Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(9):2800-2811. doi:10.7150/jca.113348 This issue Cite
Research Paper
Integrating Bulk RNA Sequencing and CRISPR-Cas9 Screening to Identify Proliferation-Related Genes for Prognostic Stratification in Breast Cancer
1. The First Department of Breast Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin's Clinical Research Center for Cancer, National Clinical Research Center for Cancer, Key Laboratory of Breast Cancer Prevention and Therapy, Tianjin Medical University Cancer Institute and Hospital, Tianjin Medical University, Tianjin, 300060, China.
2. Tianjin Medical University, Tianjin, 300070, China.
3. Immunology Department, Key Laboratory of Immune Microenvironment and Disease (Ministry of Education), Tianjin Medical University, Tianjin, 300070, China.
4. Department of General Surgery, Tianjin Medical University General Hospital, Tianjin Key Laboratory of Precise Vascular Reconstruction and Organ Function Repair, Tianjin General Surgery Institute, Tianjin, 300052, China.
# These authors contributed equally to this work.
Received 2025-3-6; Accepted 2025-5-29; Published 2025-6-12
Abstract
Breast cancer has become one of the most common malignant tumors in women. Although the emergence of molecular typing has greatly improved the prognosis of breast cancer patients, some patients still face drug resistance, recurrence and metastasis. At present, the development of effective biomarkers is still an important direction of breast cancer research. This study aims to provide new ideas for individualized treatment of breast cancer by identifying new biomarkers and constructing models to predict the prognosis of breast cancer patients. In this study, seven tumor-dependent genes associated with tumor proliferation were identified through the combined analysis of bulk-RNA sequencing and CRISPR-CAS9, and the mechanism of their potential promotion of tumor proliferation was initially analyzed. Immune infiltration analysis suggested these genes may be associated with the formation of immunosuppressive microenvironment. In addition, we constructed a gene signature based on seven genes that can predict prognostic risk in patients with breast cancer. The group with higher signature scores was associated with more GATA3 somatic mutations. Finally, we screened potential drugs suitable for high-risk groups to improve their outcomes. Our study provides potential therapeutic targets as well as individualized treatment strategies for breast cancer.
Keywords: breast cancer, tumor dependency, CRISPR-Cas9, bulk-RNA sequencing, cell proliferation
Introduction
Breast cancer (BRCA) has emerged as one of the most prevalent and lethal malignancies among women globally in recent years [1-3]. According to 2020 global cancer statistics, over 2.26 million new cases and approximately 685,000 deaths were reported, with incidence rates continuing to rise annually [4]. Although advancements in medical technologies, including surgical interventions, radiotherapy, chemotherapy, targeted therapies, and endocrine treatments, have significantly improved patient prognosis [5], the high intra- and inter-tumoral heterogeneity of breast cancer leads to variable therapeutic sensitivities [6]. Therefore, identifying novel prognostic biomarkers and potential therapeutic targets remains imperative to expand intervention strategies for breast cancer patients.
The goal of the Dependency Map (DepMap) database, integrating CRISPR screening data from hundreds of cancer cell lines, systematically identifies genes essential for tumor cell survival and proliferation across cancer types, serving as a pivotal tool for discovering cancer dependency genes [7]. Genes identified through CRISPR screenings exhibit high clinical translational value. For instance, Xiao et al. [8] analyzed DepMap CRISPR-Cas9 data to investigate olaparib sensitivity-related gene expression variations, revealing that NLRP4 upregulation enhances olaparib resistance in pancreatic cancer. Similarly, Li et al. [9] identified three proliferation-associated genes in triple-positive breast cancer using CRISPR-Cas9 and constructed a prognostic prediction model. These studies highlight the potential of DepMap-based dependency analyses in guiding precision oncology. Cross-validation with databases like The Cancer Genome Atlas (TCGA) may further strengthen the clinical relevance of candidate genes.
In this study, we identified seven tumor dependency-associated genes by integrating CRISPR-Cas9 whole-genome knockout data with TCGA transcriptomic profiles. These genes were significantly correlated with poor prognosis, low CD8 T cell infiltration, and high M2 macrophage infiltration in breast cancer. Furthermore, a tumor dependency signature constructed from these genes effectively stratified patient outcomes, with the high-dependency subgroup demonstrating enrichment in GATA3 mutations. Finally, potential therapeutic agents targeting high-dependency patients were screened to guide personalized treatment. Our findings provide novel insights and candidate targets for breast cancer therapy.
