Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(9):2946-2958. doi:10.7150/jca.114813 This issue Cite
Research Paper
The Predictive Value of the Cholesterol-to-Natural Killer Cell Ratio in Colorectal Cancer
1. Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology and Hepatology, Ministry of Health, State Key Laboratory for Oncogenes and Related Genes, Shanghai Institute of Digestive Disease, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
2. Department of Gastroenterology, Sichuan Provincial People's Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China.
* Q-Q Liu, Y Chen and Z-Q Zhan contributed equally to this work.
Received 2025-4-1; Accepted 2025-6-5; Published 2025-6-23
Abstract
Purpose: We constructed a novel biomarker cholesterol (C)-to-natural killer (NK) cell ratio (CNR) to reflect the synergistic effect of cholesterol metabolism and inflammation on colorectal cancer (CRC) outcomes. This study aimed to investigate the clinical significance and predictive value of CNR in CRC and develop a simple and reliable prognostic model for predicting OS in CRC patients.
Methods: We retrospectively collected the hematology data and medical records of 213 patients with CRC at Renji hospital and the histological data and medical records of 94 patients with CRC included in a tissue microarray. The association between tumor biomarkers and survival was evaluated using the log-rank test. The diagnostic efficacy of CNR was assessed using receiver operating characteristic curves. The overall survival (OS) rates were estimated using the Kaplan-Meier method. Cox proportional hazards regression was employed in both univariate and multivariate analyses to identify independent prognostic factors, which were subsequently utilized to develop a predictive model for OS. The performance of the model was evaluated using the concordance index (C-index) and calibration plots. The patients were stratified based on the total risk scores calculated from the model. The differences in OS among these groups were assessed using the Kaplan-Meier method. The relationship between cholesterol and NK cells was analyzed by investigating the colon cancer datasets TCGA and GSE39582.
Results: The TNM stage III-IV CRC group had significantly higher blood levels of cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), CNR, and carcinoembryonic antigen (CEA), and shorter progression-free survival (PFS) than the TNM stage I-II CRC group (all, P < 0.05). The blood CNR correlated negatively with PFS (P < 0.001). Elevated tissue CNR levels were an independent risk factor for CRC, where low-tissue CNR patients demonstrated significantly prolonged survival (P < 0.05). The area under the curve for blood CNR was 0.743. The multivariate analyses indicated that tumor location (P = 0.004), TNM stage (P = 0.004), and tissue CNR (P = 0.033) were independent prognostic factors of OS in CRC. The nomogram model was based on these variables and demonstrated good calibration and predictive performance, achieving an excellent C-index of 0.737 [95% confidence interval (CI), 0.674-0.779]. The expression of the key cholesterol biosynthesis players HMGCR, SQLE, and SREBP2 was not significantly associated with NK cell-mediated cytotoxicity-related gene signatures. HMGCR and SQLE were negatively associated with CD56, a phenotypic NK cell marker (P < 0.001).
Conclusion: This is the first study to explore the predictive value of CNR in CRC, which was a promising predictor of CRC progression. The developed nomogram model may serve as a reliable tool for predicting survival in patients with CRC, which may complement the TNM staging system.
Keywords: Colorectal cancer, Cholesterol, NK cell, Tumor markers
Introduction
Colorectal cancer (CRC) is one of the most prevalent malignant tumors worldwide and a leading cause of cancer-related deaths [1, 2]. GLOBOCAN data stated that there were more than 1.9 million new cases of CRC and more than 900,000 deaths worldwide in 2020. While developed countries have a higher CRC incidence, its incidence in some developing nations is also increasing [3, 4]. Age, gender, genetic predispositions, and lifestyle choices, including high-fat and high-red meat diets, obesity, smoking, and lack of exercise, significantly influence CRC risk. Despite advancements in surgical treatment and chemoradiotherapy, the long-term outcomes for patients with CRC remain concerning. Various factors affect CRC prognosis, including tumor staging, degree of differentiation, lymph node metastasis, and biomarker status, such as microsatellite instability [5-8]. However, the predictive performance of these factors remains unsatisfactory due to the high heterogeneity of CRC. Consequently, novel prognostic indicators and models are essential for improving the identification of patients at higher risk of mortality, allowing for more tailored treatment planning.
A notable characteristic of cancer cells is their metabolic abnormalities, including lipid metabolism alterations [9,10]. Recent studies have identified a complex relationship between serum lipids, such as total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, and cancer occurrence and progression [11-14]. CRC cells have elevated cholesterol uptake and accumulation, promoting their proliferation. Furthermore, cancer occurrence and progression appear to be linked to the consumption of circulating cholesterol by the tumor [15-17]. Additionally, systemic inflammatory responses are crucial in cancer development, as cancer-associated inflammatory mediators contribute to immune suppression and immune escape [18-20]. Immune and inflammation-related biomarkers, such as lymphocyte counts, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR), are commonly used to predict CRC long-term prognosis [21-27]. Recent studies have also demonstrated that the accumulation of cholesterol and its metabolites in the tumor microenvironment (TME) positively affect immune suppression. For example, inflammation-induced lipid-laden lung mesenchymal cells metabolically reprogram tumor cells and natural killer (NK) cells, facilitating breast cancer lung metastasis [28]. However, a study that examined the prognostic value of cholesterol and its relationship with immune signatures did not assess the combined effect of cholesterol metabolism and NK cells on the prognosis of patients with CRC [29]. Therefore, this study aimed to evaluate the prognostic value of the cholesterol-to-NK cell ratio (CNR) in CRC and develop and validate a novel prognostic model that may complement the TNM staging system.
Materials and methods
Patient population
This study included patients diagnosed with CRC. Inclusion criteria for the CRC group: (1) CRC confirmed by histopathology; (2) patients did not receive surgery or any treatment. Exclusion criteria: (1) after undergoing surgery or treatment; (2) history of other tumors or malignancies; (3) serious infection and chronic disease; (4) incomplete clinical information, laboratory data, and follow-up data. The ethics committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China) approved the data analysis (Approval no. KY2023-101-C). In accordance with institutional guidelines, informed consent was obtained from all patients before analysis.
Data collection
Each patient's clinical stage (stage I-IV) was determined based on the 8th edition of the TNM Classification of Malignant Tumors published by the American Joint Committee on Cancer. The relevant clinical and histopathological characteristics provided to the researchers were anonymized. Detailed information, including demographic characteristics (age, gender, body mass index [BMI]), clinicpathological characteristics (cholesterol level, lymphocyte ratios, serum CEA), tumor-related characteristics, treatment, and oncological outcomes, was collected from each patient. All analyses were conducted in accordance with the provisions of the Helsinki Declaration of 1975.
