Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(11):3497-3512. doi:10.7150/jca.118209 This issue Cite
Review
Latest Advancements of Natural Products in Combating Ovarian Cancer
1. The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, China.
2. School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China.
#These authors contributed equally to this work and should be considered as co-first authors.
Received 2025-5-25; Accepted 2025-7-16; Published 2025-7-28
Abstract
Ovarian cancer (OC), the seventh most common cancer in women globally, is one of the most prevalent and lethal gynecological cancers. With a high degree of malignancy and a dismal prognosis, late-stage patients frequently experience relapses and metastases. Associated with this is the high drug resistance of OC, which is a common challenge in cancer treatment. Natural products have garnered increasing attention in tumor treatment due to their relatively high safety and stable sources. Previous studies have demonstrated that various active ingredients in natural products can impact the progression of OC through diverse molecular mechanisms. These mechanisms include inducing cell cycle arrest, regulating reactive oxygen species (ROS), modulating autophagy, promoting cell apoptosis, inhibiting proliferation, invasion, and migration of OC cells, and regulating the tumor immune microenvironment (TIME). This article comprehensively elucidates the mechanisms by which natural products combat ovarian cancer and highlights both the emerging opportunities and existing challenges in their development as effective therapeutic agents.
Keywords: ovarian cancer, natural products, therapeutic strategy, molecular mechanisms, traditional Chinese medicine.
The Genetic and Epigenetic Characteristics of OC
Ovarian cancer (OC), recognized as one of the most aggressive gynecological malignancies, ranks seventh globally in incidence among female cancers [1]. While its incidence remains relatively stable, persistently high mortality rates pose significant public health challenges [2]. Updated 2024 data from the American Cancer Society reveals that the 5-year relative survival rate for OC patients remains below 50%, underscoring the disease's grave clinical prognosis [3]. According to the National Comprehensive Cancer Network (NCCN) clinical practice guidelines, surgery combined with platinum-based chemotherapy continues to serve as the first-line standard treatment regimen [4]. In recent years, immune checkpoint inhibitors (ICIs) have emerged as the most promising therapeutic innovation, demonstrating substantial clinical benefits both as monotherapy and in combination with conventional modalities including radiotherapy and chemotherapy [5]. However, treatment for OC still faces certain limitations. Advanced-stage disease frequently manifests as extra-ovarian disseminated metastases characterized by extensive peritoneal carcinomatosis and high recurrence rates [2]. Furthermore, the development of multidrug resistance in tumor cells constitutes a critical pathological mechanism underlying therapeutic failure and unfavorable prognoses [6].
OC exhibits intricate molecular pathogenesis characterized by heterogeneous genetic and epigenetic alterations, involving dysregulated signaling pathways, somatic gene mutations, and aberrant epigenetic modifications. Current studies have been identified recurrent genetic abnormalities, notably including functional inactivation of tumor suppressor genes (e.g., BRCA1/2 and TP53 mutations) [7, 8], and oncogenic amplification events (e.g., RAD21 gene amplification) [9], which collectively drive tumor progression. Epigenetic dysregulation encompasses DNA methylation abnormalities, histone post-translational modifications, and non-coding RNA (ncRNA) disturbances. These epigenetic mechanisms orchestrate the transcriptional activity of oncogenesis-related genes, thereby modulating critical carcinogenic processes including neoplastic initiation, metastatic dissemination, and therapeutic resistance [10]. Particularly noteworthy is the overexpression of miR-205 in OC tissues, which demonstrates significant associations with enhanced metastatic potential and serves as an independent prognostic biomarker for adverse outcomes [11].
Source and Phytochemical Inventory of Anti-ovarian Cancer Herbs
In recent years, the application value of traditional Chinese medicine (TCM) formulations based on holistic principles has become increasingly prominent in the comprehensive treatment system for OC. Representative classical prescriptions, such as Guizhi Fuling Formula, Lichong Shengsui Decoction, and Fufang Banmao Capsule, demonstrate synergistic antitumor effects through the scientific combination of medicinal components like Ginseng, Astragalus, and Cinnamon. Modern pharmacological research has revealed that these formulations exhibit significant advantages in inhibiting tumor proliferation, inducing apoptosis, and reversing drug resistance through multi-target regulatory mechanisms [12].
Taking Astragalus as an example, its core active component, quercetin, has been demonstrated in studies on anti-ovarian cancer mechanisms to exert its effects by inducing endoplasmic reticulum (ER) stress, apoptosis, and autophagy through the p- signal transducer and activator of transcription 3 (STAT3)/Bcl-2 axis [13]. Notably, when quercetin is combined with the PARP inhibitor (PARPi) olaparib, a synergistic enhancement effect is observed, significantly improving the anti-proliferative efficacy against BRCA wild-type OC cells. This provides a crucial theoretical foundation for integrated traditional Chinese and Western medicine treatment strategies [14].
In addressing chemotherapy resistance, the active component of ginger, 6-Shogaol, demonstrates unique advantages. It overcomes drug resistance in OC cells by inducing ER stress and cell death [15]. Ginsenosides exert their effects through multimodal mechanisms: Rg3 inhibits the migration of SKOV3 cells [16], while 20(S)-Rg3 further suppresses SKOV3 tumor metastasis by inducing autophagy [17]. Additionally, ginsenoside 20(S)-Rg3 blocks hypoxia-induced epithelial-mesenchymal transition (EMT) in OC cells by downregulating hypoxia-inducible factor-1α (HIF-1α) expression [18].
The aforementioned research findings have established a solid theoretical foundation for the clinical application of TCM formulations in the treatment of OC. The structurally diverse family of natural compounds holds significant promise as a source of novel candidate drugs for OC therapy. Among these, plant-derived natural products, owing to their unique structural complexity and chemical diversity, demonstrate irreplaceable core value in the development of innovative therapeutic agents [19].
Bioactive Components in Anti-OVC Herbs
A comprehensive overview of the chemical structures and biological actions of the natural products discussed in this study is presented in Table 1. Analysis of these natural products reveals that the majority possess polyphenolic structures, including compounds such as tanshinone, quercetin, and ellagic acid. These compounds have demonstrated efficacy in improving outcomes in OC through various mechanisms, including the modulation of cell proliferation, autophagy, apoptosis, and several other signaling pathways. These findings are consistent with structure-activity relationship analyses, which highlight the positive correlation between the number of phenolic hydroxyl groups and antioxidant activity, as well as the enhanced transmembrane permeability conferred by lactone ring structures. Furthermore, the chemical classification of compounds such as terpenoids, flavonoids, and alkaloids further supports their potential anticancer properties. Additionally, these results underscore the impact of variations in extraction processes on the proportional composition of active components [20].
This article explores the potential role of natural products in the treatment of OC. Based on their mechanisms of action, the natural products with reported anti-ovarian cancer activity are systematically classified, summarized, and discussed. These natural products primarily function in cytotoxicity, inducing cell cycle arrest, regulating reactive oxygen species (ROS), modulating autophagy, promoting cell apoptosis, inhibiting the proliferation, invasion, and migration of OC cells, inhibiting angiogenesis, and regulating the tumor immune microenvironment (TIME).
Natural plant components for anti-ovarian cancer effects.
| Compound | Formula | Structure | Experiment | Cell types | IC50 | Effect | Reference |
|---|---|---|---|---|---|---|---|
| Kadsuphilactone B | C30H42O5 |  | In vitro | A2780 | Below 25 μM | Cytotoxic action | [25] |
| Tanshinone IIA | C19H18O3 |  | In vitro | TOV-21G, SKOV3, OVCAR3 | N/A | Cytotoxic action | [26] |
| Curcumin | C21H20O6 |  | In vitro | SKOV3 | 24.8μM | Trigger cell cycle arrest, modulating the TIME, DNA damage | [34-37, 96-98] |
| Celastrol | C29H38O4 | 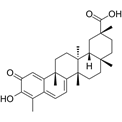 | In vitro and In vivo | A2780 | 2.11μM | Trigger cell cycle arrest, modulating the TIME, inhibit proliferation, promote apoptosis and suppress cell migration and invasion | [38] |
| SKOV3 | 2.29μM | ||||||
| Berberine | C20H18NO4+ | 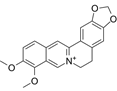 | In vitro | SKOV3 | N/A | ROS damage, promote DNA damage response | [45] |
| Glaucocalyxin B | C22H30O5 | 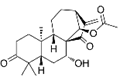 | In vitro | A2780 | N/A | ROS damage | [46] |
| Βeta-sitosterol | C29H50O | 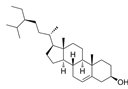 | In vitro | ES2, OV90 | N/A | ROS damage and promote apoptosis | [47] |
| Artemether | C16H26O5 | 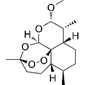 | In vitro and In vivo | SKOV3 | 23.55±3.86μM | ROS damage, regulating apoptosis and ferroptosis | [48-51] |
| HEY1 | 5.80±1.62μM | ||||||
| HEY2 | 7.34±0.56μM | ||||||
| OVCAR8 | 5.51±1.06μM | ||||||
| IGROV-1 | 8.82±1.18μM | ||||||
| OVCAR3 | 14.95±6.38μM | ||||||
| OV-90 | 31.89±4.15μM | ||||||
| TOV-21G | 6.11±0.64μM | ||||||
| Paeonol | C9H10O3 | 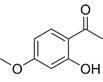 | In vitro and In vivo | A2780, SKOV3 | 1.2mM | Autophagy | [54] |
| Ellagic acid | C14H6O8 | 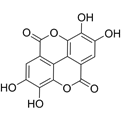 | In vitro | SKOV3 | 36.6μM | Induce autophagy, inhibit proliferation and promote apoptosis, suppress cell migration and invasion | [55] |
| Puerarin | C21H20O9 | 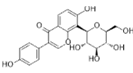 | In vitro and In vivo | SKOV3 | 157.0μg/mL | Inhibit proliferation and promote apoptosis | [59] |
| Caov-4 | 119.3μg/mL | ||||||
| Quercetin | C15H10O7 | 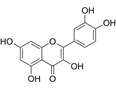 | In vitro | PA-1 | N/A | Suppress cell migration and invasion | [64] |
| Fraxetin | C10H8O5 | 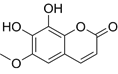 | In vitro | SKOV3, SW626 | N/A | Suppress cell migration and invasion | [65] |
| Dihydroartemisinin | C15H24O5 | 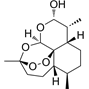 | In vitro | OVCAR3, SKOV3, A2780, OV-56 | 80μmol/L | Suppress cell migration and invasion, inhibit proliferation | [66] |
| Proanthocyanidins | Compound |  | In vitro | A2780/CP70 | N/A | Inhibit angiogenesis | [71] |
| Baicalein | C15H10O5 | 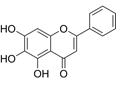 | In vitro and In vivo | SKOV3 | N/A | Inhibit angiogenesis and proliferation, autophagy | [72, 73] |
| Theasaponin E1 | C59H90O27 | 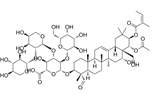 | In vitro | OVCAR-3 | 3.5μM | Inhibit angiogenesis and proliferation | [70] |
| A2780/CP70 | 2.8μM | ||||||
| Triptolide | C20H24O6 | 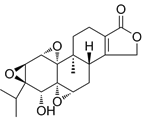 | In vitro and In vivo | SKOV3/DDP | N/A | Suppress cell migration and invasion | [80] |
In the future, natural drugs are expected to become important tools in the treatment of OC, providing more effective and safer treatment options. The molecular mechanisms are shown in Figure 1.
