Lynch DH, Namen AE, Miller RE. In vivo evaluation of the effects of interleukins 2, 4 and 7 on enhancing the immunotherapeutic efficacy of anti-tumor cytotoxic T lymphocytes. Eur J Immunol. 1991;21:2977-85 doi:10.1002/eji.1830211212
57. Touw I, Pouwels K, van Agthoven T, van Gurp R, Budel L, Hoogerbrugge H. et al. Interleukin-7 is a growth factor of precursor B and T acute lymphoblastic leukemia. Blood. 1990;75:2097-101
58. Li AL, Li C, Feng YG, Yuan GH, Wang GM, Hao J. et al. Antileukemic effect of interleukin-7-transduced bone marrow stromal cells in mice following allogeneic T-cell-depleted bone marrow transplantation. Transplant Proc. 2005;37:2297-9 doi:S0041-1345(05)00327-1 [pii] 10.1016/j.transproceed.2005.03.088
59. Consolini R, Legitimo A, Cattani M, Simi P, Mattii L, Petrini M. et al. The effect of cytokines, including IL4, IL7, stem cell factor, insulin-like growth factor on childhood acute lymphoblastic leukemia. Leuk Res. 1997;21:753-61 doi:S0145212697000489 [pii]
60. Wu B, Shen RN, Wang WX, Broxmeyer HE, Lu L. Antitumor effect of interleukin 7 in combination with local hyperthermia in mice bearing B16a melanoma cells. Stem Cells. 1993;11:412-21 doi:10.1002/stem.5530110508
61. Sica D, Rayman P, Stanley J, Edinger M, Tubbs RR, Klein E. et al. Interleukin 7 enhances the proliferation and effector function of tumor-infiltrating lymphocytes from renal-cell carcinoma. Int J Cancer. 1993;53:941-7
62. Markley JC, Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115:3508-19 doi:10.1182/blood-2009-09-241398 blood-2009-09-241398 [pii]
63. Roberti MP, Rocca YS, Amat M, Pampena MB, Loza J, Colo F. et al. IL-2- or IL-15-activated NK cells enhance Cetuximab-mediated activity against triple-negative breast cancer in xenografts and in breast cancer patients. Breast Cancer Res Treat. 2012;136:659-71 doi:10.1007/s10549-012-2287-y
64. Decot V, Voillard L, Latger-Cannard V, Aissi-Rothe L, Perrier P, Stoltz JF. et al. Natural-killer cell amplification for adoptive leukemia relapse immunotherapy: comparison of three cytokines, IL-2, IL-15, or IL-7 and impact on NKG2D, KIR2DL1, and KIR2DL2 expression. Exp Hematol. 2010;38:351-62 doi:10.1016/j.exphem.2010.02.006 S0301-472X(10)00051-2 [pii]
65. Anguille S, Lion E, Tel J, de Vries IJ, Coudere K, Fromm PD. et al. Interleukin-15-induced CD56(+) myeloid dendritic cells combine potent tumor antigen presentation with direct tumoricidal potential. PLoS One. 2012;7:e51851. doi:10.1371/journal.pone.0051851 PONE-D-12-18783 [pii]
66. Marrero B, Shirley S, Heller R. Delivery of Interleukin-15 to B16 Melanoma by Electroporation Leads to Tumor Regression and Long-term Survival. Technol Cancer Res Treat. 2013 doi:10.7785/tcrtexpress.2013.600252
67. Ochoa MC, Fioravanti J, Duitman EH, Medina-Echeverz J, Palazon A, Arina A. et al. Liver gene transfer of interkeukin-15 constructs that become part of circulating high density lipoproteins for immunotherapy. PLoS One. 2012;7:e52370. doi:10.1371/journal.pone.0052370 PONE-D-12-14672 [pii]
68. Perna SK, De Angelis B, Pagliara D, Hasan ST, Zhang L, Mahendravada A. et al. Interleukin 15 provides relief to CTLs from regulatory T cell-mediated inhibition: implications for adoptive T cell-based therapies for lymphoma. Clin Cancer Res. 2013;19:106-17 doi:10.1158/1078-0432.CCR-12-2143 1078-0432.CCR-12-2143 [pii]
