Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(12):3579-3588. doi:10.7150/jca.116142 This issue Cite
Research Paper
Impact of Huisheng Oral Solution Combined with Immune Checkpoint Inhibitors and Chemotherapy in Patients with Stage III-IV Non-Small Cell Lung Cancer: A Retrospective Analysis
1. Department of Oncology, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, 610071, Sichuan Province, China.
2. Department of Radiology, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, 610071, Sichuan Province, China.
3. Department of Thoracic Surgery, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, 610071, Sichuan Province, China.
4. Department of Breast Surgery, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, 610071, Sichuan Province, China.
5. Department of Medical Administration Division, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, 610071, Sichuan Province, China.
Received 2025-4-21; Accepted 2025-7-11; Published 2025-7-28
Abstract
Background: To evaluate the impact of Huisheng Oral Solution (HSOS) in conjunction with immune checkpoint inhibitors (ICIs) and chemotherapy on patients with stage III-IV non-small cell lung cancer (NSCLC).
Methods: This retrospective study included patients with stage III-IV NSCLC who were treated at Sichuan Provincial People's Hospital from May 2018 to June 2021. Patients were categorized into two groups: the ICIs & Chemo Group and the ICIs & Chemo & HSOS Group, based on the therapies administered. The disease control rate (DCR), objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and immune-related adverse events (irAEs) were assessed.
Results: A total of 185 patients were included, with 109 patients in ICIs & Chemo & HSOS Group. The ICIs & Chemo & HSOS Group exhibited significantly enhanced DCR (90.83% vs. 71.05%, p=0.001) compared to the ICIs & Chemo Group. The ORR was not statistically significant between the two groups (31.19% vs. 27.63%, p=0.628). Patients in the ICIs & Chemo & HSOS Group had significantly longer PFS (HR=0.47, 95% CI: 0.29-0.75, p<0.001) and OS (HR=0.58, 95% CI: 0.33-1.00, p=0.037) than the ICIs & Chemo Group. In terms of irAEs, nephrotoxicity (5.77% vs. 15.25%, p=0.044), checkpoint inhibitor-related pneumonitis (CIP) (2.75% vs. 11.84%, p=0.014), and cardiotoxicity (0% vs. 13.04%, p=0.026) were significantly lower in the ICIs & Chemo & HSOS Group.
Conclusion: The addition of HSOS to ICIs and chemotherapy may enhance DCR, PFS, and OS, while concurrently reducing irAEs in patients with stage III-IV NSCLC. These findings suggest that HSOS may serve as a promising adjunct to ICI-based therapies. Further prospective studies are warranted to validate these results.
Keywords: non-small cell lung cancer, immune checkpoint inhibitors, Huisheng oral solution, chemotherapy, immune-related adverse events
Introduction
Lung cancer remains one of the most prevalent and lethal malignancies worldwide, accounting for 11.4% of all cancer cases and 18.0% of all cancer-related deaths [1]. Non-small cell lung cancer (NSCLC) constitutes approximately 85% of all lung cancer cases [2]. In China, an estimated 800,000 individuals are diagnosed with lung cancer annually, with a 5-year overall survival (OS) rate of less than 20% [3]. Since the 1990s, platinum-based doublet chemotherapy has served as the cornerstone of first-line treatment for advanced NSCLC. However, the efficacy of chemotherapy as a monotherapy remains limited [4, 5]. Combination therapies incorporating other treatment modalities have become the standard of care, particularly for advanced-stage patients [6].
Recent advancements in immunotherapy, particularly with immune checkpoint inhibitors (ICIs), have revolutionized the treatment landscape for NSCLC [7]. ICIs, including programmed death receptor-1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors, are now widely used in the first- and second-line treatment of advanced NSCLC, as well as in the adjuvant treatment of locally advanced disease [8]. Numerous clinical studies have demonstrated that ICIs enhance the immunosuppressive tumor microenvironment (TME) and potentiate the immune system's natural anti-tumor capabilities [9]. When combined with other therapies, ICIs have been shown to increase tumor remission rates and reduce mortality compared to monotherapy. However, improvements in progression-free survival (PFS) and OS in second-line treatment and adjuvant settings remain inconsistent [10, 11]. Despite their clinical efficacy, ICIs are associated with immune-related adverse events (irAEs), which can significantly impact multiple organ systems. Common irAEs include rash, colitis, hepatitis, myocarditis, endocrine dysfunction, and checkpoint inhibitor pneumonitis (CIP) [12]. Severe irAEs, particularly grade 3-4 toxicities, are often managed with glucocorticoids and immunosuppressive agents, though these treatments have limited effectiveness and can introduce additional side effects [13].
Traditional Chinese Medicine (TCM) has a long history of complementing conventional cancer therapies by enhancing efficacy, improving tolerance, and mitigating adverse effects [14, 15]. Huisheng Oral Solution (HSOS), a Chinese patent medicine derived from the classic Qing Dynasty prescription Hua Zheng Hui Sheng Dan, has demonstrated therapeutic potential in NSCLC[16, 17]. Produced by Chengdu Di'ao Group Tianfu Pharmaceutical Co., Ltd. (Approval number: Z20025042), HSOS has been shown to improve short-term efficacy, prolong survival, and reduce adverse reactions in NSCLC patients when used in combination with chemotherapy, radiotherapy, targeted therapy, or immunotherapy [16, 18, 19]. Mechanistically, HSOS enhances immune function and modulates T lymphocyte subsets [20]. Preclinical studies have further suggested that HSOS can inhibit PD-1/PD-L1 expression and the activation of related signaling pathways, potentially enhancing the efficacy of immunotherapy while reducing irAEs [21].
To date, clinical evidence on the therapeutic benefits of HSOS combined with ICIs and chemotherapy for advanced NSCLC remains limited. This study retrospectively analyzed the impact of this combination therapy in patients with stage III-IV NSCLC.