Search Strategy
The literature search was conducted in PubMed using a combination of Medical Subject Headings (MeSH) terms and free-text keywords related to OC, natural product interventions, and mechanistic pathways (e.g., autophagy, apoptosis, PI3K/Akt/mTOR signaling). Inclusion criteria encompassed in vitro, in vivo, and clinical trial studies published within the last decade that explicitly investigated the mechanisms of natural products in OC regulation. Exclusion criteria removed incomplete data, irrelevant studies, and non-peer-reviewed publications. To ensure comprehensive coverage, citation tracking of highly cited references and backward/forward citation searches of high-impact publications were performed. After deduplication, titles and abstracts were screened to exclude non-relevant studies, followed by full-text assessment of remaining articles against predefined criteria. A standardized data extraction form was designed to systematically capture key information, including experimental models, compound concentrations, and molecular targets. Two independent researchers executed the search and screening processes to ensure rigor, with discrepancies resolved through discussion or third-party arbitration.
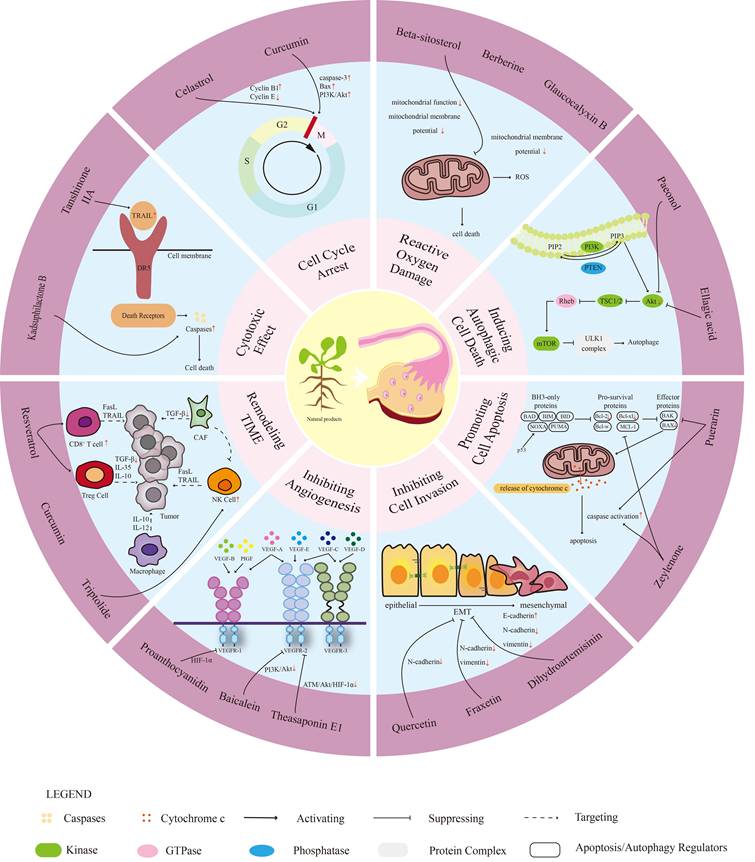
The mechanism of natural products in ovarian cancer (OC). This diagram illustrates the multifaceted mechanisms through which natural products may influence OC biology. The concentric hierarchical progression highlights the key roles of natural products: (1) exhibiting cytotoxicity, (2) inducing cell cycle arrest, (3) promoting reactive oxygen species (ROS)-mediated damage, (4) triggering autophagy, (5) activating apoptotic pathways, (6) reducing proliferation and migration, (7) inhibiting angiogenesis, and (8) modulating the tumor immune microenvironment. These mechanisms collectively act to slow tumor progression and may improve patient outcomes while preventing recurrence. Through these pathways, natural products emerge as a promising therapeutic approach for managing OC.
Biological Effects of Natural Products
Cytotoxic Effects
Cytotoxic Drug-Based Therapeutic Strategies for OC
The cytotoxic suppression of cancer cells primarily achieves synergistic antitumor effects by targeting critical biological processes such as DNA replication, cell division, metabolism, or signaling. DNA double-strand breaks (DSBs), the most lethal form of DNA damage [21], can be induced by chemotherapy or radiotherapy, triggering poly (ADP-ribose) polymerase (PARP)-dependent base excision repair (BER). When PARP inhibitors (PARPi) block BER and form "PARP-DNA trapping complexes," unrepaired single-strand breaks (SSBs) are converted into DSBs during replication, leading to selective elimination of homologous recombination repair (HRR)-deficient tumor cells via synthetic lethality [22]. Notably, PARPi not only directly kill cancer cells through DNA damage but also activate the natural immune response, driving a Th1-polarized immune response (e.g., IFN-γ secretion), promoting dendritic cell maturation and T-cell infiltration, and synergizing with immune checkpoint inhibitors [23].
Additionally, TNF-related apoptosis-inducing ligand (TRAIL) activates caspase cascades by binding death receptors (DR4/DR5), with selective induction of apoptosis [24]. Based on these mechanisms, dual-targeting strategies for OC can be designed as follows: 1) Combined Induction of DNA Damage and Repair Inhibition—using natural products to induce DSBs while inhibiting DNA damage response (DDR) key proteins (e.g., PARP), triggering synthetic lethality through irreversible damage accumulation; 2) Death Receptor Signaling Remodeling—epigenetically upregulating DR4/DR5 expression while suppressing anti-apoptotic factors (e.g., c-FLIP/XIAP) to enhance TRAIL-mediated apoptotic sensitivity, thereby overcoming therapeutic resistance in OC.
Cytotoxic Natural Products with anti-Ovarian Cancer Activity
Recent pharmacological studies delineate both direct and indirect mechanisms underlying natural compounds' anti-ovarian cancer effects. A representative example is kadsuphilactone B, a Schisandra chinensis-derived nortriterpenoid exhibiting moderate cytotoxicity against A2780 cells (IC50 <25 μM). Mechanistic investigations revealed concentration-dependent activation of both intrinsic and extrinsic apoptotic pathways, evidenced by sequential cleavage of caspase-9/-8/-3 cascade effectors and PARP. Pharmacological inhibition of caspases significantly attenuated the pro-apoptotic effects, confirming caspase-dependent apoptosis. Western blot analysis further identified concomitant modulation of B-cell lymphoma 2 (Bcl-2) family proteins and phosphorylation alterations in mitogen-activated protein kinase (MAPK) signaling pathways, suggesting multimodal regulation of programmed cell death [25].
Synergistic cytotoxic effects were observed in TOV-21G endometrial cancer models through combined therapy. While monotherapy with 100 ng/mL TRAIL induced 21.1% ± 3.6% cell death, coadministration with 2 μM Tanshinone IIA (TIIA) potentiated cytotoxicity to 88.5% (Δ +5.6% vs theoretical additive effect). This chemosensitization correlated with enhanced proteolytic processing of apoptotic mediators: immunoblotting demonstrated marked elevation of cleaved caspase-9/-7/-3 and PARP fragments in combination groups compared to single-agent treatments. These data establish that TIIA directly targets the TRAIL-induced apoptosis pathway by caspase cascade amplification, rather than indirect metabolic or immune modulation, providing rationale for combination regimens in apoptosis-resistant malignancies [26].