Immunohistochemistry
The tissues underwent immunohistochemical analysis to examine the NK cells. CD56 expression was detected in tissue microarrays (Outdo Biotech, Shanghai, China, HCo1A180Su22) containing 94 patients with colorectal cancer. Endogenous peroxidase activity was quenched with 3% H2O2 for 10 minutes, then the slide was washed and heated in a microwave with citrate buffer for antigen retrieval. Subsequently, the slide was blocked with horse serum for 30 minutes and incubated overnight at 4°C with primary antibodies against CD56 (Servicebio, Wuhan, China, GB112671, 1:500), followed by incubation with horseradish peroxidase-conjugated polyclonal anti-rabbit antibodies at room temperature for 30 minutes. Finally, the slide was developed using DAB buffer according to standard protocols. The immunohistochemical staining results were evaluated in a blinded manner (The evaluators were blinded to the clinical characteristics of individual patients). CD56 protein expression levels were quantified depending on staining intensity and extent (percentage of positive tumor cells) under a microscope (200×). Staining intensity score was evaluated on a scale of 0-3: 0 = no staining, 1 = weak staining, 2 = moderate staining, and 3 = strong staining. Further, staining extent score indicated the percentage of positively stained cells (0 = 0%-5%, 1 = 6%-25%, 2 = 26%-50%, 3 = 51%-75%, and 4 = 76%-100%). To derive the final score, staining intensity and extent scores were multiplied.
Immunofluorescence
The tissue microarrays underwent immunofluorescence analysis to examine the total cholesterol (Haling Biotech, Shanghai, China, HL80079.2.1). Briefly, the slide was dewaxed, purified, cleaned, hydrolyzed, and sealed, then stained for 30 minutes, where the fluorescent signal for cholesterol appeared blue. The fluorescence was analyzed using a panoramic scanning microscope (3D HISTECH, Hungary), and digital image processing was performed using CaseViewer 2.4.
Statistical analysis
Count data are expressed as the frequency and percentage, while measured data are expressed as means. Data were compared between groups using the independent samples t-test or the Mann-Whitney U test. Correlations were analyzed using Spearman correlation analysis. The correlation between clinical features and PFS or OS was investigated using the Kaplan-Meier method and log-rank test. Potential prognostic factors were identified using univariate and multivariate analysis with Cox proportional hazards regression. Significant factors identified in the multivariate analysis were used to construct a nomogram model predicting the patients' 3- and 5-year OS rates. Gene set enrichment analysis (GSEA) was assessed using sangerbox (http://sangerbox.com/home.html). P < 0.05 was considered statistically significant.
Results
Pathological characteristics of CRC patients
This study initially enrolled 213 patients with CRC, of which 27 (12.7%) were TNM stage I-II and 186 (87.3%) were stage III-IV. The correlation between TNM stage and clinicopathological factors (gender, age, BMI, tumor size, tumor location, histology, blood lipids) was evaluated (Table 1). Patients in the TNM stage III-IV group had significantly larger tumor size and higher serum CEA, cholesterol, triglyceride, and LDL-C levels than those in the TNM stage I-II group (P = 0.029, 0.040, 0.017, 0.044, 0.032, respectively), while the TNM stage III-IV group had shorter PFS than the stage I-II group (P = 0.023).
The association between clinical factors and PFS
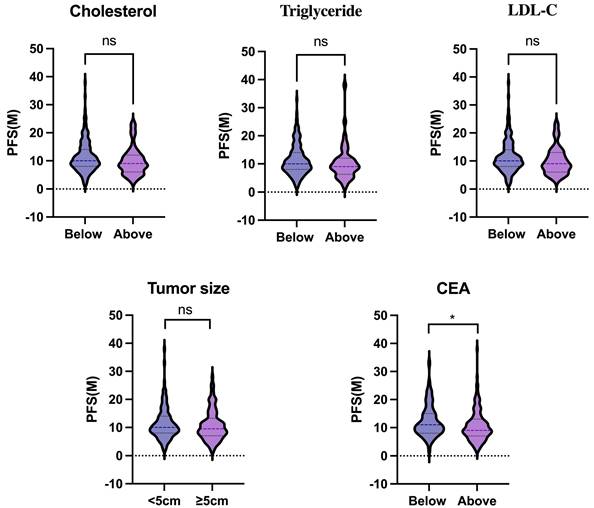
We further investigated the association between the above clinical factors and PFS. Patients were divided into two groups based on their blood cholesterol, triglyceride, LDL-C or CEA levels (below or above the upper limit) or tumor size (< or ≥5cm). Our results indicated that blood cholesterol (P = 0.075), triglyceride (P = 0.318), LDL-C (P = 0.074), and tumor size (P = 0.426) were not significantly associated with PFS (Fig. 1). However, patients with low serum CEA levels had a longer PFS compared to those with high CEA levels (P = 0.035).
Clinicopathological characteristics of 213 patients with CRC.