Methods
Study design and patients
This retrospective study analyzed data from patients with stage III-IV NSCLC who were treated in the Oncology Department of Sichuan Provincial People's Hospital between May 2018 and June 2021. This study was approved by the Institutional Review Board of Sichuan Provincial People's Hospital (Approval No.: EC Review (Research) No.11,202). As this study involved retrospective analysis using de-identified data, informed consent was not required. The inclusion criteria were as follows: (1) primary diagnosis of NSCLC confirmed by histopathology; (2) stage III-IV disease [22]; (3) all patients received a combination of ICIs and chemotherapy; (4) at least one measurable lesion; (5) age ≥18 years; and (6) complete clinical data available. Exclusion criteria included: (1) mixed small-cell lung cancer and NSCLC histology; (2) prior exposure to PD-1/PD-L1 inhibitors; and (3) current or previous use of immunosuppressive medications. Patients were divided into two groups based on their therapies. The ICIs & Chemo group comprised patients who only received ICIs in combination with chemotherapy. The ICIs & Chemo & HSOS group included patients who received the same combination of ICIs and chemotherapy, with the addition of HSOS therapy. The ICIs used in both groups were as follows: Pembrolizumab (2 mg/kg, every 3 weeks), Sintilimab (200 mg, every 3 weeks), Camrelizumab (200 mg, every 3 weeks), Toripalimab (240 mg, every 3 weeks), and Tislelizumab (200 mg, every 3 weeks). Chemotherapy regimens included either paclitaxel or pemetrexed combined with platinum-based agents. Patients in the ICIs & Chemo & HSOS Group received 10 mL of HSOS orally three times daily. All patients underwent at least four treatment cycles, with each cycle lasting approximately 21 days.
Outcome
The outcome measures included the objective response rate (ORR), disease control rate (DCR), OS, PFS, and immune-related adverse events (irAEs). Objective response was assessed using RECIST v1.1 criteria, and evaluated by investigators based on imaging studies obtained at intervals of approximately two immune cycles [23]. According to the RECIST v1.1 criteria, Complete Response (CR) is defined as the disappearance of all target lesions, with no new lesions appearing. Partial Response (PR) is characterized by a reduction of at least 30% in the sum of the diameters of target lesions, using baseline measurements as a reference. Stable Disease (SD) refers to a condition where there is neither sufficient shrinkage to qualify for PR nor sufficient increase to meet the criteria for Progressive Disease (PD). Progressive Disease (PD) is defined as a 20% or greater increase in the sum of the diameters of target lesions, with an absolute increase of at least 5 mm or the appearance of new lesions. The ORR is calculated as the proportion of patients who achieve either CR or PR. The DCR was defined as the proportion of patients achieving CR, PR, or SD.
Follow-up was conducted through outpatient appointments and telephone consultations until October 2022. OS was defined as the time from treatment initiation to the date of death. Patients who were lost to follow-up or alive at the end of the study were censored at their last follow-up date. PFS was defined as the time from treatment initiation to disease progression or death. For patients who were lost to follow-up or had no documented progression, the cut-off time was the last follow-up date. According to previous studies on the impact of PD-1/PD-L1 inhibitors in small-cell lung cancer, the median PFS was around 7 to 8 months, and the median OS was around 18 to 19 months [24, 25]. Therefore, poor prognosis was defined as the occurrence of events (death or progression) within 6 months, while good prognosis was defined as the absence of events (no death or progression) beyond 18 months in this study. The differences in prognosis between the two groups were analyzed.
The evaluation of immune-related toxicities included liver function (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), renal function (creatinine [CRE]), thyroid function (thyroid-stimulating hormone [TSH]), cardiac toxicity (myocardial enzyme levels), and CIP. The NCCN Guidelines: Management of Immunotherapy-Related Toxicities, Version 1.2020 [26] were used to define the evaluation standards for hepatorenal toxicity, cardiotoxicity, and thyroid dysfunction. CIP and myocarditis were assessed using the Common Terminology Criteria for Adverse Events (CTCAE Version 4.03) [24] and were primarily determined by the lead clinical physician for each patient. The manifestations of CIP were categorized into four types: nonspecific interstitial pneumonia (NSIP), cryptogenic organizing pneumonia (COP), acute interstitial pneumonia/acute respiratory distress syndrome (AIP/ARDS), and hypersensitivity pneumonia (HP). The diagnostic criteria for myocardial infarction considered patients with normal high-sensitivity troponin levels (<1.5 ng/L) during baseline screening and a significant elevation in troponin levels following immunotherapy.
Statistical analysis
All statistical analyses were performed using SPSS 24.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). Quantitative data were described using medians and ranges, while categorical data were presented as counts (N) and percentages (frequencies). Comparisons of categorical variables were conducted using χ² tests. Kaplan-Meier survival curves were constructed to evaluate PFS and OS, and differences between groups were assessed using the log-rank test. Univariate and multivariate Cox proportional-hazards regression models were used to identify prognostic factors for PFS and OS. Statistical significance was defined as a two-sided p-value < 0.05.
Results
Baseline characteristics of the patients
A total of 185 patients with stage III-IV NSCLC were included in the study, with 76 patients in the ICIs & Chemo group and 109 patients in the ICIs & Chemo & HSOS group. No statistically significant differences were observed between the two groups regarding gender, age, pathological type, disease stage, PD-L1 expression, driver gene expression, or the choice of ICIs and antiangiogenic drugs (all p > 0.05). The baseline characteristics of the patients are summarized in Table 1.
ORR and DCR
In the ICIs & chemotherapy group, no patients achieved CR, while 21 patients achieved PR, 33 had SD, and 22 experienced PD. In the ICIs & chemotherapy & HSOS group, similarly, no CR were observed. However, 34 patients achieved PR, 65 had SD, and 10 developed PD. The ORR in the ICIs & Chemo Group and the ICIs & Chemo & HSOS Group was not statistically significant (31.19% vs. 27.63%, p = 0.628). However, the DCR was significantly higher in the ICIs & Chemo & HSOS Group compared to the ICIs & Chemo Group (90.83% vs. 71.05%, p = 0.001). Further analysis of clinical factors revealed that patients with squamous cell carcinoma (42.11% vs. 21.10%, p = 0.003) or lobulation (32.64% vs. 8.70%, p = 0.024) were more likely to have a higher ORR. Driver gene expression was identified as an influencing factor for DCR (p = 0.004) (Table 2).