Cell Cycle Arrest
Inducing Cell Cycle Arrest as a Therapeutic Strategy for OC
The progression of OC is closely linked to dysregulation of cell cycle control, which is tightly governed by three critical checkpoints: G1/S phase, intra-S phase, and G2/M phase checkpoints [27]. Upon DNA damage, checkpoint kinase systems—including the Rad3-related serine/threonine kinase (ATR)-checkpoint kinase 1 (CHK1) signaling axis and WEE1 G2 checkpoint kinase (WEE1)—are activated to induce cell cycle arrest, providing a time window for DNA repair and maintaining genomic stability [28]. In OC pathogenesis, despite its multifactorial complexity, disruption of the cell cycle regulatory network has been identified as a core pathogenic mechanism. This dysregulation disrupts the dynamic equilibrium between aberrant proliferation and programmed cell death, ultimately driving malignant proliferation and metastatic progression in ovarian tumors [29].
Targeted therapies centered on cell cycle regulation have become pivotal in OC treatment. CDK4/6 inhibitors, suppress tumor proliferation by blocking the G1/S phase transition through competitive binding to the ATP domain of CDK4/6, thereby inhibiting phosphorylation of the retinoblastoma protein (Rb) and inducing irreversible G1 arrest. This arrest not only halts cell cycle progression but also amplifies antitumor effects by driving metabolic reprogramming and transcriptomic remodeling [30]. In parallel, WEE1 inhibitors release the G2/M phase arrest by preventing Tyr15 phosphorylation of CDK1/2, forcing replication-stressed tumor cells into abnormal division [31]. Clinically, combining CDK4/6 inhibitors with PARP inhibitors has shown synergistic efficacy, as this dual strategy simultaneously disrupts cell cycle checkpoints and DNA repair pathways—particularly effective in tumors with HRR deficiencies [32]. By leveraging synthetic lethality and precision biomarker-guided approaches, these therapeutic advancements offer new avenues to improve clinical outcomes for OC patients [33].
Anti-Ovarian Cancer Natural Products Inducing Cell Cycle Arrest
Emerging evidence highlights the cell cycle-modulating potential of phytochemicals in OC therapeutics. Curcumin, a bioactive polyphenol isolated from Curcuma longa rhizomes (Zingiberaceae family), demonstrates multimodal cell cycle disruption in SKOV-3 ovarian adenocarcinoma. Pharmacological studies revealed that 30 μM curcumin triggered a time-dependent accumulation of apoptotic sub-G1-phase cells (no significant change at 6 hours, but marked increases at 24 and 48 hours). At 6 hours, S- and G2/M-phase populations increased, followed by reductions at 24 and 48 hours. Notably, curcumin treatment significantly decreased the percentage of G1/G0-phase cells across all timepoints. However, prolonged exposure (24-48 hours) to 30 μM curcumin resulted in a global reduction of cells in G1/G0, S, and G2/M phases, suggesting progressive cell cycle arrest and apoptotic commitment [34]. Mechanistically, this biphasic cell cycle arrest coordinates with pro-apoptotic signaling amplification through PI3K/Akt axis modulation, evidenced by upregulation of caspase-3 activity and elevation of Bcl-2-associated X protein (Bax), effectively impairing cancer cell self-renewal capacity [35].
Furthermore, curcumin directly induces cancer cell apoptosis by triggering mitochondrial accumulation, oxidative stress, and elevated DNA damage [36]. Additionally, it selectively suppresses the hyperactivated Wnt/β-catenin signaling pathway in OC, synergistically enhancing chemotherapeutic drug sensitivity [37].
The triterpenoid celastrol, derived from Tripterygium wilfordii radix, emerges as another potent cell cycle modulator. The compound demonstrates multifaceted antiproliferative effects, inducing cell cycle arrest through distinct molecular targets while simultaneously provoking direct cell cycle perturbation via complementary pathways. Flow cytometric analysis of A2780/SKOV-3 co-cultures exposed to 0.3, 1, and 3 μM celastrol revealed an increase in the sub-G1 population accompanied by proportional reduction of G0/G1 phase cells, demonstrating significant cell cycle progression disruption. Molecular profiling revealed cyclin network reprogramming upregulation of p27 and Cyclin B1 contrasted with reduction in Cyclin E expression. This confirms celastrol's ability to induce dual-phase cell cycle arrest through cyclin-dependent kinase (CDK) inhibitor (CDKi) activation and G2/M checkpoint potentiation [38].
Inducing Reactive Oxygen Damage
Inducing Reactive Oxygen Damage as a Therapeutic Strategy for OC
In cells, oxidative stress arises from an imbalance between ROS generation and antioxidant defenses. ROS—including superoxide radicals, hydrogen peroxide, and hydroxyl radicals—induce genomic instability through DNA alkylation, phosphodiester bond cleavage, and strand breaks, leading to mutations, chromosomal translocations, and impaired repair mechanisms. These lesions drive carcinogenesis by disrupting cell cycle checkpoints, suppressing apoptosis, and promoting oncogenic activation (e.g., KRAS, PI3K) while silencing tumor suppressors (e.g., TP53, BRCA1), collectively fostering a pro-tumorigenic microenvironment [39-42].
Ferroptosis is a form of regulated cell death characterized by iron-dependent lipid peroxidation, with its molecular basis involving dysregulation of glutathione (GSH) metabolism and collapse of the membrane phospholipid repair system. Mechanistic studies have shown that GSH peroxidase 4 (GPX4) maintains membrane stability by reducing phospholipid hydroperoxides (PLOOH), while GPX4 inhibitors can induce PLOOH accumulation and trigger oxidative disintegration of the plasma membrane [43]. Research has found that combined treatment with GPX4 inhibitors and PARPi exhibits synergistic antitumor efficacy in BRCA1-deficient OC cells, patient-derived organoids (PDOs), and xenografts [44], providing an important direction for drug development and clinical treatment of OC.
Anti-Ovarian Cancer Natural Products Inducing Reactive Oxygen Damage
Berberine, a quaternary protoberberine alkaloid isolated from Berberis species, exhibits potent cytotoxic effects in OC cells. At a concentration of 30 μM, berberine induces cell death in nearly 90% of SKOV-3 cells. Furthermore, when combined with gamma-ray irradiation, berberine enhances oxidative stress, as evidenced by elevated ROS levels and reduced GSH content. This dual targeting of cancer cell viability (direct cytotoxicity) and metabolic adaptation (indirect microenvironment modulation) establishes berberine as a promising radiosensitizer for OC therapy [45].
Glaucocalyxin B, a bioactive diterpenoid isolated from the aerial parts of Rabdosia japonica, demonstrates direct anticancer properties mediated through ROS-dependent pathways in OC cells. Studies demonstrate that glaucocalyxin B significantly elevates intracellular ROS levels in both cisplatin-sensitive (A2780) and cisplatin-resistant (A2780/DDP) cell lines. This ROS accumulation induces DNA damage and suppresses cancer cell proliferation via activation of the c-Jun N-terminal kinase (JNK) signaling pathway, which triggers mitochondrial apoptosis. These findings highlight glaucocalyxin B as a promising therapeutic candidate, particularly for overcoming cisplatin resistance in OC [46].
Beta-sitosterol exerts anti-ovarian cancer effects through both direct mitochondrial targeting and indirect stress-inducing mechanisms. This phytosterol not only triggers loss of mitochondrial membrane potential (MMP) but also activates key pro-apoptotic factors including Bax, Bcl-2 homologous antagonist/killer (BAK), caspase-3, caspase-9, and cytochrome C release. These coordinated events directly execute mitochondrial-mediated apoptosis in OC cells. Furthermore, Notably, beta-sitosterol also initiates indirect tumor-suppressive effects by disturbing cellular homeostasis. It significantly elevates intracellular ROS levels while disrupting calcium homeostasis, collectively generating oxidative stress that exacerbates mitochondrial damage and ultimately leads to programmed cell death in OC cells [47].
Artemisinin and its derivatives, natural compounds derived from Artemisia annua with established antimalarial and anticancer properties [48], demonstrate significant potential as ferroptosis inducers in OC therapy. These compounds exhibit concentration-dependent dual mechanisms: at lower concentrations, artemether induces ROS-independent G1 phase cell cycle arrest through mTOR signaling inhibition, while higher concentrations trigger ROS-mediated apoptosis and ferroptosis [49]. The ferroptotic effects are primarily mediated through iron metabolism dysregulation, characterized by increased ferrous ion accumulation, GSH depletion, and elevated oxidative stress. As clinically viable ferroptosis-targeting agents, artemisinin compounds exert potent anti-tumor effects by promoting iron-dependent lipid peroxidation and subsequent cancer cell death [50]. As a representative derivative of artemisinin compounds, preclinical studies have demonstrated that artesunate not only effectively inhibits the in vitro proliferation of OC cell lines and primary patient-derived cells but also significantly suppresses tumor growth in murine xenograft models by modulating the tumor microenvironment (TME)[51]. These findings systematically elucidate the molecular mechanisms through which artemisinin and its derivatives exert anti-ovarian cancer effects via spatiotemporal dynamic regulation of cell cycle progression, oxidative stress homeostasis, and ferroptosis signaling networks. This comprehensive evidence offers promising therapeutic potential for OC treatment either as monotherapy or in combination regimens.