| Patients (n=213) | |||
|---|---|---|---|
| Characteristics | I-II (n=27) | III-IV (n=186) | P value |
| Gender-no. (%) | |||
| Male | 21 (77.7%) | 120 (64.5%) | 0.198 |
| Female | 6 (22.3%) | 66 (35.5%) | |
| Age (mean, y) | 62.41 | 61.30 | 0.661 |
| BMI (mean, kg/m2) | 23.31 | 23.35 | 0.951 |
| Tumor size (mean, cm) | 3.93 | 4.68 | 0.029 |
| Tumor location-no. (%) | |||
| Left colon | 6 (22.2%) | 73 (39.2%) | 0.039 |
| Right colon | 11 (40.7%) | 37 (19.9%) | |
| Rectum | 10 (37.1%) | 76 (40.9%) | |
| Histological type-no. (%) | |||
| Adenocarcinoma | 26 (96.3%) | 183 (98.4%) | 0.421 |
| Others | 1 (3.7%) | 3 (1.6%) | |
| Fecal occult blood test-no. (%) | |||
| Positive | 25 (92.6%) | 168 (90.3%) | 0.999 |
| Negative | 2 (7.4%) | 18 (9.7%) | |
| Serum CEA-no. (%) | |||
| Positive | 14 (51.9%) | 136 (73.1%) | 0.040 |
| Negative | 13 (48.1%) | 50 (26.9%) | |
| Ki67 (mean) | 59.63% | 67.46% | 0.062 |
| Cholesterol (mean, mmol/L) | 4.35 | 4.96 | 0.017 |
| Triglyceride (mean, mmol/L) | 1.12 | 1.43 | 0.044 |
| HDL-C (mean, mmol/L) | 1.12 | 1.09 | 0.729 |
| LDL-C (mean, mmol/L) | 2.61 | 3.09 | 0.032 |
| PFS (mean, m) | 16.33 | 10.41 | 0.023 |
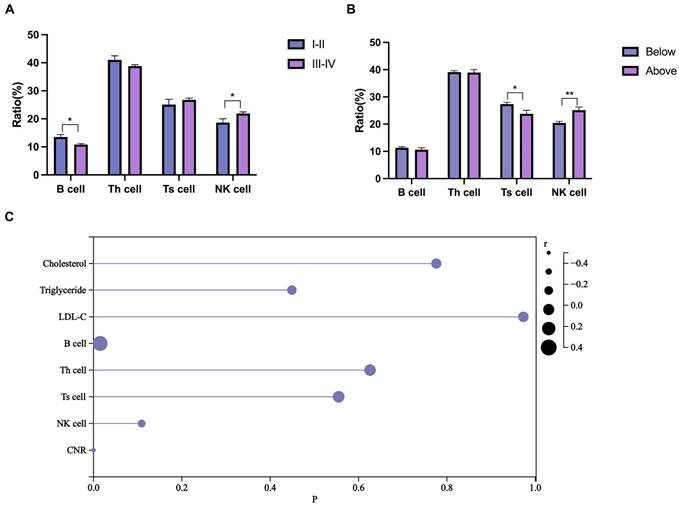
Inflammatory biomarker analysis
The systemic inflammatory response is vital in cancer. Compared to TNM stages I-II, the TNM stage III-IV group had a reduced B cell ratio and increased NK cell ratio, while the T helper (Th) and T suppressor (Ts) cell ratios remained unchanged (Fig. 2A). To further investigate the synergistic effect of blood lipids on the lymphatic system, patients with colorectal cancer were divided into two groups based on their blood cholesterol, triglyceride, or LDL-C levels (below or above the upper limit). Patients in the high-cholesterol group (above the upper limit) had a higher NK cell ratio and a lower Ts cell ratio (Fig. 2B), while no effect was demonstrated for triglycerides (Supplementary Fig. S1A) and LDL-C levels (Supplementary Fig. S1B). Combined effect of cholesterol metabolism and lymphocyte on the prognosis is promising [29]. We then analyzed the correlation of blood lipids and lymphocyte ratios with PFS of CRC patients. The Spearman correlation analysis (Fig. 2C) demonstrated significant associations between B cells (r = 0.324, P = 0.015) and the CNR (r = -0.468, P < 0.001) with PFS.
The correlation between blood CNR and clinical factors
The blood CNR was classified into low and high levels based on the optimal cut-off point of 29.99, as determined by BioLadder software (Supplementary Fig. S2), which yielded an area under the curve of 0.743. The correlation between the blood CNR and clinicopathological factors (gender, age, BMI, tumor size, tumor location, histology) was evaluated (Table 2). Notably, the blood CNR-high group had a poorer pathological stage than the blood CNR-low group (P = 0.041). Furthermore, the blood CNR-high group had a significantly shorter PFS than the blood CNR-low group (P = 0.003). The results indicated that blood CNR may be a promising predictor in CRC. Additionally, the proportions of females in the blood CNR-high group were significantly higher than those in the blood CNR-low group (P = 0.021). Why blood CNR is influenced by gender remains unclear. A large-scale study and additional analyses are needed to confirm these findings.
The correlation of tissue CNR with OS in human colorectal cancer
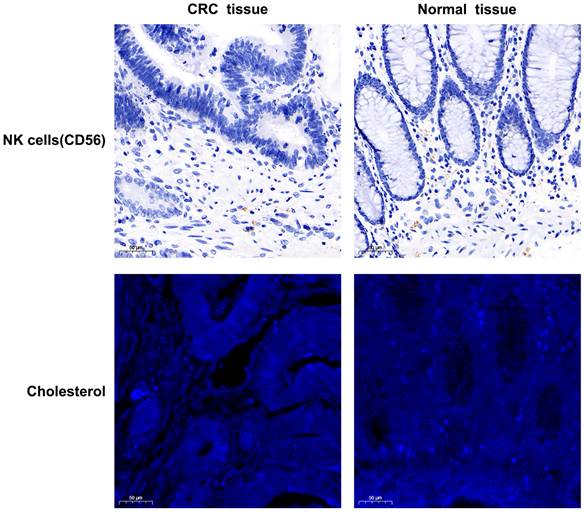
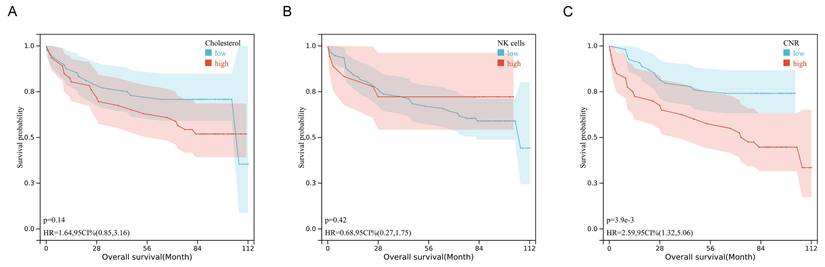
The tissue expression profiles of cholesterol and NK cells in human colorectal cancer were investigated through immunofluorescence staining and immunohistochemical staining in 94 colorectal cancer specimens. TNM stage I, II, III, and IV disease was observed in 7 (7.4%), 44 (46.8%), 40 (42.6%), and 3 (3.2%) of the patients, respectively. The clinicopathological data are presented in Supplementary Table 1 and Supplementary Table 2. Notably, the tissue CNR-high group had a significantly poorer survival rate and shorter OS than the tissue CNR-low group (P = 0.003, 0.031, respectively). High tissue CD56 expression (indicative of NK cells) or cholesterol was observed in 18 (19.1%) and 46 (48.9%) of the 94 specimens, respectively. Figure 3 depicts representative images of the tissue CD56 immunohistochemical staining and the tissue cholesterol immunofluorescence staining. The human CRC tissue had lower NK cell and cholesterol levels than the normal tissue. However, the data showed no significant differences in the expression of tissue NK cells and cholesterol across TNM stages I to IV, which might have been limited by the relatively small sample size (Supplementary Fig. S3). The Kaplan-Meier curves revealed that tissue cholesterol (P = 0.14) and NK cells (P = 0.42) expression was not significantly associated with OS (Fig. 4). However, patients with low tissue CNR had a longer OS compared to those with high tissue CNR (P < 0.001).