Basic clinical information of included patients (n, %)
| ICIs & Chemo Group (n=76) | ICIs & Chemo & HSOS Group (n=109) | p | |
|---|---|---|---|
| Gender | 0.447 | ||
| Male | 64 (84.21) | 86 (78.90) | |
| Female | 12 (15.79) | 23 (21.10) | |
| Age (years) | 0.375 | ||
| <60 | 36 (47.37) | 59 (54.13) | |
| ≥60 | 40 (52.63) | 50 (45.87) | |
| Pathological type | 0.762 | ||
| Adenocarcinoma | 46 (60.53) | 63 (57.80) | |
| Squamous cell carcinoma | 30 (39.47) | 46 (42.20) | |
| Staging of disease | 0.152 | ||
| IIIA | 8 (10.53) | 7 (6.42) | |
| IIIB | 23 (30.26) | 21 (21.10) | |
| IIIC | 5 (6.58) | 11 (10.09) | |
| IVA | 24 (31.58) | 51 (46.79) | |
| IVB | 16 (21.05) | 19 (17.43) | |
| PD-L1 expression | 0.969 | ||
| Negative | 10 (13.16) | 14 (12.84) | |
| Positive | 9 (11.84) | 15 (13.76) | |
| Unknown | 57 (75.00) | 80 (73.40) | |
| Driver gene expression | 0.319 | ||
| Negative | 3 (3.95) | 5 (4.59) | |
| Positive | 2 (2.63) | 9 (8.25) | |
| Unknown | 71 (93.42) | 95 (87.16) | |
| Antiangiogenic drugs | 0.128 | ||
| None | 58 (76.32) | 82 (75.23) | |
| Endostar | 6 (7.89) | 17 (15.60) | |
| Bevacuzumab | 10 (13.16) | 10 (9.17) | |
| Anlotinib | 2 (2.63) | 0 (0) | |
| ICIs | 0.359 | ||
| Sintilimab | 23 (30.26) | 26 (23.85) | |
| Camrelizumab | 32 (42.11) | 59 (54.13) | |
| Pembrolizumab | 8 (10.53) | 13 (11.93) | |
| Tislelizumab | 11 (14.47) | 8 (7.34) | |
| Toripalimab | 2 (2.63) | 3 (2.75) |
Abbreviations: ICIs: Immune checkpoint inhibitors; HSOS: Huisheng oral solution; PD-L1: Programmed death-ligand 1
PFS and OS
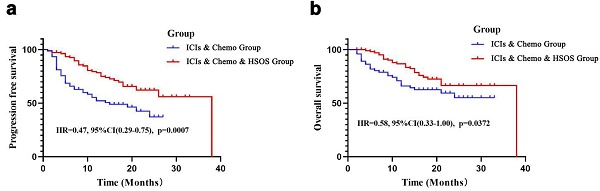
The median follow-up time was 14 months (range: 2-33 months) for the ICIs & Chemo Group and 16 months (range: 2-38 months) for the ICIs & Chemo & HSOS Group. By the end of the follow-up period, disease progression (including death) had occurred in 39 patients in the ICIs & Chemo Group and 34 patients in the ICIs & Chemo & HSOS Group. The median PFS was 19 months in the ICIs & Chemo Group, while the estimated median PFS was not reached in the ICIs & Chemo & HSOS Group. Patients in the ICIs & Chemo & HSOS Group had significantly longer PFS compared to those in the ICIs & Chemo Group (HR = 0.47, 95% CI: 0.29-0.75, p < 0.001) (Figure 1a). For OS, the estimated median OS was not reached in either group by the end of the follow-up. However, the ICIs & Chemo & HSOS Group exhibited better OS than the ICIs & Chemo Group (HR = 0.58, 95% CI: 0.33-1.00, p = 0.037) (Figure 1b). Prognosis analysis based on predefined criteria showed that the ICIs & Chemo & HSOS Group had a higher proportion of patients with good prognosis compared to the ICIs & Chemo Group for both PFS (78.95% [30/38] vs. 41.86% [18/43], p < 0.001) and OS (92.11% [35/38] vs. 64.10% [25/39], p = 0.003), based on follow-up results.
Survival analysis of PFS and OS in the two groups. a) PFS in the ICIs & Chemo Group and the ICIs & Chemo & HSOS Group; b) OS in thethe ICIs & Chemo Group and the ICIs & Chemo & HSOS Group.

Prognostic factors for PFS and OS
In multivariate Cox regression analysis, the ICIs & Chemo & HSOS Group was independently associated with better PFS (HR = 0.44, 95% CI: 0.27-0.72, p = 0.001) and OS (HR = 0.48, 95% CI: 0.27-0.85, p = 0.011) (Tables 3 and 4). Other prognostic factors for PFS included lymphangitis carcinomatosa (HR = 0.40, 95% CI: 0.21-0.76, p = 0.005), tracheobronchial sign (HR = 0.59, 95% CI: 0.35-0.99, p = 0.045), and hydrothorax (HR = 0.56, 95% CI: 0.33-0.96, p = 0.036) (Figure S1 a, b, c). Similarly, lymphangitis carcinomatosa (HR = 0.27, 95% CI: 0.13-0.64, p < 0.001), tracheobronchial sign (HR = 0.54, 95% CI: 0.30-0.99, p = 0.045), and hydrothorax (HR = 0.46, 95% CI: 0.25-0.83, p = 0.009) were associated with OS (Figure S1 d, e, f).
Immune-related adverse events
There were no significant differences between the two groups in terms of hepatotoxicity (25.86% vs. 13.73%, p = 0.055) or endocrine toxicity (3.92% vs. 3.61%, p = 0.927). However, nephrotoxicity (5.77% vs. 15.25%, p = 0.044), CIP (2.75% vs. 11.84%, p = 0.014), and cardiotoxicity (TN) (0% vs. 13.04%, p = 0.026) was significantly lower in the ICIs & Chemo & HSOS Group compared to the ICIs & Chemo Group (Table 5). Further analysis indicated that lymph node metastasis, tracheobronchial signs, and chronic bronchitis were significant risk factors for the development of CIP (all p < 0.05, Table S1).