Inducing Autophagic Cell Death
Inducing Autophagic Cell Death as a Therapeutic Strategy for OC
In the pathogenesis and progression of OC, aberrant activation of the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway is closely associated with genetic alterations. Gain-of-function mutations in the PIK3CA gene enhance PI3K lipid kinase activity, significantly elevating phosphorylation levels of Akt and Mek1/2, thereby promoting tumor cell survival and malignant transformation. Concurrently, copy number alterations resulting from PIK3CA gene amplification are strongly linked to hyperactivation of the PI3K pathway in tumor tissues. Oncogenic mutations in downstream effectors of the AKT family (AKT1/2/3) disrupt interactions between the pleckstrin homology domain (PH) and kinase domain (KD), leading to constitutive activation and driving mTOR complex 1 (mTORC1)-mediated anabolic reprogramming. As a critical negative regulator, inactivating mutations or epigenetic silencing of phosphatase and tensin homolog (PTEN) impair its phosphatase function, causing abnormal accumulation of phosphatidylinositol-3,4,5-trisphosphate (PIP3) and sustained activation of the Akt/mTOR signaling cascade, ultimately fostering tumor proliferation and metabolic adaptation. Notably, PTEN also modulates homologous recombination repair efficiency by regulating RAD51 expression. The mTORC1 complex suppresses autophagosome formation by phosphorylating Unc-51-like kinase 1 (ULK1) and the master lysosomal gene regulator transcription factor EB (TFEB), while simultaneously inhibiting lysosomal biogenesis to impair cellular clearance capacity. Under stress conditions, the p53 protein dynamically balances these processes through subcellular localization-dependent mechanisms, with nuclear translocation activating a pro-autophagy transcriptional network [52, 53].
Natural products hold promising potential to modulate tumor cell autophagy by targeting the PI3K/Akt/mTOR signaling pathway. Specifically, these compounds are expected to inhibit mTORC1 activity, thereby relieving its phosphorylation-mediated suppression of key autophagy factors. This dynamic regulation of lysosome-dependent degradation mechanisms helps maintain intracellular homeostasis. Notably, in malignant OC cells, such compounds may selectively inhibit hyperactivated autophagic pathways, impairing the clearance efficiency of damaged organelles and abnormal protein aggregates. Ultimately, this process activates apoptotic signaling to delay the progression of malignant phenotypes in OC.
Anti-Ovarian Cancer Natural Products Inducing Autophagic Cell Death
Paeonol, a natural compound extracted from Moutan Cortex, was found to significantly upregulate the expression of the autophagy marker Bax protein in treated A2780 and SKOV-3 OC cells, accompanied by the accumulation of autophagosomes and lysosomes, confirming the activation of intact autophagic flux. Paeonol relieves negative regulation on the autophagy initiation complex initiates by inhibiting the Akt/mTOR signaling pathway. Notably, moderate activation of paeonol-induced autophagy promotes stress adaptation in tumor cells, whereas prolonged intervention leads to autophagy-associated programmed cell death. Further validation demonstrated that combined application of autophagy inhibitors blocks the Akt/mTOR-dependent compensatory autophagy mechanism, significantly enhancing the anti-ovarian cancer efficacy of paeonol through synergistic amplification of apoptotic signaling [54].
Ellagic acid inhibited cell proliferation (IC50 = 36.6 μmol/L), migration, and invasion in a concentration-dependent manner, and significantly induced caspase-dependent apoptosis. It upregulated the expression of Beclin-1, ATG-5, and LC3-I/II while reducing p62 protein levels, indicating the complete activation of autophagic flux. Additionally, ellagic acid markedly increased the expression of the pro-apoptotic protein Bax and suppressed Bcl-2 levels, synergistically activating caspase-3/8 cleavage and modulating the balance of Bcl-2 family proteins. Further mechanistic analysis revealed that ellagic acid inhibited the PI3K/Akt/mTORC1 signaling axis, as evidenced by significant reductions in phosphorylated Akt and downstream targets of mTORC1, while activating the energy-sensing adenosine monophosphate-activated protein kinase (AMPK) pathway. Importantly, co-treatment with the autophagy inhibitor chloroquine partially abrogated the biological effects of ellagic acid, suggesting autophagy-dependence of its mechanism [55].
Inhibiting Cell Proliferation and Promoting Cell Apoptosis
Targeting Cell Growth and Apoptosis in OC Therapy
The Bcl-2 protein family plays a central role in tumorigenesis by regulating the apoptosis pathway. Pro-apoptotic Bcl-2 homology 3 (BH3)-only proteins antagonize anti-apoptotic members such as Bcl-2, B-cell lymphoma-extra large (Bcl-xL), myeloid cell leukemia 1 (MCL-1), and Bcl-2-like protein 2 (Bcl-w) through high-affinity binding. This interaction activates Bax/BAK oligomerization, alters MMP, triggers cytochrome c release, and activates the caspase cascade, ultimately inducing apoptosis [56]. This process is regulated through multiple layers by transcription factors such as E2F transcription factor 1 (E2F-1) and nuclear factor-kappa B (NF-κB). The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway enhances tumor cell survival signaling by promoting pro-survival members of the Bcl-2 protein family transcription. Additionally, post-translational modifications dynamically regulate protein stability [57].
In OC, natural products targeting apoptosis regulators, activating BAX/BAK to induce oligomerization, MMP, and cytochrome c release, thereby reactivating the caspase cascade and significantly increasing apoptosis rates in OC SKOV-3 cells. These natural products concurrently suppress the JAK/STAT3 pathway, offering synergistic intervention strategies to overcome chemotherapy resistance. Furthermore, combining such natural products with platinum-based chemotherapy agents enhances therapeutic potential through apoptosis signal reprogramming in OC treatment.
Anti-Ovarian Cancer Natural Products Inhibiting cell proliferation and promoting cell apoptosis
The polyketide compound Zeylenone, derived from Uvaria grandiflora, significantly activated the mitochondrial apoptosis pathway in OC cells after 24-hour treatment at concentrations of 2.5, 5, and 10 μg/mL. This effect was characterized by increased levels of the apoptosis-inducing factor (AIF) and suppression of Bcl-2 expression. Additionally, it initiates the extrinsic death receptor pathway via upregulating Fas/FasL expression, thereby triggering caspase cascade activation. Mechanistically, this dual pro-apoptotic effect is achieved through indirect regulation of the tumor microenvironment. Zeylenone significantly inhibits JAK/STAT survival signaling, and this upstream blockade subsequently disrupts downstream survival pathways [58].
In vitro experimental results demonstrated that puerarin exhibits growth inhibitory effects on OC cell lines SKOV-3 and Caov-4, with IC50 values of 157.0 μg/mL and 119.3 μg/mL, respectively. Notably, at a subtoxic concentration of 50 μg/mL, puerarin significantly induced apoptosis in SKOV-3 OC cells. Further analysis revealed that puerarin treatment markedly increased early and late apoptosis rates in both SKOV-3 cells and their cisplatin-resistant counterpart SKOV-3/DDP. Its direct mechanism of action is manifested as follows: puerarin reshaped the regulatory balance of the mitochondrial apoptosis pathway by downregulating anti-apoptotic proteins Bcl-2 and Bcl-xL, while reducing the expression of pro-apoptotic protein Bax. Morphological observations confirmed characteristic apoptotic changes, accompanied by cleavage of PARP protein, indicating activation of the caspase-dependent apoptosis pathway. Importantly, the mechanism of drug resistance reversal is related to the indirect regulation of the TME. Puerarin reversed cisplatin resistance in OC cells, a mechanism closely linked to inhibition of aberrant Wnt/β-catenin signaling pathway activation [59].
Inhibiting Cell Migration and Invasion
Limiting Cell Migration and Invasion in OC Therapy
EMT, a core process driving tumor invasion and metastasis, induces transcriptional reprogramming via TGF-β/Smad and Wnt/β-catenin signaling pathways [60]. This leads to reduced E-cadherin expression, elevated vimentin levels, and ultimately loss of cell polarity and enhanced stromal invasiveness [61, 62]. Studies have demonstrated that using the TGF-β receptor kinase inhibitor Galunisertib[63] or Wnt pathway inhibitors [60] can suppress EMT progression and reduce metastatic lesions. Clinical research aims to leverage natural products to restore epithelial phenotypes in OC cells by targeting mesenchymal markers and inhibiting EMT, thereby significantly lowering the risk of OC metastasis.
Anti-Ovarian Cancer Natural Products Inhibiting cell migration and invasion
Quercetin, a natural flavonoid compound, exerts direct anti-metastatic effects by targeting EMT core mechanisms in OC PA-1 cells. Specifically, it reverses the EMT phenotype through significant downregulation of the mesenchymal marker N-cadherin, a key driver of cellular motility and invasiveness [64].
The natural coumpound fraxetin, a coumarin derivative from Fraxinus rhynchophylla Hance, exerts direct anti-EMT effects in OC through primary targeting of the Toll-like receptor 4/STAT3 (TLR4/STAT3) signaling axis. This direct inhibition leads to significant downregulation of mesenchymal markers (N-cadherin, vimentin, c-Myc) and cell cycle regulators (Cyclin D1) in SKOV-3 and SW626 cells treated with 80 μM fraxetin for 12 hours. Notably, the anti-EMT effects of fraxetin could be partially reversed by the STAT3 activator Colivelin, confirming the critical role of STAT3 signaling in mediating its activity [65].
Dihydroartemisinin demonstrates various anti-metastatic mechanisms in OC through both direct molecular targeting and indirect microenvironmental modulation. Mechanistically, dihydroartemisinin directly suppresses EMT in A2780 and SKOV-3 cells by regulation of key EMT markers: upregulating epithelial signature protein E-cadherin while downregulating mesenchymal markers N-cadherin and vimentin. This EMT inhibition is synergistically reinforced through dihydroartemisinin's direct modulation of apoptotic machinery, specifically by reducing anti-apoptotic Bcl-2 expression, elevating pro-apoptotic Bax/Bcl-2 ratio, and activating executioner caspase-3 - constituting a cancer cell apoptosis pathway. Notably, dihydroartemisinin extends its therapeutic effects through indirect regulation of TME components. It significantly enhances expression of reversion-inducing cysteine-rich protein with Kazal motifs (RECK), a tumor suppressor that indirectly inhibits metastasis. These coordinated actions on both cancer cell-intrinsic pathways (direct EMT/apoptosis regulation) and extrinsic microenvironmental factors establish dihydroartemisinin as a multi-targeted therapeutic agent against OC progression [66].