69. Mishra A, Liu S, Sams GH, Curphey DP, Santhanam R, Rush LJ. et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell. 2012;22:645-55 doi:10.1016/j.ccr.2012.09.009 S1535-6108(12)00394-7 [pii]
70. Zhao N, Li X, He X, Qiu Y, Zhu L, Qi F. IL-15 gene therapy and the mTOR inhibitor everolimus inhibit growth of metastatic breast cancer. J Gene Med. 2013 doi:10.1002/jgm.2739
71. Li B, VanRoey MJ, Jooss K. Recombinant IL-7 enhances the potency of GM-CSF-secreting tumor cell immunotherapy. Clin Immunol. 2007;123:155-65 doi:S1521-6616(07)00005-8 [pii] 10.1016/j.clim.2007.01.002
72. Carnemolla B, Borsi L, Balza E, Castellani P, Meazza R, Berndt A. et al. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99:1659-65
73. Schwartz RN, Stover L, Dutcher J. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park). 2002;16:11-20
74. Halin C, Rondini S, Nilsson F, Berndt A, Kosmehl H, Zardi L. et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol. 2002;20:264-9 doi:10.1038/nbt0302-264 nbt0302-264 [pii]
75. Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today. 2012;17:583-90 doi:10.1016/j.drudis.2012.01.007 S1359-6446(12)00009-8 [pii]
76. Kontermann RE. Antibody-cytokine fusion proteins. Arch Biochem Biophys. 2012;526:194-205 doi:10.1016/j.abb.2012.03.001 S0003-9861(12)00083-5 [pii]
77. Johannsen M, Spitaleri G, Curigliano G, Roigas J, Weikert S, Kempkensteffen C. et al. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46:2926-35 doi:10.1016/j.ejca.2010.07.033 S0959-8049(10)00728-8 [pii]
78. Gilman AL, Ozkaynak MF, Matthay KK, Krailo M, Yu AL, Gan J. et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: a report from the Children's Oncology Group. J Clin Oncol. 2009;27:85-91 doi:10.1200/JCO.2006.10.3564 JCO.2006.10.3564 [pii]
79. Baluna R, Rizo J, Gordon BE, Ghetie V, Vitetta ES. Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc Natl Acad Sci U S A. 1999;96:3957-62
80. Munger W, DeJoy SQ, Jeyaseelan R Sr, Torley LW, Grabstein KH, Eisenmann J. et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell Immunol. 1995;165:289-93 doi:S0008-8749(85)71216-6 [pii] 10.1006/cimm.1995.1216
81. Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J. et al. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011;118:3301-10 doi:10.1182/blood-2011-02-336321 blood-2011-02-336321 [pii]
82. Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357-68 doi:10.1111/j.1600-065X.2008.00604.x IMR604 [pii]
83. Zdrenghea MT. Could interleukin-15 potentiate histone deacetylase inhibitor effects in haematological malignancy? Med Hypotheses. 2013;81:311-5 doi:10.1016/j.mehy.2013.04.021 S0306-9877(13)00191-6 [pii]
84. Mazzucchelli RI, Warming S, Lawrence SM, Ishii M, Abshari M, Washington AV. et al. Visualization and identification of IL-7 producing cells in reporter mice. PLoS One. 2009;4:e7637. doi:10.1371/journal.pone.0007637
85. Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR. et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701-14 doi:10.1084/jem.20071681 jem.20071681 [pii]