The correlation between blood CNR and clinicopathological factors.
| Patients (n=213) | |||
|---|---|---|---|
| Characteristics | CNR-low (n=110) | CNR-high (n=103) | P value |
| Gender-no. (%) | |||
| Male | 81 (73.6%) | 60 (58.3%) | 0.021 |
| Female | 29 (26.4%) | 43 (41.7%) | |
| Age (mean, y) | 62.07 | 60.77 | 0.437 |
| BMI (mean, kg/m2) | 23.62 | 23.06 | 0.184 |
| Tumor size (mean, cm) | 4.44 | 4.74 | 0.199 |
| TNM stage-no. (%) | |||
| I-II | 19 (17.3%) | 8 (7.8%) | 0.041 |
| III-IV | 91 (82.7%) | 95 (92.2%) | |
| Tumor location-no. (%) | |||
| Left colon | 41 (37.3%) | 38 (36.9%) | 0.032 |
| Right colon | 32 (29.1%) | 16 (15.5%) | |
| Rectum | 37 (33.6%) | 49 (47.6%) | |
| Histological type-no. (%) | |||
| Adenocarcinoma | 108 (98.2%) | 101 (98.1%) | 0.999 |
| Others | 2 (1.8%) | 2 (1.9%) | |
| Fecal occult blood test-no. (%) | |||
| Positive | 102 (92.7%) | 91 (88.3%) | 0.349 |
| Negative | 8 (7.3%) | 12 (11.7%) | |
| Serum CEA-no. (%) | |||
| Positive | 82 (74.5%) | 68 (66.0%) | 0.180 |
| Negative | 28 (25.5%) | 35 (34.0%) | |
| Ki67 (mean) | 65.09% | 67.94% | 0.311 |
| PFS (mean, m) | 12.42 | 9.83 | 0.003 |
The association between clinical factors (blood lipids, tumor size, serum CEA) and PFS. Statistical significance was determined by unpaired Student's t test. *, P < .05.
Inflammatory biomarker analysis. (A) Differences in blood lymphocyte ratios between patients with stage I-II CRC and patients with stage III-IV CRC. (B) Differences in blood lymphocyte ratios between high- and low-cholesterol (blood) patients with CRC. (C) Lollipop chart of the correlation of blood lipids, lymphocytes, and CNR with PFS. Data are expressed as the mean ± SEM (A and B). Statistical significance was determined by unpaired Student's t test (A and B) and Spearman's rank-sum test (C). *, P < .05; **, P < .01.
The NK cells (tissue, immunohistochemical staining) and cholesterol (tissue, immunofluorescence staining) expression profiles in human colorectal cancer samples (200× magnifications).
Prognostic factors in the univariate and multivariate analyses
The effect of clinicopathological characteristics on survival was investigated using Cox proportional hazards regression (Table 3). The univariate analysis results revealed that tumor location (left vs. right, P = 0.021), TNM stage (III-IV vs. I-II, P = 0.011), and tissue CNR (high vs. low, P = 0.008) were significantly associated with OS. Furthermore, the multivariate analysis confirmed that tumor location (left vs. right, hazard ratio [HR]: 2.811, 95% CI [1.414-5.794], P = 0.004), TNM stage (III-IV vs. I-II, HR: 2.722, 95% CI [1.382-5.543], P = 0.004), and tissue CNR (high vs. low, HR: 2.493, 95% CI [1.106-5.997], P = 0.033) were significant independent predictors of OS. The predictive ability of the tissue CNR and TNM stage was compared using the concordance index (C-index). The C-index of tissue CNR and TNM stage was 0.661 and 0.635 respectively, with no statistically significant difference. Wang's team compared the prognostic value of TNM stage and NLR in patients with colorectal cancer [29]. Results showed that the C-index of TNM stage and NLR was 0.663 and 0.551 respectively, which corroborates the superior predictive value of CNR.
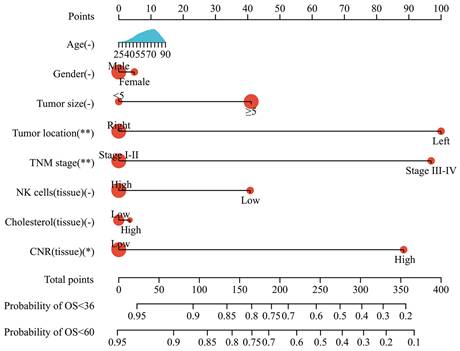
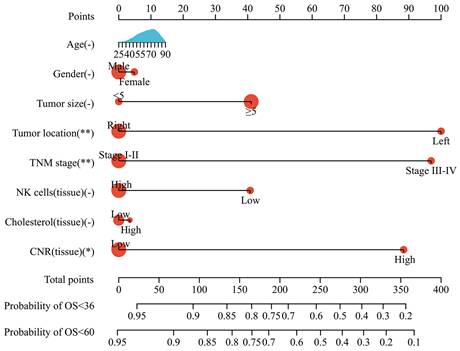
Prognostic nomogram model
Based on the multivariate analysis results, a nomogram model incorporating tumor size, tumor location, TNM stage, and tissue CNR was constructed to predict OS in CRC patients (Fig. 5). Each variable was assigned a point. The patients' prognosis was assessed using a total risk score. The nomogram model was transformed from an online calculator, sangerbox [30], where the corresponding predicted survival rate was obtained by inputting clinical factors and selecting a time point.
The predictive ability of the nomogram and non-tissue CNR nomogram was compared using the C-index (Table 4). The C-index of the nomogram was 0.737, which indicated a good predictive accuracy. Furthermore, the comparison demonstrated that incorporating the tissue CNR significantly enhanced the predictive performance (C-index: nomogram: 0.737, 95%CI [0.674-0.779] vs. non-tissue CNR nomogram: 0.692, 95%CI [0.620-0.765], P < 0.05). Additionally, the calibration curves demonstrated that the predicted survival rates calculated by the nomogram model closely aligned with the actual observed values, suggesting strong consistency between the predictions and real-world outcomes (Supplementary Fig. S4).
Univariate and multivariate Cox proportion hazard regression analysis for OS.