Inhibiting Angiogenesis
Inhibiting Angiogenesis as a Therapeutic Strategy for OC
Based on Folkman's tumor angiogenesis theory, the progression of OC relies on vascular endothelial growth factor (VEGF)-mediated pathological angiogenesis [67], with serum VEGF levels significantly elevated in OC patients compared to healthy controls [68]. Current anti-angiogenic therapies inhibit endothelial cell proliferation and vascular permeability by targeting VEGF/VEGF receptor (VEGFR) binding [69]. Emerging strategies are exploring the application of natural products to modulate the VEGF/VEGFR pathway, aiming to enhance the vascular normalization index and thereby improve therapeutic efficacy against OC.
Anti-Ovarian Cancer Natural Products Inhibiting angiogenesis
Theasaponin E1, a camellane-type saponin derived from Camellia seeds, exerts anti-angiogenic effects through modulation of the ATM/Akt/HIF-1α signaling axis. At the direct-action level, this compound specifically induces phosphorylation of ataxia-telangiectasia mutated (ATM) kinase (a key DNA damage response kinase) while upregulating PTEN expression. These dual mechanisms collectively suppress the Akt/mTOR signaling cascade, leading to reduced nuclear translocation of HIF-1α and inhibition of VEGF transcriptional activity. In vivo chorioallantoic membrane (CAM) assays demonstrated reduced neovascular density, with synergistic effects observed when combined with the Notch1 inhibitor DAPT or Akt inhibitors [70].
As a polyphenolic natural product, Myrica rubra leaf-derived proanthocyanidins demonstrate direct therapeutic mechanisms in OC intervention. The primary action involves targeting the AKT/mTOR/p70S6K/4E-BP1 signaling axis, which specifically downregulates hypoxia-inducible factor HIF-1α, thereby blocking VEGF transcriptional activation. Experimental data revealed that 10 μg/mL proanthocyanidins treatment significantly inhibited VEGF secretion in A2780/CP70 cells by 80.3%, accompanied by reduced in vitro angiogenesis capacity [71].
Baicalein, a natural flavonoid derived from Scutellaria baicalensis Georgi roots, exerts direct anti-tumor effects on OC by targeting the PI3K/Akt signaling axis. This primary mechanism leads to the direct suppression of angiogenesis through VEGF downregulation and inhibits metastatic progression by reducing Cyclin D1 and matrix metalloproteinase-2 (MMP2) expression. Furthermore, baicalein induces protective autophagy in OC cells by upregulating Beclin 1 and activating extracellular signal-regulated kinase (ERK)-mediated pathways, a dual-edged mechanism that initially promotes cancer cell survival but ultimately sensitizes cells to apoptosis under prolonged treatment [72]. Notably, the compound may also indirectly modulate tumor progression, as evidenced by its significant tumor volume reduction following oral administration [73].
Notably, the anti-angiogenic mechanisms described above—particularly HIF-1α/VEGF suppression via PI3K/Akt/mTOR inhibition—have been substantiated by in vivo and early-phase clinical studies. For example, proanthocyanidins and baicalein, through these pathways, have demonstrated tumor growth inhibition in xenograft models. Such results underscore the feasibility of leveraging mechanistic insights to guide preclinical candidate selection and rational design of future clinical trials targeting angiogenesis in OC.
Tumor Immune Microenvironment
The TIME in OC is characterized by a dynamic interplay of tumor cells, immune infiltrates, stromal components, and soluble mediators. Immune evasion within this ecosystem is a major barrier to durable therapeutic responses [74]. Natural products offer promising immunomodulatory effects that can reprogram the TIME from an immunosuppressive to an immune-active state.
Modulation of adaptive immune cells
Several natural products have been shown to enhance cytotoxic T lymphocyte (CTL) activity while simultaneously suppressing regulatory T cell (Treg) function. For example, resveratrol promotes dendritic cell (DC) maturation (upregulating CD80/CD86) and CD8⁺ T cell activation by reducing lactate accumulation in the tumor milieu, which otherwise dampens T cell function. This dual metabolic and immunological remodeling leads to decreased TGF-β levels and increased IFN-γ expression. Such compounds restore antigen presentation and reinvigorate CTL-mediated anti-tumor immunity [75, 76].
Reprogramming tumor-associated macrophages (TAMs)
In OC, TAMs are often skewed toward the M2 phenotype, supporting tumor progression via immunosuppressive cytokine production (e.g., IL-10, TGF-β) and suppression of T cell activity [77]. Natural compounds such as curcumin have demonstrated the ability to reprogram TAMs from the M2 to M1 phenotype, enhancing pro-inflammatory cytokine release (TNF-α, IL-12) and antigen presentation [78]. This polarization shift is frequently mediated via suppression of STAT3 and activation of NF-κB pathways, ultimately supporting a more robust immune response [79].
Enhancement of natural killer (NK) cell cytotoxicity
Natural products may also reverse NK cell dysfunction induced by factors such as TGF-β within the TME. Triptolide has been shown to elevate systemic IL-2 and TNF-α levels, thereby restoring NK cell cytotoxic function. This boost innate immune surveillance and supports early tumor elimination [80].
Interference with immune checkpoint pathways
Some phytochemicals may indirectly inhibit immune checkpoint molecules such as PD-L1 via modulation of oncogenic pathways like PI3K/Akt or STAT3. Although direct evidence in OC is still emerging, these interactions present an opportunity for combining natural products with immune checkpoint inhibitors to synergistically remodel the immune landscape.
Together, these findings suggest that natural compounds exert multi-dimensional control over the TIME by restoring CTL activity, inhibiting Treg infiltration, reprogramming TAM polarization, and reviving NK cell function. This immunological rebalancing offers a promising avenue to overcome immune escape and enhance the efficacy of conventional and immunotherapeutic strategies in OC.
The Molecular Mechanism of Natural Products
Emerging preclinical evidence highlights the multifaceted pharmacological mechanisms of natural products in OC therapeutics, particularly through their dual capacity as pathway modulators and chemo-sensitizing agents. Mechanistically, these bioactive compounds demonstrate oncogenic pathway activation potential via selective potentiation of STAT3 signaling and PI3K/Akt/mTOR axis regulation [81-83], while concurrently enhancing platinum-based chemotherapy through coordinated p53 modifying, thereby synergistically overcoming chemoresistance and amplifying cytotoxic efficacy [84]. Through multi-target synergistic mechanisms, natural products exert therapeutic effects in suppressing tumor proliferation and inducing apoptosis by regulating pathway interactions, while concurrently remodeling the chemoresistant TME. This integrated pharmacological action ultimately enhances therapeutic response rates and optimizes clinical outcomes for OC patients.
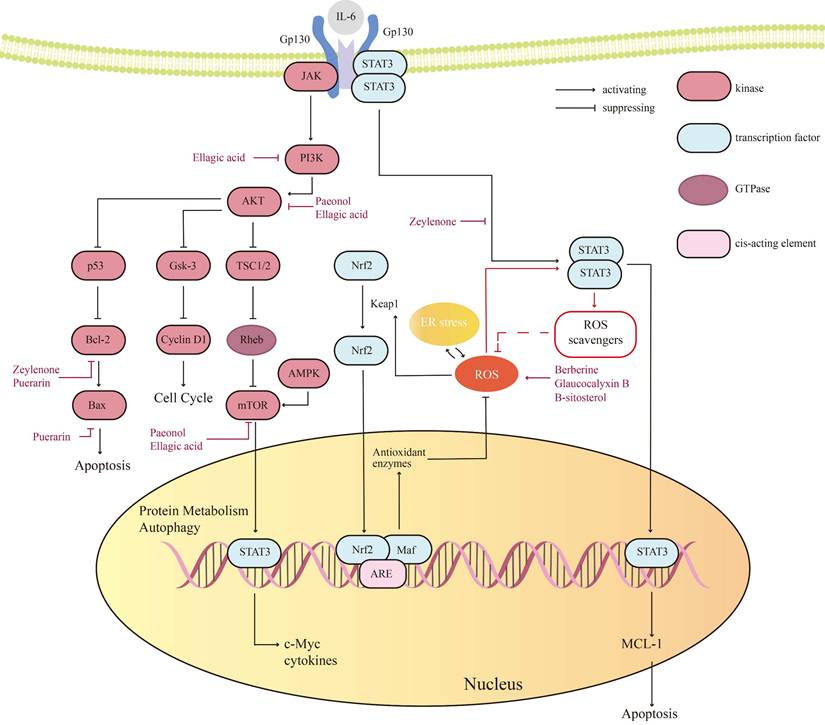
The sophisticated regulation of cellular homeostasis relies on the Kelch-like ECH-associated protein 1-nuclear factor erythroid 2-related factor 2-Antioxidant Response Elements (Keap1-Nrf2-ARE) pathway governing redox equilibrium, mTOR-driven anabolic metabolism coupled with autophagy suppression, the Bcl-2 family-modulated apoptosis switch, and the JAK/STAT-mediated transcriptional regulatory network. Specifically, Keap1 dynamically regulates Nrf2 protein stability through ROS-sensing mechanisms to activate the antioxidant defense system [85, 86], while the mTOR pathway integrates growth factor and nutrient signals to sustain proliferative dominance via metabolic reprogramming. Concurrently, the Bcl-2 family coordinates mitochondrial membrane permeability (MOMP) regulation with JAK/STAT-mediated transcriptional activation of pro-survival factors to establish apoptotic resistance. These interconnected pathways collectively dictate tumor cell proliferation, stress adaptation capacity, and therapeutic resistance phenotypes, thereby delineating critical molecular interfaces for developing targeted combination therapies against OC (Figure 2).