Influence of treatment and clinical features on the efficacy of solid tumors (n, %)
| CR | PR | SD | PD | ORR (%) | DCR (%) | p for ORR | p for DCR | |
|---|---|---|---|---|---|---|---|---|
| Group | 0.628 | 0.001 | ||||||
| ICIs & Chemo Group | 0 | 21 | 33 | 22 | 21 (27.63) | 54 (71.05) | ||
| ICIs & Chemo & HSOS Group | 0 | 34 | 65 | 10 | 34 (31.19) | 99 (90.83) | ||
| Gender | 0.099 | 0.329 | ||||||
| Female | 0 | 6 | 21 | 8 | 6 (17.14) | 27 (77.14) | ||
| Male | 0 | 49 | 77 | 24 | 49 (32.67) | 126 (84.00) | ||
| Age (years) | 0.261 | 0.848 | ||||||
| <60 | 0 | 32 | 46 | 17 | 32 (33.68) | 78 (82.11) | ||
| ≥60 | 0 | 23 | 52 | 15 | 23 (25.56) | 75 (83.33) | ||
| Staging of disease | 0.168 | 0.204 | ||||||
| IIIA | 0 | 5 | 6 | 4 | 5 (33.33) | 11 (73.33) | ||
| IIIB | 0 | 16 | 22 | 6 | 16 (36.36) | 38 (86.36) | ||
| IIIC | 0 | 8 | 7 | 1 | 8 (50.00) | 15 (93.75) | ||
| IVA | 0 | 19 | 45 | 11 | 19 (25.33) | 64 (85.33) | ||
| IVB | 0 | 7 | 18 | 10 | 7 (20.00) | 25 (71.43) | ||
| Pathological type | 0.003 | 0.117 | ||||||
| Squamous cell carcinoma | 0 | 32 | 35 | 9 | 32 (42.11) | 67 (88.16) | ||
| Adenocarcinoma | 0 | 23 | 63 | 23 | 23 (21.10) | 86 (78.9) | ||
| PD-L1 expression | 0.057 | 0.052 | ||||||
| Negative | 0 | 5 | 11 | 8 | 5 (20.83) | 16 (66.67) | ||
| Positive | 0 | 3 | 16 | 5 | 3 (12.50) | 19 (79.17) | ||
| Unknown | 0 | 47 | 71 | 19 | 47 (34.31) | 118 (86.13) | ||
| Driver gene expression | 0.231 | 0.004 | ||||||
| Negative | 0 | 1 | 5 | 2 | 1 (12.50) | 6 (75.00) | ||
| Positive | 0 | 1 | 4 | 6 | 1 (9.09) | 5 (45.45) | ||
| Unknown | 0 | 53 | 89 | 24 | 53 (31.93) | 142 (85.54) | ||
| ICIs | 0.924 | 0.035 | ||||||
| Sintilimab | 0 | 14 | 27 | 8 | 14 (28.57) | 41 (83.67) | ||
| Camrelizumab | 0 | 27 | 52 | 12 | 27 (29.67) | 79 (86.81) | ||
| Pembrolizumab | 0 | 5 | 8 | 8 | 5 (23.81) | 13 (61.90) | ||
| Tislelizumab | 0 | 7 | 14 | 2 | 7 (36.84) | 21 (110.53) | ||
| Toripalimab | 0 | 2 | 1 | 2 | 2 (40.00) | 3 (60.00) | ||
| Antiangiogenic drugs | 0.568 | 0.679 | ||||||
| None | 0 | 44 | 70 | 26 | 44 (31.43) | 114 (81.43) | ||
| Endostar | 0 | 6 | 15 | 2 | 6 (26.09) | 21 (91.3) | ||
| Bevacuzumab | 0 | 4 | 12 | 4 | 4 (20.00) | 16 (80.00) | ||
| Anlotinib | 0 | 1 | 1 | 0 | 1 (50.00) | 2 (100.00) | ||
| Lymph node condition | 0.153 | 0.074 | ||||||
| None | 0 | 6 | 21 | 12 | 6 (15.38) | 27 (69.23) | ||
| 1 | 0 | 10 | 14 | 4 | 10 (35.71) | 24 (85.71) | ||
| 2 | 0 | 26 | 45 | 9 | 26 (32.50) | 71 (88.75) | ||
| 3 | 0 | 7 | 10 | 3 | 7 (35.00) | 17 (85.00) | ||
| Airway spread | 0.194 | 0.096 | ||||||
| No | 0 | 46 | 90 | 22 | 46 (31.08) | 136 (91.89) | ||
| Yes | 0 | 3 | 10 | 6 | 3 (15.79) | 13 (68.42) | ||
| Pleural spread | 0.567 | 0.643 | ||||||
| No | 0 | 38 | 64 | 22 | 38 (30.65) | 102 (82.26) | ||
| Yes | 0 | 11 | 26 | 6 | 11 (25.58) | 37 (86.05) | ||
| Lobulation | 0.024 | 0.073 | ||||||
| No | 0 | 2 | 14 | 7 | 2 (8.70) | 16 (69.57) | ||
| Yes | 0 | 47 | 76 | 21 | 47 (32.64) | 123 (85.42) | ||
| Burr | 0.877 | 0.67 | ||||||
| No | 0 | 18 | 33 | 9 | 18 (30.00) | 51 (85.00) | ||
| Short burr | 0 | 24 | 43 | 15 | 24 (29.27) | 67 (81.71) | ||
| Long burr | 0 | 0 | 2 | 1 | 0 (0.00) | 2 (66.67) | ||
| Short burr+Long burr | 0 | 7 | 12 | 3 | 7 (31.82) | 19 (86.36) | ||
| Vacuoles | 0.502 | >0.999 | ||||||
| No | 0 | 48 | 89 | 28 | 48 (29.09) | 137 (83.03) | ||
| Yes | 0 | 1 | 1 | 0 | 1 (50.00) | 2 (100.00) | ||
| Cavity | 0.337 | 0.693 | ||||||
| No | 0 | 44 | 84 | 27 | 44 (28.39) | 128 (82.58) | ||
| Yes | 0 | 5 | 6 | 1 | 5 (41.67) | 11 (91.67) | ||
| Vessel convergence sign | >0.999 | 0.684 | ||||||
| No | 0 | 26 | 47 | 16 | 26 (29.21) | 73 (82.02) | ||
| Yes | 0 | 23 | 43 | 12 | 23 (29.49) | 66 (84.62) | ||
| Lymphangitis carcinomatosa | 0.157 | 0.168 | ||||||
| No | 0 | 47 | 80 | 23 | 47 (31.33) | 127 (84.67) | ||
| Yes | 0 | 2 | 10 | 5 | 2 (11.76) | 12 (70.59) | ||
| Pleural indentation | >0.999 | 0.83 | ||||||
| No | 0 | 18 | 32 | 11 | 18 (29.51) | 50 (81.97) | ||
| Yes | 0 | 31 | 58 | 17 | 31 (29.25) | 89 (83.96) | ||
| Tracheobronchial sign | 0.839 | >0.999 | ||||||