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (≥ 65 vs. < 65) | 1.002 (0.978-1.028) | 0.858 | ||
| Gender (male vs. female) | 0.951 (0.486-1.869) | 0.883 | ||
| Tumor size (≥ 5 cm vs. < 5cm) | 1.529 (0.736-3.433) | 0.275 | ||
| Tumor location (left vs. right) | 2.157 (1.083-4.296) | 0.021 | 2.811 (1.414-5.794) | 0.004 |
| TNM stage (stage III-IV vs. stage I-II) | 2.424 (1.254-4.688) | 0.011 | 2.722 (1.382-5.543) | 0.004 |
| tissue NK cells (low vs. high) | 1.524 (0.459-4.497) | 0.459 | ||
| tissue Cholesterol (high vs. low) | 1.036 (0.498-2.174) | 0.925 | ||
| tissue CNR (high vs. low) | 2.275 (1.194-4.334) | 0.008 | 2.493 (1.106-5.997) | 0.033 |
The C-indices of prognostic models for predicting OS.
| Models | C-index | 95% CI | P value |
|---|---|---|---|
| Nomogram | 0.737 | 0.674-0.779 | <0.05 |
| Non-tissue CNR nomogram | 0.692 | 0.620-0.765 |
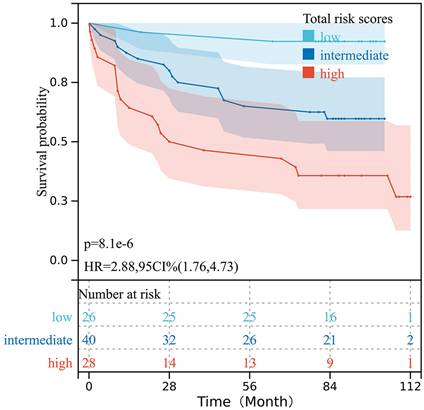
The total risk scores of all 94 patients with CRC were obtained based on the prognostic nomogram model. All risk scores were arranged in ascending order, then points were set at the 30% and 70% percentiles as the cut-off values, and the patients were categorized into low-, intermediate-, and high-risk groups. The Kaplan-Meier analysis indicated that patients with different risk levels had significantly different OS (P < 0.001) (Fig. 6).
Gene expression of cholesterol synthesis and NK cells in colon cancer datasets
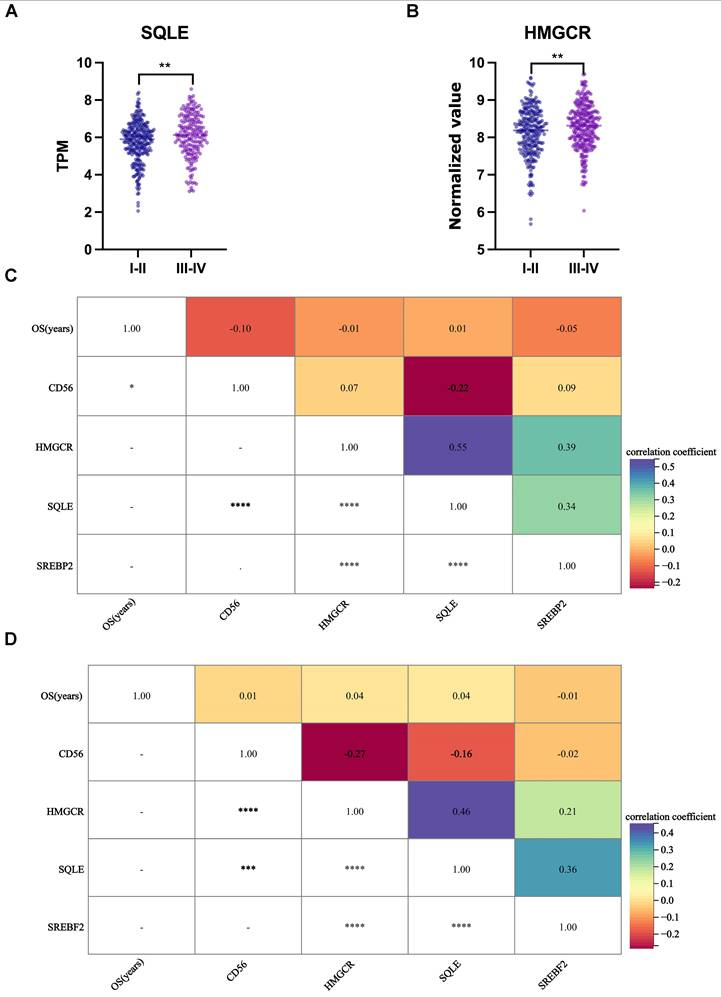
The cellular cholesterol level reflects the dynamic balance between biosynthesis, uptake, export, and esterification [31]. The relationship between cholesterol and NK cells was investigated by analyzing two independent colon cancer datasets (The Cancer Genome Atlas [TCGA], 475 colon cancer cases; GSE39582, 558 colon cancer cases). Almost all cells can synthesize cholesterol; therefore, we briefly examined three crucial players of the cholesterol biosynthetic pathway: two rate-limiting enzymes of the biosynthetic pathway: 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) and squalene monooxygenase (SQLE), and sterol regulatory element-binding protein 2 (SREBP2), a master transcriptional regulator of cholesterol biosynthesis. Compared to the TNM stage I-II group, the TNM stage III-IV group had significantly increased gene expression of SQLE (Fig. 7A) and HMGCR (Fig. 7B) (P = 0.001, 0.005, respectively), while CD56 and SREBP2 gene expression was unchanged (Supplementary Fig. S5). Furthermore, CD56 and SQLE (Figs. 7C, 7D) or HMGCR (Fig. 7D) were negatively related (r = -0.22, -0.27, respectively, P < 0.001).
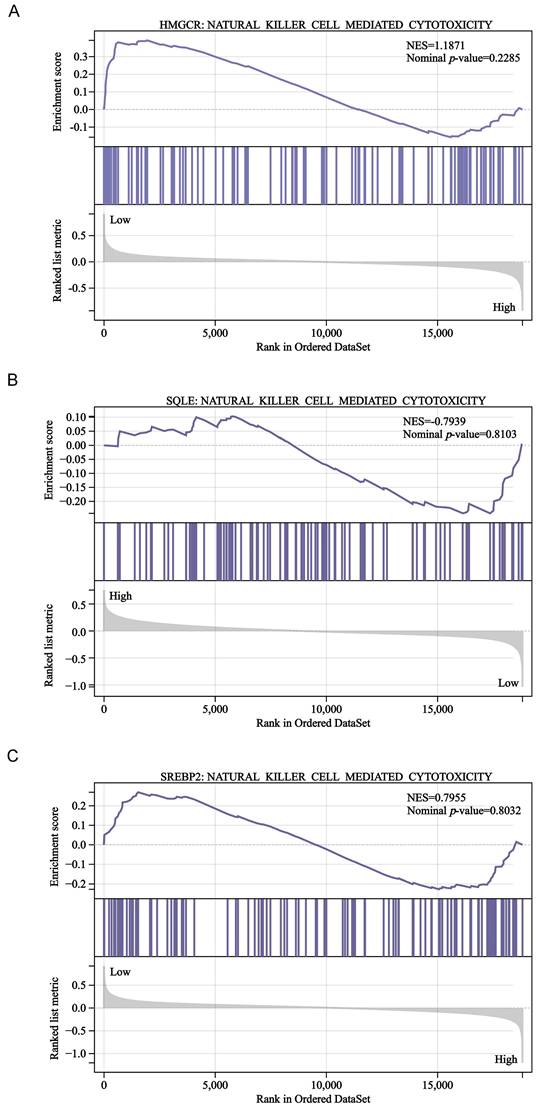
To further explore the association between cholesterol synthesis and NK cells, we performed GSEA in the GSE39582 dataset. However, our results revealed that HMGCR, SQLE, and SREBP2 expression was not significantly associated with NK cell-mediated cytotoxicity-related gene signatures (Fig. 8A-C). Therefore, more studies are needed to confirm whether and how cholesterol regulates NK cells in CRC.