Bridging Mechanisms and Clinical Applications
To enhance translational relevance, we highlight several natural compounds with dual evidence from both mechanistic and clinical/preclinical studies. These compounds represent promising candidates for integration into evidence-based, multi-targeted OC treatment strategies.
Natural Products in Clinical Applications of OC
Natural products with well-defined mechanisms are increasingly transitioning from preclinical research to clinical applications in ovarian cancer treatment. Notably, compounds such as epigallocatechin gallate (EGCG), resveratrol—previously shown to modulate apoptosis, and immune remodeling—have demonstrated promising outcomes in early clinical trials. Below, we summarize representative studies that bridge mechanistic understanding with translational and clinical validation in OC patients. While conventional chemotherapy regimens like paclitaxel-based combinations continue to demonstrate efficacy in trials such as ICON4/AGO-OVAR-2.2[87] and GCGS-01[88], showing improved progression-free survival (PFS) and overall survival (OS), emerging evidence highlights the complementary potential of natural product-based interventions. Notably, clinical studies have shown that EGCG-rich green tea consumption induced complete remission in advanced OC patients [89], while mistletoe extracts improved quality of life with minimal adverse effects [90, 91]. Agaricus blazei mushroom extracts have exhibited immunomodulatory properties by enhancing NK cell activity and mitigating chemotherapy toxicity [92]. Resveratrol has demonstrated particular promise through its multifaceted mechanisms - modulating glycolytic enzymes like PKM2 and GLUT1, suppressing TGF-β signaling, and enhancing cytotoxic T-cell responses - with preclinical studies confirming reduced tumor burden and early-phase clinical trials supporting its safety as an adjunct therapy [93]. These findings collectively underscore how natural products may offer dual benefits in ovarian cancer management: potentiating conventional treatments while reducing their adverse effects, thereby presenting new opportunities for integrative therapeutic approaches.
Conclusion and Perspectives
Natural products have demonstrated considerable anti-ovarian cancer potential through diverse mechanisms, yet face clinical translation challenges including poor bioavailability, limited tumor specificity, and potential toxicity [94]. To address these limitations, researchers are developing structurally optimized derivatives through systematic structure-activity relationship studies - for instance, artemisinin derivatives exhibit enhanced pharmacokinetics and potent ferroptosis induction in OC cells [50] - while advanced delivery systems like liposomes and polymeric nanoparticles improve solubility, stability, and targeted delivery. Particularly promising are nanocarriers co-loaded with natural products and chemotherapeutics that show synergistic effects against drug-resistant tumors [94]. However, critical challenges remain including variability in sourcing and processing methods, potential development of resistance during prolonged treatment, and most importantly the need for validation in large-scale clinical trials [95]. Future research must prioritize comprehensive structure-activity relationship (SAR) studies to guide rational compound design, continued development of targeted delivery technologies, and rigorous clinical evaluation through well-designed trials to establish safety and efficacy. By integrating these chemical, pharmaceutical and clinical approaches, natural product derivatives may emerge as valuable components of ovarian cancer therapy with improved effectiveness and reduced toxicity compared to current treatments.
Key signaling pathways and proteins of natural products in ovarian cancer (OC).
Abbreviations
AIF: apoptosis-inducing factor; Akt: protein kinase B; AMPK: adenosine monophosphate-activated protein kinase; ARE: antioxidant response elements; ATM: ataxia telangiectasia mutated; ATR: Rad3-related serine/threonine kinase; BAK: Bcl-2 homologous antagonist/killer; Bax: Bcl-2-associated X protein; Bcl-2: B-cell lymphoma 2; Bcl-w: Bcl-2-like protein 2; Bcl-xL: B-cell lymphoma extra-large; BER: base excision repair; BH3: Bcl-2 homology 3; CAM: chorioallantoic membrane; CDK: cyclin-dependent kinase; CDKi: CDK inhibitor; CHK1: checkpoint kinase 1; CTLs: cytotoxic T lymphocytes; DCs: dendritic cells; DDR: DNA damage response; DSBs: DNA double-strand breaks; E2F-1: E2F transcription factor 1; EGCG: epigallocatechin gallate; EMT: epithelial-mesenchymal transition; EOC: epithelial ovarian cancer; ER: endoplasmic reticulum; ERK: extracellular signal-regulated kinase; Fas: fatality-associated protein; FBXW7: F-box and WD-40 structural domain protein 7; GPX4: glutathione peroxidase 4; GSH: glutathione; Her2: human epidermal growth factor receptor 2; HRR: homologous recombination repair; HIF-1α: hypoxia inducible factor 1 α; IC50: the half-maximal inhibitory concentration; ICIs: immune checkpoint inhibitors; JAK: Janus kinase; JNK: c-Jun N-terminal kinase; KD: kinase domain; Keap1/Nrf2: Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2; MAPK: mitogen-activated protein kinase; MCL-1: C-Myeloid cell leukemia 1; MeSH: Medical Subject Headings; MIC: minimum inhibitory concentration; miRNAs: microRNAs; MMP: mitochondrial membrane potential; MMP2: matrix metalloproteinase-2; MOMP: mitochondrial membrane permeability; mTOR: mammalian target of rapamycin; mTORC1: mTOR complex 1; MyD88: myeloid differentiation primary response 88; NCCN: National Comprehensive Cancer Network; ncRNA: non-coding RNA; NF-κB: nuclear factor-kappa B; NK: natural killer; OC: ovarian cancer; OS: overall survival; PARP: poly(ADP-ribose) polymerase; PARPi: PARP inhibitors; PDOs: patient-derived organoids; PFS: progression-free survival; PH: pleckstrin homology domain; PI3K: phosphatidylinositol 3-kinase; PIP3: phosphatidylinositol-3,4,5-trisphosphate; PLOOH: phospholipid hydroperoxides; PTEN: phosphorylation and phosphatase and tensin homolog; RECK: reversion-inducing cysteine-rich protein with Kazal motifs; ROS: reactive oxygen species; SAR: structure-activity relationship; SSBs: single-strand breaks; STAT: signal transducer and activator of transcription; STAT3: signal transducer and activator of transcription 3; TIIA: Tanshinone IIA; TAMs: tumor-associated macrophages; TCM: traditional Chinese medicine; TFEB: transcription factor EB; TGF-β: transforming growth factor-β; TIME: tumor immune microenvironment; TLR4: Toll-like receptor 4; TME: tumor microenvironment; TRAIL: TNF-related apoptosis-inducing ligand; Tregs: regulatory T cells; ULK1: unc-51-like kinase 1; VEGF: vascular endothelial growth factor; VEGFR: VEGF receptor; WEE1: WEE1 G2 checkpoint kinase.
Acknowledgements
Funding
This study is supported by National Natural Science Foundation of China (Grant No. 82474460, 82204875).
Data availability
All data supported this study are available from the corresponding author upon request.