86. Yoshioka A, Okamoto R, Oshima S, Akiyama J, Tsuchiya K, Nakamura T. et al. Flagellin stimulation suppresses IL-7 secretion of intestinal epithelial cells. Cytokine. 2008;44:57-64 doi:10.1016/j.cyto.2008.06.004 S1043-4666(08)00182-8 [pii]
87. Vincent M, Bessard A, Cochonneau D, Teppaz G, Sole V, Maillasson M. et al. Tumor targeting of the IL-15 superagonist RLI by an anti-GD2 antibody strongly enhances its antitumor potency. Int J Cancer. 2013;133:757-65 doi:10.1002/ijc.28059
88. Silva A, Girio A, Cebola I, Santos CI, Antunes F, Barata JT. Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells. Leukemia. 2011;25:960-7 doi:10.1038/leu.2011.56 leu201156 [pii]
89. Wendelbo O, Glenjen N, Bruserud O. Interleukin-7 (IL-7) in patients receiving intensive chemotherapy for acute myelogenous leukemia: studies of systemic IL-7 Levels and IL-7 responsiveness of circulating T lymphocytes. J Interferon Cytokine Res. 2002;22:1057-65 doi:10.1089/107999002760624297
90. Zarogoulidis P, Yarmus L, Darwiche K, Walter R, Huang H, Li Z. et al. Interleukin-6 cytokine: a multifunctional glycoprotein for cancer. Immunome Res. 2013;9:16535. doi:10.1186/2090-5009-9-1
91. LeBlanc FR, Hasanali ZS, Loughran TP Jr. Does IL-15 have a causative role in large granular lymphocyte leukemia? Immunotherapy. 2013;5:231-4 doi:10.2217/imt.13.7
92. Guarini A, Riera L, Reato G, Carbone A, Cignetti A, Tos A. et al. Human lung carcinoma cells engineered to release IL2, IL7, GM-CSF and TNF alpha. Int J Oncol. 1996;8:765-72
93. Zarogoulidis K, Ziogas E, Boutsikou E, Zarogoulidis P, Darwiche K, Kontakiotis T. et al. Immunomodifiers in combination with conventional chemotherapy in small cell lung cancer: a phase II, randomized study. Drug Des Devel Ther. 2013;7:611-7 doi:10.2147/DDDT.S43184 dddt-7-611 [pii]
94. Mylonaki E, Manika K, Zarogoulidis P, Domvri K, Voutsas V, Zarogoulidis K. et al. In vivo synergistic cytogenetic effects of aminophylline on lymphocyte cultures from patients with lung cancer undergoing chemotherapy. Mutat Res. 2012;740:1-5 doi:10.1016/j.mrfmmm.2012.10.002 S0027-5107(12)00206-0 [pii]
95. Paradkar PH, Joshi JV, Mertia PN, Agashe SV, Vaidya RA. Role of cytokines in genesis, progression and prognosis of cervical cancer. Asian Pac J Cancer Prev. 2014;15(9):3851-64
96. Ladenstein R, Pötschger U, Siabalis D, Garaventa A, Bergeron C, Lewis IJ, Stein J, Kohler J, Shaw PJ, Holter W, Pistoia V, Michon J. Dose finding study for the use of subcutaneous recombinant interleukin-2 to augment natural killer cell numbers in an outpatient setting for stage 4 neuroblastoma after megatherapy and autologous stem-cell reinfusion. J Clin Oncol. 2011Feb1;29(4):441-8 doi: 10.1200/JCO.2009.23.5465. Epub 2010 Dec 13
Author contact
![]() Corresponding author: Paul Zarogoulidis, M.D, Ph.D. Pulmonary Department-Oncology Unit, ``G. Papanikolaou`` General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece. Fax: 00302310992433 Mobile: 00306977271974 E-mail: pzarogcom.
Corresponding author: Paul Zarogoulidis, M.D, Ph.D. Pulmonary Department-Oncology Unit, ``G. Papanikolaou`` General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece. Fax: 00302310992433 Mobile: 00306977271974 E-mail: pzarogcom.

 Global reach, higher impact
Global reach, higher impact