Discussion
In the present study, more than 42% patients exhibited an elevated CNR, which we determined was significantly associated with decreased PFS or OS. To our knowledge, this is the first study to describe the correlation between CNR and survival rates in CRC. Therefore, pre-treatment CNR represents a novel biomarker that can effectively predict the prognosis of patients with CRC and can be a valuable supplement to the TNM staging system, aiding clinicians in more accurate treatment planning.
Kaplan-Mier survival curves of the OS in patients with CRC stratified by tissue cholesterol (A), tissue NK cells (B), and tissue CNR (C).
Nomogram model for predicting the OS of patients with CRC.
Survival curves for the OS of patients with CRC stratified according to the total risk score obtained from the nomogram model.
Cholesterol is an important cell membrane component and participates in signal transduction, cell proliferation, and apoptosis processes. Lipid disequilibrium, especially abnormal cholesterol metabolism, is closely related to various diseases, especially tumor occurrence and development [32,33]. CRC is a common malignant tumor of the digestive system that is closely related to cholesterol metabolism [15,34-36]. Reportedly, excessive intake of cholesterol is correlated with CRC occurrence. Mutations in the cholesterol metabolism-related genes are associated with an increased risk of CRC [37,38]. While elevated cholesterol is associated with the incidence of colorectal adenomas and tumors, the conclusions on the relationship between serum cholesterol levels and CRC prognosis have been inconsistent. Ren et al. reported that statin use was associated with a significantly lower risk of incident cancer and cancer-related mortality [39]. Conversely, another study reported that the statin-induced reduction of plasma cholesterol levels did not significantly affect recurrence and survival rates in patients with stage III cancer [40]. In the present study, cholesterol alone had no predictive value for survival, but effectively distinguished CRC patients with poor prognosis when combined with immune markers.
Gene expression of cholesterol synthesis and NK cells in colon cancer datasets. (A) SQLE gene expression was increased in the TNM stage III-IV group compared to the TNM stage I-II group (TCGA). (B) HMGCR gene expression was increased in the TNM stage III-IV group compared to the TNM stage I-II group (GSE39582). (C) CD56 and SQLE were negatively related (TCGA). (D) CD56 and SQLE or HMGCR were negatively related (GSE39582). Statistical significance was determined by unpaired Student's t test (A and B) and Spearman's rank-sum test (C and D). **, P < .01; ***, P < .001; ****, P < .0001.
GSEA of the GSE39582 dataset. HMGCR (A), SQLE (B), and SREBP2 (C) expression was not significantly associated with NK cell-mediated cytotoxicity- related gene signatures.
The immune system has recently received extensive attention. Cholesterol and its metabolites within the TME are crucial in cancer progression, as they regulate cancer cell proliferation, migration, and invasion, and modulate immunity and inflammation [41-45]. A high-cholesterol diet may promote inflammation and tumor formation by affecting the gut microbiota composition [46]. Previous studies analyzed and combined several serum lipid indices and inflammation-related biomarkers to predict the prognosis of CRC. The cholesterol-to-lymphocyte ratio (CLR) and monocyte-to- HDL-C ratio (MHR) have been correlated with long-term prognosis in CRC [29,47]. In the present study, the hematological data of 213 patients with CRC and the tissue microarray-based histological data of 94 patients with CRC revealed that patients at more advanced TNM stages had significantly higher CNR, which is dependent on the combination of cholesterol and NK cells. Additionally, the CNR level was identified as an independent prognostic factor of OS in CRC, and showed slightly superior prognostic value than TNM stage, which is one of the most widely used indictor for prognosis. All of the data indicated that CNR had a reliable prognostic value.
NK cells are integral innate immune system components, pivotal in anti-tumor immunity, and crucial in tumor immunology. Numerous studies have demonstrated that NK cells exert anti-tumor cytotoxic effects through specific functional receptors on their surface, such as NKG2 and the KIR family [48]. NK cells secrete cytokines and chemokines, such as CCL5, XCL1, and XCL2, which promote dendritic cell migration into solid tumors, and enhance the anti-tumor activity of CD8+ T cells [49]. Various substances within the TME influence the biological activity of NK cells. Lynch's team was the first to discover that obesity significantly induces the activation of peroxisome proliferator-activated receptors (PPARs) in NK cells, leading to fat accumulation and resulting in the failure of NK cell-mediated immune surveillance, thereby inhibiting their normal tumor cell-killing effects [50]. Liu and colleagues confirmed that FASN, which was upregulated in CRC tissues, suppressed the antitumor response of NK cells through the SP1/PLA2G4B axis [51]. Accordingly, it is important to consider the synergistic effects of cholesterol metabolism and NK cells on CRC prognosis. Our results demonstrated the predictive value of CNR and we have incorporated it into a prognostic model. This model, with a high accuracy and stability, is expected to provide a novel approach to optimizing risk stratification for patients with CRC.
Our study has several limitations. First, due to the modest sample size, we were unable to perform propensity score matching (PSM) to balance baseline characteristics, despite some differences observed between groups (e.g., gender, and tumor location). While PSM is a robust method for reducing selection bias, its application in small cohorts risks significant sample loss and compromised statistical power. Instead, we controlled for potential confounders through univariate and multivariate Cox proportional hazards regression analyses, which yielded consistent results. Nevertheless, residual confounding may persist, and future larger-scale studies should employ PSM or randomized designs to corroborate our findings. Second, as biochemical and immunohistochemical analyses of lipids and lymphocytes were not routinely performed for all patients, several individuals who did not undergo lipid and lymphocyte measurements were excluded. Both blood and tissue levels of cholesterol and NK cells must be assessed to evaluate their influence on CRC prognosis, which may help us to determine whether blood CNR or tissue CNR is more effective in predicting OS. Furthermore, why blood CNR is influenced by gender remains unclear; the regulatory mechanism by cholesterol of NK cells remains largely unknown. Therefore, a large-scale multi-center study and additional experimental analyses are needed to confirm these findings.