Author Contributions
FT and YZ: Conceptualization, Writing - review and editing. YL and HH: Writing - original draft, Investigation. YC: Writing - original draft, Methodology. MF and KH: Writing - original draft, Resources, Software. YX: Writing - original draft, Supervision. All authors viewed and accepted the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. 2019;11:287-99
2. Gaona-Luviano P, Medina-Gaona LA, Magaña-Pérez K. Epidemiology of ovarian cancer. Chin Clin Oncol. 2020;9:47
3. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49
4. Armstrong DK, Alvarez RD, Backes FJ, Bakkum-Gamez JN, Barroilhet L, Behbakht K. et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J Natl Compr Canc Netw. 2022;20:972-80
5. Yang C, Xia BR, Zhang ZC, Zhang YJ, Lou G, Jin WL. Immunotherapy for Ovarian Cancer: Adjuvant, Combination, and Neoadjuvant. Front Immunol. 2020;11:577869
6. Zhang S, Cheng J, Quan C, Wen H, Feng Z, Hu Q. et al. circCELSR1 (hsa_circ_0063809) Contributes to Paclitaxel Resistance of Ovarian Cancer Cells by Regulating FOXR2 Expression via miR-1252. Mol Ther Nucleic Acids. 2020;19:718-30
7. Launonen IM, Lyytikäinen N, Casado J, Anttila EA, Szabó A, Haltia UM. et al. Single-cell tumor-immune microenvironment of BRCA1/2 mutated high-grade serous ovarian cancer. Nat Commun. 2022;13:835
8. Chakraborty R, Dutta A, Mukhopadhyay R. TP53 mutations and MDM2 polymorphisms in breast and ovarian cancers: amelioration by drugs and natural compounds. Clin Transl Oncol. 2025;27(7):2789-2800
9. Deng P, Wang Z, Chen J, Liu S, Yao X, Liu S. et al. RAD21 amplification epigenetically suppresses interferon signaling to promote immune evasion in ovarian cancer. J Clin Invest. 2022;132:e159628
10. Hu Q, Chen J, Yang W, Xu M, Zhou J, Tan J. et al. GPX3 expression was down-regulated but positively correlated with poor outcome in human cancers. Front Oncol. 2023;13:990551
11. Monroig-Bosque Pdel C, Rivera CA, Calin GA. MicroRNAs in cancer therapeutics: "from the bench to the bedside". Expert Opin Biol Ther. 2015;15:1381-5
12. Yali Liu, Xiaoxia Wang, Li H. A Review of Studies on Effective Components of Chinese Medicine against Ovarian Cancer. Advances in Clinical Medicine. 2020;10:1457-62
13. Shafabakhsh R, Asemi Z. Quercetin: a natural compound for ovarian cancer treatment. J Ovarian Res. 2019;12:55
14. Liu Y, Guo Z, Lang F, Li J, Jiang J. Anticancer Effect of Active Component of Astragalus Membranaceus Combined with Olaparib on Ovarian Cancer Predicted by Network-Based Pharmacology. Appl Biochem Biotechnol. 2023;195:6994-7020
15. Kim TW, Lee HG. 6-Shogaol Overcomes Gefitinib Resistance via ER Stress in Ovarian Cancer Cells. Int J Mol Sci. 2023;24:2639
16. Zhao L, Sun W, Zheng A, Zhang Y, Fang C, Zhang P. Ginsenoside Rg3 suppresses ovarian cancer cell proliferation and invasion by inhibiting the expression of lncRNA H19. Acta Biochim Pol. 2021;68:575-82
17. Zheng X, Chen W, Hou H, Li J, Li H, Sun X. et al. Ginsenoside 20(S)-Rg3 induced autophagy to inhibit migration and invasion of ovarian cancer. Biomed Pharmacother. 2017;85:620-6
18. Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou H. et al. Ginsenoside 20(S)-Rg3 targets HIF-1α to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS One. 2014;9:e103887
19. Ram Sharma D, Sheikh I Sankhyan A, Diwakar A Sharma A. "Anti-cancer potential of natural products: recent trends, scope and relevance". Letters in Applied NanoBioScience. 2020;9:902-7
20. Wei Z, Chen J, Zuo F, Guo J, Sun X, Liu D. et al. Traditional Chinese Medicine has great potential as candidate drugs for lung cancer: A review. J Ethnopharmacol. 2023;300:115748
21. Tang M, Li S, Chen J. Ubiquitylation in DNA double-strand break repair. DNA Repair (Amst). 2021;103:103129
22. Lee EK, Konstantinopoulos PA. Combined PARP and Immune Checkpoint Inhibition in Ovarian Cancer. Trends Cancer. 2019;5:524-8
23. Li A, Yi M, Qin S, Chu Q, Luo S, Wu K. Prospects for combining immune checkpoint blockade with PARP inhibition. J Hematol Oncol. 2019;12:98
24. Cullen SP, Martin SJ. Fas and TRAIL 'death receptors' as initiators of inflammation: Implications for cancer. Semin Cell Dev Biol. 2015;39:26-34
25. Jeong M, Kim HM, Kim HJ, Choi JH, Jang DS. Kudsuphilactone B, a nortriterpenoid isolated from Schisandra chinensis fruit, induces caspase-dependent apoptosis in human ovarian cancer A2780 cells. Arch Pharm Res. 2017;40:500-8
26. Lin JY, Ke YM, Lai JS, Ho TF. Tanshinone IIA enhances the effects of TRAIL by downregulating survivin in human ovarian carcinoma cells. Phytomedicine. 2015;22:929-38
27. Icard P, Fournel L, Wu Z, Alifano M, Lincet H. Interconnection between Metabolism and Cell Cycle in Cancer. Trends Biochem Sci. 2019;44:490-501
28. Gralewska P, Gajek A, Marczak A, Rogalska A. Participation of the ATR/CHK1 pathway in replicative stress targeted therapy of high-grade ovarian cancer. J Hematol Oncol. 2020;13:39
29. Scavone G, Ottonello S, Blondeaux E, Arecco L, Scaruffi P, Stigliani S. et al. The Role of Cyclin-Dependent Kinases (CDK) 4/6 in the Ovarian Tissue and the Possible Effects of Their Exogenous Inhibition. Cancers (Basel). 2023;15:4923
30. Gao X, Leone GW, Wang H. Cyclin D-CDK4/6 functions in cancer. Adv Cancer Res. 2020;148:147-69
31. Li Z, Pinch BJ, Olson CM, Donovan KA, Nowak RP, Mills CE. et al. Development and Characterization of a Wee1 Kinase Degrader. Cell Chem Biol. 2020;27:57-65.e9
32. Yi J, Liu C, Tao Z, Wang M, Jia Y, Sang X. et al. MYC status as a determinant of synergistic response to Olaparib and Palbociclib in ovarian cancer. EBioMedicine. 2019;43:225-37
33. Tian C, Wei Y, Li J, Huang Z, Wang Q, Lin Y. et al. A Novel CDK4/6 and PARP Dual Inhibitor ZC-22 Effectively Suppresses Tumor Growth and Improves the Response to Cisplatin Treatment in Breast and Ovarian Cancer. Int J Mol Sci. 2022;23:2892
34. Almosa H, Alqriqri M, Denetiu I, Baghdadi MA, Alkhaled M, Alhosin M. et al. Cytotoxicity of Standardized Curcuminoids Mixture against Epithelial Ovarian Cancer Cell Line SKOV-3. Scientia Pharmaceutica. 2020;2020:1
35. Yu Z, Wan Y, Liu Y, Yang J, Li L, Zhang W. Curcumin induced apoptosis via PI3K/Akt-signalling pathways in SKOV3 cells. Pharm Biol. 2016;54:2026-32
36. Bao Q, Wang Z, Yang T, Su X, Chen Y, Liu L. et al. Curcumin induces mitochondrial dysfunction-associated oxidative DNA damage in ovarian cancer cells. PLoS One. 2025;20:e0319846
37. Yen HY, Tsao CW, Lin YW, Kuo CC, Tsao CH, Liu CY. Regulation of carcinogenesis and modulation through Wnt/β-catenin signaling by curcumin in an ovarian cancer cell line. Sci Rep. 2019;9:17267
38. Xu LN, Zhao N, Chen JY, Ye PP, Nan XW, Zhou HH. et al. Celastrol Inhibits the Growth of Ovarian Cancer Cells in vitro and in vivo. Front Oncol. 2019;9:2
39. Baird L, Yamamoto M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol Cell Biol. 2020;40:e00099-20
40. Deshmukh P, Unni S, Krishnappa G, Padmanabhan B. The Keap1-Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys Rev. 2017;9:41-56
41. Klaunig JE. Oxidative Stress and Cancer. Curr Pharm Des. 2018;24:4771-8
42. Lingappan K. NF-κB in Oxidative Stress. Curr Opin Toxicol. 2018;7:81-6
43. Liu S, Zhao X, Shui S, Wang B, Cui Y, Dong S. et al. PDTAC: Targeted Photodegradation of GPX4 Triggers Ferroptosis and Potent Antitumor Immunity. J Med Chem. 2022;65:12176-87
44. Xie X, Chen C, Wang C, Guo Y, Sun B, Tian J. et al. Targeting GPX4-mediated ferroptosis protection sensitizes BRCA1-deficient cancer cells to PARP inhibitors. Redox Biol. 2024;76:103350
45. Aleissa MS, Al-Zharani M, Alneghery LM, Aleissa AM. Berberine enhances the sensitivity of radiotherapy in ovarian cancer cell line (SKOV-3). Saudi Pharm J. 2023;31:110-8
46. Zhang T, Xu C, Zheng P, Zhang X, Qiu C, Wu F. et al. Glaucocalyxin B Attenuates Ovarian Cancer Cell Growth and Cisplatin Resistance In vitro via Activating Oxidative Stress. Oxid Med Cell Longev. 2022;2022:6324292
47. Bae H, Park S, Ham J, Song J, Hong T, Choi JH. et al. ER-Mitochondria Calcium Flux by β-Sitosterol Promotes Cell Death in Ovarian Cancer. Antioxidants (Basel). 2021;10:1583
48. Wu Z, Zhong M, Liu Y, Xiong Y, Gao Z, Ma J. et al. Application of natural products for inducing ferroptosis in tumor cells. Biotechnol Appl Biochem. 2022;69:190-7
49. Tan Z, Huang H, Sun W, Li Y, Jia Y. Current progress of ferroptosis study in ovarian cancer. Front Mol Biosci. 2022;9:966007
50. Hu Y, Guo N, Yang T, Yan J, Wang W, Li X. The Potential Mechanisms by which Artemisinin and Its Derivatives Induce Ferroptosis in the Treatment of Cancer. Oxid Med Cell Longev. 2022;2022:1458143
51. Greenshields AL, Shepherd TG, Hoskin DW. Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol Carcinog. 2017;56:75-93
52. Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol. 2022;12:819128
53. Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24:560-75
54. Gao L, Wang Z, Lu D, Huang J, Liu J, Hong L. Paeonol induces cytoprotective autophagy via blocking the Akt/mTOR pathway in ovarian cancer cells. Cell Death Dis. 2019;10:609
55. Elsaid FG, Alshehri MA, Shati AA, Al-Kahtani MA, Alsheri AS, Massoud EE. et al. The anti-tumourigenic effect of ellagic acid in SKOV-3 ovarian cancer cells entails activation of autophagy mediated by inhibiting Akt and activating AMPK. Clin Exp Pharmacol Physiol. 2020;47:1611-21
56. Radha G, Raghavan SC. BCL2: A promising cancer therapeutic target. Biochim Biophys Acta Rev Cancer. 2017;1868:309-14
57. Kaloni D, Diepstraten ST, Strasser A, Kelly GL. BCL-2 protein family: attractive targets for cancer therapy. Apoptosis. 2023;28:20-38
58. Xu X, Shi J, Gao H, Li Q. Zeylenone inhibits proliferation and promotes apoptosis in ovarian carcinoma cells via Janus kinase 2 / signal transducers and activators of transcription 3 pathways. J Obstet Gynaecol Res. 2018;44:1451-7
59. Duan J, Yin M, Shao Y, Zheng J, Nie S. Puerarin induces platinum-resistant epithelial ovarian cancer cell apoptosis by targeting SIRT1. J Int Med Res. 2021;49:3000605211040762
60. Zhang J, Tian XJ, Xing J. Signal Transduction Pathways of EMT Induced by TGF-β, SHH, and WNT and Their Crosstalks. J Clin Med. 2016;5:41
61. Kuburich NA, den Hollander P, Pietz JT, Mani SA. Vimentin and cytokeratin: Good alone, bad together. Semin Cancer Biol. 2022;86:816-26
62. Paolillo M, Schinelli S. Extracellular Matrix Alterations in Metastatic Processes. Int J Mol Sci. 2019;20:4947
63. Tschernia NP, Gulley JL. Tumor in the Crossfire: Inhibiting TGF-β to Enhance Cancer Immunotherapy. BioDrugs. 2022;36:153-80
64. Dhanaraj T, Mohan M, Arunakaran J. Quercetin attenuates metastatic ability of human metastatic ovarian cancer cells via modulating multiple signaling molecules involved in cell survival, proliferation, migration and adhesion. Arch Biochem Biophys. 2021;701:108795
65. Xu R, Ruan Y, Zhang L, Gu Y, Liu M. Fraxetin suppresses the proliferation, migration, and invasion of ovarian cancer cells by inhibiting the TLR4/STAT3 signaling pathway. Immunopharmacol Immunotoxicol. 2023;45:287-94
66. Zheng J, Li X, Yang W, Zhang F. Dihydroartemisinin regulates apoptosis, migration, and invasion of ovarian cancer cells via mediating RECK. J Pharmacol Sci. 2021;146:71-81
67. Elebiyo TC, Rotimi D, Evbuomwan IO, Maimako RF, Iyobhebhe M, Ojo OA. et al. Reassessing vascular endothelial growth factor (VEGF) in anti-angiogenic cancer therapy. Cancer Treat Res Commun. 2022;32:100620
68. Nusrat O, Belotte J, Fletcher NM, Memaj I, Saed MG, Diamond MP. et al. The Role of Angiogenesis in the Persistence of Chemoresistance in Epithelial Ovarian Cancer. Reprod Sci. 2016;23:1484-92
69. Singh N, Badrun D, Ghatage P. State of the art and up-and-coming angiogenesis inhibitors for ovarian cancer. Expert Opin Pharmacother. 2020;21:1579-90
70. Li B, Tong T, Ren N, Rankin GO, Rojanasakul Y, Tu Y. et al. Theasaponin E(1) Inhibits Platinum-Resistant Ovarian Cancer Cells through Activating Apoptosis and Suppressing Angiogenesis. Molecules. 2021;26:1681
71. Zhang Y, Chen S, Wei C, Rankin GO, Rojanasakul Y, Ren N. et al. Dietary Compound Proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc. ) leaves inhibit angiogenesis and regulate cell cycle of cisplatin-resistant ovarian cancer cells via targeting Akt pathway. J Funct Foods. 2018;40:573-81
72. Wang YF, Xu YL, Tang ZH, Li T, Zhang LL, Chen X. et al. Baicalein Induces Beclin 1- and Extracellular Signal-Regulated Kinase-Dependent Autophagy in Ovarian Cancer Cells. Am J Chin Med. 2017;45:123-36
73. Chuang TC, Fang GS, Hsu SC, Lee YJ, Shao WS, Wang V. et al. Baicalein suppresses HER2-mediated malignant transformation of HER2-overexpressing ovarian cancer cells by downregulating HER2 gene expression. Environ Toxicol. 2023;38:1609-17
74. Lv B, Wang Y, Ma D, Cheng W, Liu J, Yong T. et al. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front Immunol. 2022;13:844142
75. Zhang Y, Yang S, Yang Y, Liu T. Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect Agent Cancer. 2019;14:27
76. Chen J, Huang ST, Chen JG, He JH, Lin WM, Huang ZH. et al. Resveratrol reduces lactate production and modifies the ovarian cancer immune microenvironment. Neoplasma. 2022;69:1129-37
77. Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021;81:1201-8
78. Li X, Su L, Qian C, Qiu W, Tao L, Guo Z. et al. Curcumin suppresses malignant behaviors of ovarian cancer through regulation of tumor-associated macrophages. Med Oncol. 2025;42:151
79. Deswal B, Bagchi U, Kapoor S. Curcumin Suppresses M2 Macrophage-derived Paclitaxel Chemoresistance through Inhibition of PI3K-AKT/STAT3 Signaling. Anticancer Agents Med Chem. 2024;24:146-56
80. Hu H, Huang G, Wang H, Li X, Wang X, Feng Y. et al. Inhibition effect of triptolide on human epithelial ovarian cancer via adjusting cellular immunity and angiogenesis. Oncol Rep. 2018;39:1191-6
81. Yang J, Wang L, Guan X, Qin JJ. Inhibiting STAT3 signaling pathway by natural products for cancer prevention and therapy: In vitro and in vivo activity and mechanisms of action. Pharmacol Res. 2022;182:106357
82. Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin Cancer Biol. 2022;80:1-17
83. Park SH, Kim M, Lee S, Jung W, Kim B. Therapeutic Potential of Natural Products in Treatment of Cervical Cancer: A Review. Nutrients. 2021;13:154
84. Dasari S, Njiki S, Mbemi A, Yedjou CG, Tchounwou PB. Pharmacological Effects of Cisplatin Combination with Natural Products in Cancer Chemotherapy. Int J Mol Sci. 2022;23:1532
85. Zhou H, Wang Y, You Q, Jiang Z. Recent progress in the development of small molecule Nrf2 activators: a patent review (2017-present). Expert Opin Ther Pat. 2020;30:209-25
86. Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol Rev. 2018;98:1169-203
87. Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB. et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-106
88. Ngoi NYL, Heong V, Tang JI, Choo BA, Kumarakulasinghe NB, Lim D. et al. Phase 1 Study of Low-Dose Fractionated Whole Abdominal Radiation Therapy in Combination with Weekly Paclitaxel for Platinum-Resistant Ovarian Cancer (GCGS-01). Int J Radiat Oncol Biol Phys. 2021;109:701-11
89. Trudel D, Labbé DP, Araya-Farias M, Doyen A, Bazinet L, Duchesne T. et al. A two-stage, single-arm, phase II study of EGCG-enriched green tea drink as a maintenance therapy in women with advanced stage ovarian cancer. Gynecol Oncol. 2013;131:357-61
90. Piao BK, Wang YX, Xie GR, Mansmann U, Matthes H, Beuth J. et al. Impact of complementary mistletoe extract treatment on quality of life in breast, ovarian and non-small cell lung cancer patients. A prospective randomized controlled clinical trial. Anticancer Res. 2004;24:303-9
91. Steele ML, Axtner J, Happe A, Kröz M, Matthes H, Schad F. Adverse Drug Reactions and Expected Effects to Therapy with Subcutaneous Mistletoe Extracts (Viscum album L.) in Cancer Patients. Evid Based Complement Alternat Med. 2014;2014:724258
92. Ahn WS, Kim DJ, Chae GT, Lee JM, Bae SM, Sin JI. et al. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int J Gynecol Cancer. 2004;14:589-94
93. Mozafaryan MJ, Rezaei P, Masoudpoor F, Bahri A, Samini M, Farkhondeh T. et al. Resveratrol in the Treatment of Gynecological Cancer: Mechanisms and Therapeutic Potential. Curr Med Chem. 2024
94. Dwivedi K, Sahoo A, Almalki WH, Almujri SS, Aodah A, Alruwaili NK. et al. Innovative nanocarrier systems for enhanced delivery of phyto-active compounds in cancer therapy. Nanomedicine (Lond). 2025;20:91-116
95. Naeem A, Hu P, Yang M, Zhang J, Liu Y, Zhu W. et al. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules. 2022;27:8367
96. Shirley SA, Montpetit AJ, Lockey RF, Mohapatra SS. Curcumin prevents human dendritic cell response to immune stimulants. Biochem Biophys Res Commun. 2008;374:431-6
97. Lv J, Shao Q, Wang H, Shi H, Wang T, Gao W. et al. Effects and mechanisms of curcumin and basil polysaccharide on the invasion of SKOV3 cells and dendritic cells. Mol Med Rep. 2013;8:1580-6
98. Zhang J, Liu J, Xu X, Li L. Curcumin suppresses cisplatin resistance development partly via modulating extracellular vesicle-mediated transfer of MEG3 and miR-214 in ovarian cancer. Cancer Chemother Pharmacol. 2017;79:479-87
Author contact
![]() Corresponding authors: Yuan Zhou, Email address: zhouyedu.cn; Fangfang Tao, Email address: taoffedu.cn.
Corresponding authors: Yuan Zhou, Email address: zhouyedu.cn; Fangfang Tao, Email address: taoffedu.cn.

 Global reach, higher impact
Global reach, higher impact