| No | 0 | 39 | 69 | 22 | 39 (30.00) | 108 (83.08) | ||
| Yes | 0 | 10 | 21 | 6 | 10 (27.03) | 31 (83.78) | ||
| Chronic bronchitis | 0.603 | >0.999 | ||||||
| No | 0 | 42 | 80 | 25 | 42 (28.57) | 122 (82.99) | ||
| Yes | 0 | 7 | 10 | 3 | 7 (35.00) | 17 (85.00) | ||
| Tuberculosis | 0.420 | >0.999 | ||||||
| No | 0 | 46 | 87 | 27 | 46 (28.75) | 133 (83.13) | ||
| Yes | 0 | 3 | 3 | 1 | 3 (42.86) | 6 (85.71) | ||
| Emphysema | 0.861 | 0.085 | ||||||
| No | 0 | 30 | 53 | 22 | 30 (28.57) | 83 (79.05) | ||
| Yes | 0 | 19 | 37 | 6 | 19 (30.65) | 56 (90.32) | ||
| Lung bullae | 0.796 | >0.999 | ||||||
| No | 0 | 44 | 78 | 25 | 44 (29.93) | 122 (82.99) | ||
| Yes | 0 | 5 | 12 | 3 | 5 (25.00) | 17 (85.00) | ||
| Exudation | 0.059 | 0.647 | ||||||
| No | 0 | 30 | 71 | 19 | 30 (25.00) | 101 (84.17) | ||
| Yes | 0 | 19 | 19 | 9 | 19 (40.43) | 38 (80.85) | ||
| Hydrothorax | >0.999 | >0.999 | ||||||
| No | 0 | 39 | 71 | 22 | 39 (29.55) | 110 (83.33) | ||
| Yes | 0 | 10 | 19 | 6 | 10 (28.57) | 29 (82.86) |
Abbreviations: CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease; ORR: Objective response rate; DCR: Disease control rate
Discussion
The results of this study suggest that the addition of HSOS to ICIs and chemotherapy may improve DCR, PFS, and OS, while reducing irAEs in patients with stage III-IV NSCLC. These findings underscore the clinical value of integrating HSOS into standard treatment regimens for advanced NSCLC, providing a promising strategy to enhance therapeutic efficacy and reduce irAEs.
This study demonstrated that the addition of HSOS, a Chinese patent medicine, to ICIs and chemotherapy might significantly improve clinical outcomes in patients with stage III-IV NSCLC. These findings are consistent with prior studies highlighting the advantages of combining HSOS for the treatment of advanced NSCLC [16, 17]. The significant improvements in DCR, PFS, and OS observed in the ICIs & Chemo & HSOS Group compared to the ICIs & Chemo Group suggest that HSOS may act as a valuable adjunct by modulating the tumor microenvironment, reducing systemic inflammation, and enhancing immune function, as supported by preclinical studies [20, 21].
Univariate and multivariable Cox regression analysis of PFS
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Group (ICIs & Chemo & HSOS Group vs. ICIs & Chemo Group) | 0.47 (0.29-0.75) | 0.0007 | 0.44 (0.27-0.72) | 0.001 |
| Gender (Female vs Male) | 0.92 (0.51-1.68) | 0.784 | ||
| Age (<60 vs ≥60) | 0.72 (0.45-1.14) | 0.158 | ||
| Staging of disease (Ⅲ vs Ⅳ) | 0.78 (0.48-1.25) | 0.298 | ||
| Pathological type (Squamous cell carcinoma vs Adenocarcinoma) | 0.74 (0.45-1.20) | 0.218 | ||
| Lymph node condition (No vs Yes) | 1.44 (0.84-2.45) | 0.184 | ||
| Airway spread (No vs Yes) | 0.66 (0.33-1.34) | 0.25 | ||
| Pleural spread (No vs Yes) | 1.58 (0.86-2.91) | 0.14 | ||
| Lobulation (No vs Yes) | 1.27 (0.65-2.50) | 0.486 | ||
| Burr (No vs Yes) | 1.40 (0.86-2.28) | 0.177 | ||
| Vacuoles (No vs Yes) | 0.90 (0.12-6.46) | 0.913 | ||
| Cavity (No vs Yes) | 1.60 (0.50-5.11) | 0.426 | ||
| Vessel convergence sign (No vs Yes) | 1.50 (0.92-2.46) | 0.108 | ||
| Lymphangitis carcinomatosa (No vs Yes) | 0.40 (0.21-0.76) | 0.005 | 0.50 (0.25-0.97) | 0.04 |
| Pleural indentation (No vs Yes) | 1.18 (0.72-1.93) | 0.513 | ||
| Tracheobronchial sign (No vs Yes) | 0.59 (0.35-0.99) | 0.045 | 0.69 (0.40-1.20) | 0.189 |
| Chronic bronchitis (No vs Yes) | 0.70 (0.36-1.38) | 0.301 | ||
| Tuberculosis (No vs Yes) | 1.32 (0.32-5.39) | 0.702 | ||
| Emphysema (No vs Yes) | 0.91 (0.56-1.49) | 0.707 | ||
| Lung bullae (No vs Yes) | 1.86 (0.75-4.64) | 0.181 | ||
| Exudation (No vs Yes) | 0.89 (0.52-1.50) | 0.654 | ||
| Hydrothorax (No vs Yes) | 0.56 (0.33-0.96) | 0.036 | 0.57 (0.34-0.98) | 0.018 |
Abbreviations: PFS: Progression-free survival; OS: Overall survival; HR: Hazard ratio
Univariate and multivariable Cox regression analysis of OS
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Group (ICIs & Chemo & HSOS Group vs. ICIs & Chemo Group) | 0.58 (0.33-1.00) | 0.0372 | 0.48 (0.27-0.85) | 0.011 |