Conclusions
This is the first study to explore the predictive value of CNR, which was an independent risk factor for CRC progression. The nomogram model incorporating CNR and other clinical features may be a valuable tool for predicting survival in patients with CRC.
Abbreviations
C-index: concordance index; CEA: carcinoembryonic antigen; CI: confidence interval; CLR: cholesterol-to-lymphocyte ratio; CNR: cholesterol-to-natural killer cell ratio; CRC: colorectal cancer; GEO: gene expression omnibus; GSEA: gene set enrichment analysis; HDL-C: high-density lipoprotein cholesterol; HMGCR: 3-hydroxy-3-methylglutaryl coenzyme A reductase; HR: hazard ratio; LDL-C: low-density lipoprotein cholesterol; LMR: lymphocyte-to-monocyte ratio; MHR: monocyte-to-HDL-C ratio; NK: natural killer; NLR: neutrophil-to-lymphocyte ratio; OS: overall survival; PFS: progression-free survival; PLR: platelet-to-lymphocyte ratio; PPARs: peroxisome proliferator-activated receptors; ROC: receiver operating characteristic; SQLE: squalene monooxygenase; SREBP2: sterol regulatory element- binding protein 2; TCGA: the cancer genome atlas; Th: T helper; TME: tumor microenvironment; TNM: Tumor Node Metastasis; Ts: T suppressor.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
We thank the staff at the State Key Laboratory for Oncogenes and Related Genes, Shanghai Institute of Digestive Disease for providing support on research conditions in this study.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82303215, 82203269).
Author contributions
Q-Q Liu and Y-X Chen designed and oversaw the study. Q-Q Liu, Y Chen, and H-L Wang initiated or participated in data acquisition and analyses. Q-Q Liu, Z-Q Zhan, and Y-X Chen contributed to interpret and annotate the results. Q-Q Liu, Z-Q Zhan, and Y-X Chen drafted the manuscript. All authors reviewed and approved the final version of manuscript.
Data availability statement
For reasons of patient privacy, the data sets analyzed in the current study are not publicly available, but it can be obtained from the corresponding author (yingxuanchen71@sjtu.edu.cn) under reasonable requirements.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-54
2. Xia C, Dong X, Li H, Cao M, Sun D, He S. et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-90
3. Mahmoud NN. Colorectal Cancer: Preoperative Evaluation and Staging. Surg Oncol Clin N Am. 2022;31:127-41
4. Chen K, Collins G, Wang H, Toh JWT. Pathological Features and Prognostication in Colorectal Cancer. Curr Oncol. 2021;28:5356-83
5. Zygulska AL, Pierzchalski P. Novel Diagnostic Biomarkers in Colorectal Cancer. Int J Mol Sci. 2022;23:852
6. Wang H, Huo R, He K, Cheng L, Zhang S, Yu M. et al. Perineural invasion in colorectal cancer: mechanisms of action and clinical relevance. Cell Oncol (Dordr). 2024;47:1-17
7. Qin Y, Li M, Lin Q, Pan X, Liang Y, Huang Z. et al. Colorectal Cancer Cell Differentiation Trajectory Predicts Patient Immunotherapy Response and Prognosis. Cancer Control. 2022;29:10732748221121382
8. Pakarinen S, Varpe P, Carpelan A, Koivisto M, Huhtinen H. Mobile-CEA - A Novel Surveillance Method for Patients with Colorectal Cancer. Cancer Control. 2022;29:10732748221102780
9. Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2:132-41
10. Broadfield LA, Pane AA, Talebi A, Swinnen JV, Fendt SM. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev Cell. 2021;56:1363-93
11. van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH. et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European prospective investigation into cancer and nutrition. Gut. 2011;60:1094-102
12. Cao LQ, Xie Y, Fleishman JS, Liu X, Chen ZS. Hepatocellular carcinoma and lipid metabolism: Novel targets and therapeutic strategies. Cancer Lett. 2024;597:217061
13. Liu J, Geng W, Sun H, Liu C, Huang F, Cao J. et al. Integrative metabolomic characterisation identifies altered portal vein serum metabolome contributing to human hepatocellular carcinoma. Gut. 2022;71:1203-13
14. Ecker J, Benedetti E, Kindt ASD, Höring M, Perl M, Machmüller AC. et al. The Colorectal Cancer Lipidome: Identification of a Robust Tumor-Specific Lipid Species Signature. Gastroenterology. 2021;161:910-23
15. He X, Lan H, Jin K, Liu F. Cholesterol in colorectal cancer: an essential but tumorigenic precursor? Front Oncol. 2023;13:1276654
16. Zhan ZQ, Chen YZ, Huang ZM, Luo YH, Zeng JJ, Wang Y. et al. Metabolic syndrome, its components, and gastrointestinal cancer risk: a meta-analysis of 31 prospective cohorts and Mendelian randomization study. J Gastroenterol Hepatol. 2024;39:630-41
17. Wong CC, Wu JL, Ji F, Kang W, Bian X, Chen H. et al. The cholesterol uptake regulator PCSK9 promotes and is a therapeutic target in APC/KRAS-mutant colorectal cancer. Nat Commun. 2022;13:3971
18. Iftimie S, Escribano A, Díez-Sans A, Albiciuc I, Hernández-Aguilera A, Fort-Gallifa I. et al. Influence of surgical procedures on serum paraoxonase-1-related variables and markers of inflammation in hospitalized patients. J Invest Surg. 2021;34:216-24
19. Venakteshaiah SU, Kumar KH. Inflammation and cancer. Endocr Metab Immune Disord Drug Targets. 2021;21:193-94
20. Yamamoto T, Kawada K, Obama K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int J Mol Sci. 2021;22:8002
21. Rossi S, Basso M, Strippoli A, Schinzari G, D'Argento E, Larocca M. et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer. 2017;16:264-74
22. Liu Q, Luo D, Cai S, Li Q, Li X. Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer. Clin Transl Med. 2020;9:6
23. Schwartz PB, Poultsides G, Roggin K, Howard JH, Fields RC, Clarke CN. et al. PLR and NLR are poor predictors of survival outcomes in sarcomas: a new perspective from the USSC. Surg Res. 2020;251:228-38