| Gender (Female vs Male) | 0.84 (0.41-1.72) | 0.636 | ||
| Age (<60 vs ≥60) | 0.53 (0.30-0.92) | 0.025 | 0.58 (0.32-1.06) | 0.076 |
| Staging of disease (III vs IV) | 0.85 (0.49-1.47) | 0.558 | ||
| Pathological type (Squamous cell carcinoma vs Adenocarcinoma) | 0.88 (0.50-1.54) | 0.657 | ||
| Lymph node condition (No vs Yes) | 1.17 (0.62-2.21) | 0.625 | ||
| Airway spread (No vs Yes) | 0.66 (0.29-1.46) | 0.301 | ||
| Pleural spread (No vs Yes) | 1.57 (0.79-3.15) | 0.209 | ||
| Lobulation (No vs Yes) | 0.89 (0.38-2.10) | 0.795 | ||
| Burr (No vs Yes) | 1.69 (0.96-2.96) | 0.067 | ||
| Vacuoles (No vs Yes) | 0.59 (0.08-4.30) | 0.605 | ||
| Cavity (No vs Yes) | 1.09 (0.34-3.52) | 0.88 | ||
| Vessel convergence sign (No vs Yes) | 1.44 (0.81-2.57) | 0.211 | ||
| Lymphangitis carcinomatosa (No vs Yes) | 0.27 (0.13-0.64) | <0.001 | 0.29 (0.13-0.61) | 0.001 |
| Pleural indentation (No vs Yes) | 1.12 (0.63-1.99) | 0.695 | ||
| Tracheobronchial sign (No vs Yes) | 0.54 (0.30-0.99) | 0.045 | 0.82 (0.43-1.57) | 0.544 |
| Chronic bronchitis (No vs Yes) | 0.62 (0.29-1.33) | 0.219 | ||
| Tuberculosis (No vs Yes) | 0.88 (0.21-3.63) | 0.859 | ||
| Emphysema (No vs Yes) | 0.74 (0.42-1.30) | 0.289 | ||
| Lung bullae (No vs Yes) | 3.84 (0.93-15.80) | 0.063 | ||
| Exudation (No vs Yes) | 0.90 (0.49-1.65) | 0.736 | ||
| Hydrothorax (No vs Yes) | 0.46 (0.25-0.83) | 0.009 | 0.42 (0.24-0.80) | 0.007 |
Incidence of irAEs (n, %)
| Indexes | ICIs & Chemo Group | ICIs & Chemo & HSOS Group | p |
|---|---|---|---|
| Hepatotoxicity (AST and ALT) | 15/58 (25.86) | 14/102 (13.73) | 0.055 |
| Nephrotoxicity (BUN and CRE) | 9/59 (15.25) | 6 /104 (5.77) | 0.044 |
| Pulmonary toxicity (CIP) | 9/76 (11.84) | 3/109 (2.75) | 0.014 |
| Endocrine toxicity (TSH) | 2/51 (3.92) | 3/83 (3.61) | 0.927 |
| Cardiotoxicity (TN) | 3 /23 (13.04) | 0/52 (0) | 0.026 |
Abbreviations: AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; BUN: Blood urea nitrogen; CRE: Creatinine; TSH: Thyroid-stimulating hormone; TN: Troponin; CIP: Checkpoint inhibitor pneumonitis; irAEs: Immune-related adverse event
The reduction in irAEs observed in this study, including pulmonary toxicity, nephrotoxicity, and cardiotoxicity, underscores the potential role of HSOS in mitigating ICI-related toxicities. These findings align with the established role of Traditional Chinese Medicine (TCM) in reducing the adverse effects of cancer therapies [16, 18]. Mechanistically, HSOS has been shown to regulate inflammatory and coagulation pathways, reduce fibrosis, and promote immune homeostasis [27]. These effects make HSOS a promising candidate for addressing the challenges associated with ICI-induced toxicities, which are often managed with glucocorticoids and immunosuppressive agents that carry their own risks.
Compared to previous studies, the survival outcomes in our cohort are notably more favorable. For example, Wang et al. [28] reported a median PFS of 12.8 months for patients receiving ICIs and chemotherapy, while L. Paz-Ares et al. [29] and Martin Reck et al. [30] observed median PFS durations of 8.7 months and 6.7 months, respectively, with the same treatment approach. In contrast, our study demonstrated a median PFS of 19 months in the ICIs & Chemo Group, with an even longer PFS observed in the ICIs & Chemo & HSOS Group. The improved outcomes in our study may be attributed to the unique composition of HSOS, which includes immune-enhancing and anti-inflammatory components that complement the effects of ICIs. Additionally, differences in patient populations, and treatment durations may account for the observed discrepancies.
Our analysis also identified specific imaging and clinical features, such as malignant lymphangitis, tracheobronchial signs, and hydrothorax, as prognostic factors for worse outcomes. These findings are consistent with prior studies linking these features to advanced disease burden, distant metastasis, or impaired respiratory function [31]. However, the role of these factors in predicting treatment response and toxicity in the context of HSOS requires further investigation.
While our results align broadly with the current literature, some differences should be noted. Studies evaluating ICIs alone or in combination with chemotherapy frequently report higher rates of severe irAEs, including CIP and myocarditis [12]. The significantly lower rates of these toxicities in our study may reflect the protective effects of HSOS, which has been shown to inhibit PD-1/PD-L1 signaling pathways and modulate immune-related cytokines [27]. These findings suggest that HSOS may not only enhance therapeutic efficacy but also improve the tolerability of ICIs, making it a promising addition to combination regimens. The therapeutic potential of HSOS is grounded in its complex composition, which includes 34 herbal and invertebrate-based ingredients. These components work synergistically to address the complex pathogenesis of lung cancer, which involves phlegm, blood stasis, blood toxicity, and deficiency syndromes. For instance, Trionycis Carapax and Leonuri Herba dissipate pathological masses, while aromatic herbs such as Chuanxiong Rhizoma, Angelica sinensis, and Cortex Cinnamomi Cassiae reduce turbidity and activate Qi to relieve pain. Blood-activating herbs, including Semen Persicae and Carthami Flos, promote circulation and resolve stasis, while immune-enhancing ingredients such as Ginseng and Rehmanniae Radix Praeparata improve systemic resistance and immunity.
Mechanistically, HSOS has been shown to regulate key pathways involved in tumor progression and immune modulation. It inhibits the Wnt/β-catenin pathway, reducing β-catenin and CyclinD1 expression to suppress tumor proliferation. It also modulates the miR-200b/ZEB-1 and TGF-β1-Smad3 pathways, alleviating EMT, a process closely associated with fibrosis and tumor metastasis [32]. Additionally, HSOS reduces hypercoagulability and inflammation in tumor blood by lowering levels of tissue factor (TF), fibrinogen (FIB), and interleukin-6 (IL-6), while inhibiting angiogenesis-related factors such as CD44, metalloproteinase-2 (MMP-2), and vascular endothelial growth factor (VEGF) [33]. These effects contribute to its anti-tumor, anti-inflammatory, and anti-metastatic properties, which likely underlie the observed improvements in DCR, PFS, and OS in this study.
Furthermore, HSOS enhances immune response by inhibiting PD-1/PD-L1 pathways, promoting macrophage function, and increasing the secretion of interleukin-12 (IL-12) and interleukin-18 (IL-18) [29]. These effects lead to thymus and spleen enlargement and improved systemic immunity, which may explain the significant reduction in irAEs observed in our cohort. Clinical evidence also supports its ability to regulate serum levels of VEGF, IL-6, MMP-9, TNF-α, and TGF-β, further contributing to its efficacy in combination with ICIs [27].
Despite its promising findings, this study has several limitations. First, as a retrospective, single-center study, it is subject to inherent biases, including selection bias and unmeasured confounders. Additionally, the retrospective design of the study led to incomplete documentation of imaging characteristics for some patients. Consequently, the number of evaluable cases varied across different clinical parameters. Second, the follow-up period was relatively short, and many patients had not yet experienced disease progression or death by the end of the study, limiting the ability to estimate median PFS and OS. Third, certain key clinical characteristics, such as PD-L1 expression rates, tumor mutation burden, and driver gene mutations, were not comprehensively analyzed, which may have influenced treatment responses and survival outcomes. Additionally, the small sample size in certain subgroups, such as those receiving antiangiogenic drugs, limits the generalizability of the findings. Future research should include larger, multicenter, prospective studies with longer follow-up periods to validate these results and provide more robust conclusions.
Conclusion
This study demonstrates that the combination of HSOS with ICIs and chemotherapy significantly improves clinical outcomes in patients with stage III-IV NSCLC. The addition of HSOS may be associated with higher DCR, prolonged PFS and OS, and a reduced incidence of irAEs, including nephrotoxicity, pulmonary toxicity and cardiotoxicity. These findings suggest that HSOS may serve as a promising adjunct to standard treatment regimens for advanced NSCLC. Further prospective studies are needed to confirm these results and to explore the underlying mechanisms by which HSOS modulates immune responses and reduces irAEs.
Supplementary Material
Supplementary figure and table.
Acknowledgements
We are grateful for the help from Bo Jia who is the head of the teaching and research section of Formulaology in Chengdu University of Traditional Chinese Medicine, Chengdu, China, provided theoretical guidance for prescription about Huisheng oral solution.
Funding
This work was supported by Key research and development project of science and technology department of Sichuan province (Number 2022YFS0156) to Yifeng Bai, Key research and development project of science and technology department of Sichuan province (Number 2022YFS0074) and Sichuan Provincial People's hospital youth talent fund (Number 2021QN08) to Shengkun Peng. The funder had no role in study design, data collection, analysis, interpretation, writing of the manuscript, or the decision to submit the manuscript for publication.
Author contributions
YB and SP designed the study; JW, HH and LY, collected the clinical data; LS and SP evaluated the imaging features; YB, JW and HH evaluated the outcome indicators; JC and YB, analysed the data, and wrote the original manuscript; YB and SP, reviewed and edited the manuscript; GF and HP supervised the study, and finalized the manuscript.