24. Xia LJ, Li W, Zhai JC, Yan CW, Chen JB, Yang H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to- monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer. BMC Cancer. 2020;20:208
25. Lu J, Xu BB, Zheng ZF, Xie JW, Wang JB, Lin JX. et al. CRP/prealbumin, a novel inflammatory index for predicting recurrence after radical resection in gastric cancer patients: post hoc analysis of a randomized phase III trial. Gastric Cancer. 2019;22:536-45
26. Liao CK, Yu YL, Lin YC, Hsu YJ, Chern YJ, Chiang JM. et al. Prognostic value of the C-reactive protein to albumin ratio in colorectal cancer: an updated systematic review and meta-analysis. World J Surg Oncol. 2021;19:139
27. Xu H, You G, Zhang M, Song T, Zhang H, Yang J. et al. Association of pre-surgery to pre-radiotherapy lymphocyte counts ratio with disease-free survival in rectal cancer patients receiving neoadjuvant concurrent chemoradiotherapy. World J Surg Oncol. 2019;17:199
28. Gong Z, Li Q, Shi J, Liu ET, Shultz LD, Ren G. Lipid-laden lung mesenchymal cells foster breast cancer metastasis via metabolic reprogramming of tumor cells and natural killer cells. Cell Metab. 2022;34:1960-76
29. Zhou S, He Q, Sheng N, Gong J, Ren J, Wang Z. Blood cholesterol-to- lymphocyte ratio as a novel prognostic marker to predict postoperative overall survival in patients with colorectal cancer. World J Surg Oncol. 2022;20:18
30. Shen WT, Song ZG, Zhong X, Huang M, Shen DT, Gao PP. et al. Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. Imeta. 2022;1:e36
31. Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21:225-45
32. Chimento A, Casaburi I, Avena P, Trotta F, De Luca A, Rago V. et al. Cholesterol and its metabolites in tumor growth: therapeutic potential of statins in cancer treatment. Front Endocrinol (Lausanne). 2019;9:807
33. Ding X, Zhang W, Li S, Yang H. The role of cholesterol metabolism in cancer. Am J Cancer Res. 2019;9:219-27
34. Zhang KL, Zhu WW, Wang SH, Gao C, Pan JJ, Du ZG. et al. Organ-specific cholesterol metabolic aberration fuels liver metastasis of colorectal cancer. Theranostics. 2021;11:6560-72
35. Jin Y, Chen Z, Dong J, Wang B, Fan S, Yang X. et al. SREBP1/FASN/cholesterol axis facilitates radioresistance in colorectal cancer. FEBS Open Bio. 2021;11:1343-52
36. Wang Y, Liu C, Hu L. Cholesterol regulates cell proliferation and apoptosis of colorectal cancer by modulating miR-33a-PIM3 pathway. Biochem Biophys Res Commun. 2019;511:685-92
37. Rzasa P, Whelan S, Farahmand P, Cai H, Guterman I, Palacios-Gallego R. et al. BRAFV600E-mutated serrated colorectal neoplasia drives transcriptional activation of cholesterol metabolism. Commun Biol. 2023;6:962
38. Tian Y, Wang K, Li J, Wang J, Wang Z, Fan Y. et al. The association between serum lipids and colorectal neoplasm: a systemic review and meta-analysis. Public Health Nutr. 2015;18:3355-70
39. Ren QW, Yu SY, Teng TK, Li X, Cheung KS, Wu MZ. et al. Statin associated lower cancer risk and related mortality in patients with heart failure. Eur Heart J. 2021;42:3049-59
40. Ng K, Ogino S, Meyerhardt JA, Chan JA, Chan AT, Niedzwiecki D. et al. Relationship between statin use and colon cancer recurrence and survival: results from CALGB 89803. J Natl Cancer Inst. 2011;103:1540-51
41. Xu H, Zhou S, Tang Q, Xia H, Bi F. Cholesterol metabolism: new functions and therapeutic approaches in cancer. Biochim Biophys Acta Rev Cancer. 2020;1874:188394
42. Yang Y, Liu X, Ma W, Xu Q, Chen G, Wang Y. et al. Light-activatable liposomes for repetitive on-demand drug release and immunopotentiation in hypoxic tumor therapy. Biomaterials. 2021;265:120456
43. Yan C, Zheng L, Jiang S, Yang H, Guo J, Jiang LY. et al. Exhaustion-associated cholesterol deficiency dampens the cytotoxic arm of antitumor immunity. Cancer Cell. 2023;41:1276-93
44. Yang Z, Huo Y, Zhou S, Guo J, Ma X, Li T. et al. Cancer cell-intrinsic XBP1 drives immunosuppressive reprogramming of intratumoral myeloid cells by promoting cholesterol production. Cell Metab. 2022;34:2018-35
45. Mao W, Cai Y, Chen D, Jiang G, Xu Y, Chen R. et al. Statin shapes inflamed tumor microenvironment and enhances immune checkpoint blockade in non-small cell lung cancer. JCI Insight. 2022;7:e161940
46. Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int J Mol Sci. 2019;20:1214
47. Zhang X, Qin H, Tan X, Mo Y, Li Z, Huang G. et al. Predictive value of monocyte to high-density lipoprotein cholesterol ratio and tumor markers in colorectal cancer and their relationship with clinicopathological characteristics. World J Surg Oncol. 2023;21:200
48. Quatrini L, Della Chiesa M, Sivori S, Mingari MC, Pende D, Moretta L. Human NK cells, their receptors and function. Eur J Immunol. 2021;51:1566-79
49. Guillerey C. NK Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1273:69-90
50. Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K. et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol. 2018;19:1330-40
51. Liu X, Lu J, Ni X, He Y, Wang J, Deng Z. et al. FASN promotes lipid metabolism and progression in colorectal cancer via the SP1/PLA2G4B axis. Cell Death Discov. 2025;11:122
Author contact
![]() Corresponding author: Prof Y X Chen, Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology and Hepatology, Ministry of Health, State Key Laboratory for Oncogenes and Related Genes, Renji Hospital, School of Disease, 145 Middle Shandong Road, Shanghai 200001, China, yingxuanchen71edu.cn.
Corresponding author: Prof Y X Chen, Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology and Hepatology, Ministry of Health, State Key Laboratory for Oncogenes and Related Genes, Renji Hospital, School of Disease, 145 Middle Shandong Road, Shanghai 200001, China, yingxuanchen71edu.cn.

 Global reach, higher impact
Global reach, higher impact