Data availability statement
All the data generated or analyzed during this study are included in this article.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Li C, Lei S, Ding L, Xu Y, Wu X, Wang H. et al. Global burden and trends of lung cancer incidence and mortality. Chin Med J (Engl). 2023;136:1583-90
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021;71:209-49
3. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-91
4. Tong Y, Wen J, Yang T, Li H, Wei S, Jing M. et al. Clinical efficacy and safety of Tanreqing injection combined with antibiotics versus antibiotics alone in the treatment of pulmonary infection patients after chemotherapy with lung cancer: A systematic review and meta-analysis. Phytother Res. 2021;35:122-37
5. Griesinger F, Korol EE, Kayaniyil S, Varol N, Ebner T, Goring SM. Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: A meta-analysis. Lung Cancer. 2019;135:196-204
6. Wu L, Zhang Z, Bai M, Yan Y, Yu J, Xu Y. Radiation combined with immune checkpoint inhibitors for unresectable locally advanced non-small cell lung cancer: synergistic mechanisms, current state, challenges, and orientations. Cell communication and signaling: CCS. 2023;21:119
7. Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer. 2020;20:1185
8. Qu J, Jiang M, Wang L, Zhao D, Qin K, Wang Y. et al. Mechanism and potential predictive biomarkers of immune checkpoint inhibitors in NSCLC. Biomed Pharmacother. 2020;127:109996
9. Memmott RM, Wolfe AR, Carbone DP, Williams TM. Predictors of Response, Progression-Free Survival, and Overall Survival in Patients With Lung Cancer Treated With Immune Checkpoint Inhibitors. J Thorac Oncol. 2021;16:1086-98
10. Ren S, Xiong X, You H, Shen J, Zhou P. The Combination of Immune Checkpoint Blockade and Angiogenesis Inhibitors in the Treatment of Advanced Non-Small Cell Lung Cancer. Front Immunol. 2021;12:689132
11. Zhang Q, Tang L, Zhou Y, He W, Li W. Immune Checkpoint Inhibitor-Associated Pneumonitis in Non-Small Cell Lung Cancer: Current Understanding in Characteristics, Diagnosis, and Management. Front Immunol. 2021;12:663986
12. Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M, Reynolds KL. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr Oncol Rep. 2020;22:39
13. Bai X, Hu J, Betof Warner A, Quach HT, Cann CG, Zhang MZ. et al. Early Use of High-Dose Glucocorticoid for the Management of irAE Is Associated with Poorer Survival in Patients with Advanced Melanoma Treated with Anti-PD-1 Monotherapy. Clin Cancer Res. 2021;27:5993-6000
14. Yu X, Zhang X, Yao T, Zhang Y, Zhang Y. Fatal Adverse Events Associated With Immune Checkpoint Inhibitors in Non-small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2021;8:627089
15. Geraud A, Gougis P, Vozy A, Anquetil C, Allenbach Y, Romano E. et al. Clinical Pharmacology and Interplay of Immune Checkpoint Agents: A Yin-Yang Balance. Annu Rev Pharmacol Toxicol. 2021;61:85-112
16. Huang J, Wang Z, Xue H, Cao A, Turner C, Wang J. et al. Huisheng Oral Solution Adjunct With Platinum-Based Chemotherapy for the Treatment of Advanced Non-Small Cell Lung Cancer: A Meta-Analysis and Systematic Review. Frontiers in pharmacology. 2020;11:476165
17. Jia K, Zhang C, Li F, He B, Xie S, Du J. et al. A retrospective study on the effect of Chinese patent medicine combined with conventional treatment on the survival outcomes of 313 patients with stage II-III NSCLC. Aging. 2024;16:6212-28
18. Wang L, Jia K, Li F, Zhang C, Feng G, Du J. Comparison of Improvement in 2-Year Survival Rate of Patients with Stage II-III Non-Small Cell Lung Cancer Treated with Different Durations of Chinese Patent Medicine: A Retrospective Cohort Study. Frontiers in pharmacology. 2021;12:719802
19. Yang X, Zhang H, Kong F, Wang G, Gu Q, Zhao Z. et al. Effect of Huisheng oral solution on coagulation function in perioperative period in patients with primary lung cancer. J Thorac Dis. 2017;9:1891-902
20. Chen Z, Liu M, Xie K, Chen H, Wang J, Liu X. Huisheng Oral Solution exerts anti-tumor effects by downregulating tissue factor and inhibiting the expression of metastasis-related factors, CD44, MMP2, and VEGF. Transl Cancer Res. 2019;8:2602-12
21. Chen L, Zhang L, Zhao R, Shen J, Wang Y, Zhu J. et al. Oral delivery of anti-PD-L1 antibody for cancer immunotherapy against orthotopic colorectal tumors. Nano Today. 2023;50:101834
22. Li HY, Wang YY, Liu H, Liu HX, Jiang LY, Han YC. et al. [The ninth edition of TNM staging for lung cancer: precise staging for precise diagnosis and treatment]. Zhonghua Wai Ke Za Zhi. 2024;62:537-42
23. Fournier L, de Geus-Oei LF, Regge D, Oprea-Lager DE, D'Anastasi M, Bidaut L. et al. Twenty Years On: RECIST as a Biomarker of Response in Solid Tumours an EORTC Imaging Group - ESOI Joint Paper. Front Oncol. 2021;11:800547
24. Nishino M, Hatabu H, Hodi FS, Ramaiya NH. Drug-Related Pneumonitis in the Era of Precision Cancer Therapy. JCO Precis Oncol. 2017 1
25. Yang G, Sun H, Sun N, Huang W, Wang Z, Zhang H. et al. Efficacy and safety comparison of PD-1 inhibitors vs. PD-L1 inhibitors in extensive-stage small-cell lung cancer: a retrospective comparative cohort study. J Thorac Dis. 2022;14:4925-37
26. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S. et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J Natl Compr Canc Netw. 2020;18:230-41
27. Wang W, Wang H, Wang CM, Gou S, Chen ZH, Guo J. Treatment with Huisheng oral solution inhibits the development of pulmonary thromboembolism and metastasis in mice with Lewis lung carcinoma. Oncol Lett. 2014;7:87-94
28. Wang Y, Zhang Q, Chen Y, Liang CL, Liu H, Qiu F. et al. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother. 2020;121:109570
29. Paz-Ares L, Ciuleanu TE, Yu X, Salman P, Pluzanski A, Nagrial A. et al. LBA3 Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as first-line (1L) treatment (tx) for advanced non-small cell lung cancer (aNSCLC): CheckMate 227 - part 2 final analysis. Annals of Oncology. 2019;30:xi67-xi8
30. Reck M, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J. et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open. 2021;6:100273
31. Curtis L. Understanding the Prognosis of Non-Small Cell Lung Cancer. 2024.
32. Li W, Wu Y, Zhu C, Wang Z, Gao R, Wu Q. Anti-fibrosis effects of Huisheng oral solution in CCl4-induced hepatic fibrosis in rat. Indian J Pharmacol. 2014;46:216-21
33. Liu SQ, Guo JY, Du J, Deng Q, He ZJ, Lin HY. et al. Anticoagulant effect of Huisheng Oral Solution in a rat model of thrombosis. Indian J Pharmacol. 2013;45:359-64
Author contact
![]() Corresponding author: Shengkun Peng, E-mail: pengshengkunuestc.edu.cn; Tel.: 0086-28-87393999.
Corresponding author: Shengkun Peng, E-mail: pengshengkunuestc.edu.cn; Tel.: 0086-28-87393999.

 Global reach, higher impact
Global reach, higher impact