Materials and Methods
Data acquisition and preprocessing
The workflow of this study is illustrated in Figure 1. The DepMap database provides genome-wide CRISPR-Cas9 knockout data across multiple cancer cell lines. CERES matrices from 42 breast cancer cell lines post-CRISPR-Cas9 knockout were downloaded and processed. To identify tumor proliferation dependency genes, the following criteria were applied: 1) Genes meeting CERES scores < -1 in ≥75% of breast cancer cell lines; 2) Genes with average CERES scores < -1 across all breast cancer cell lines. Lower CERES scores indicate stronger tumor proliferation dependency. Filtered genes were preliminarily designated as proliferation-associated genes for downstream analyses.
Transcriptomic profiles and somatic mutation data of breast cancer were retrieved from the TCGA database via the 'TCGAbiolinks' (v2.26.0) package [10], while clinical data were obtained from the XENA database. Transcriptomic data were processed using TPM (transcripts per million)-normalized expression matrices to minimize biases from sequencing depth and gene length. Inclusion criteria for samples were: 1) Availability of estrogen receptor (ER), progesterone receptor (PR), and Erb-B2 receptor tyrosine kinase 2 (HER2) status confirmed by immunohistochemistry (IHC) or immunofluorescence; 2) Complete survival information (overall survival time and status); 3) Transcriptomic data availability. After quality control, 721 TCGA-BRCA samples were retained for subsequent analyses.
WGCNA analysis
A total of 698 proliferation-associated genes from 721 samples were included in weighted gene co-expression network analysis (WGCNA) through 'WGCNA' (v1.72-1) package [11]. Outlier samples were excluded prior to constructing an unsigned topological matrix. The soft thresholding power was set to 5, with a minimum module size of 30 genes. Three gene modules were ultimately identified.
Survival and differential expression analysis
Univariate Cox regression analysis was performed to evaluate the prognostic value of each module. Differential expression analysis between 113 normal breast tissues and 721 tumor samples was conducted using the 'limma' (3.54.2) package [12]. Genes with log2(fold change) > 0.585 and P < 0.05 were defined as differentially expressed. Intersection analysis between proliferation-associated genes and differentially expressed genes was performed by 'venn' (v1.11) package. Kaplan-Meier survival curves were generated to assess associations between gene expression levels and overall survival (P < 0.05 considered statistically significant). Of note, 'survminer' (v0.4.9) package was used to determine the best cut-off value of grouping.
Immune infiltration and correlation analysis
CIBERSORTx [13] was employed to estimate immune cell infiltration in TCGA-BRCA samples using the LM22 signature matrix as a reference. Spearman correlation analysis was performed to evaluate relationships between the seven proliferation-associated genes and immune cell abundance, immune checkpoint molecule expression, and inter-gene correlations (P < 0.05 considered statistically significant).
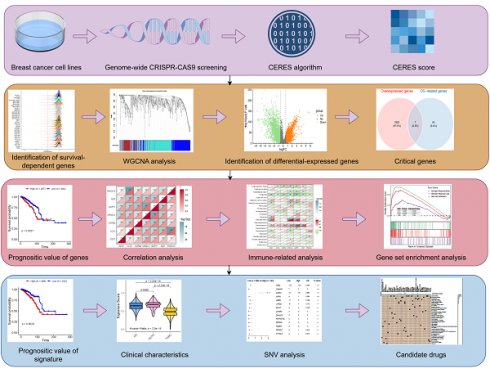
Schematic design of the study. WGCNA, Weighted Gene Co-Expression Network Analysis; SNV, single nucleotide variants.
ssGSEA and GSEA
A gene signature score comprising the seven candidate genes was established using single-sample gene set enrichment analysis (ssGSEA) via 'GSVA' (v1.46.0) package. Subsequently, the signature scores were standardized. Samples were stratified into high- and low-proliferation groups based on median signature scores. 'genesets.v2023.1.hallmark.Hs' was obtained from GSEA database. Differentially expressed genes identified via 'limma' were input into gene set enrichment analysis (GSEA) with the " genesets.v2023.1.hallmark.Hs" gene sets as reference. Pathways with P < 0.05 were considered statistically enriched.
Clinical feature and somatic mutation analysis
Kaplan-Meier analysis evaluated the prognostic value of the gene signature. Associations between signature scores and molecular subtypes were assessed via Kruskal-Wallis test, while correlations with clinical features (T stage, M stage, tumor stage) were analyzed using Wilcoxon test (P < 0.05). The 'maftools' (v2.14.0) package was utilized to compare genomic heterogeneity between high- and low-signature groups. Fisher's exact test identified differential somatic mutations, and mutation landscapes of the top 15 mutated genes were visualized (P < 0.05).
Enrichment analysis and drug identification
The top 1500 genes in the high-signature group underwent functional enrichment analysis using the 'clusterProfiler' (v4.6.2) package [14]. The CAMP database [15] screened candidate therapeutic compounds by comparing top 100 genes from high- and low-signature groups (version 1.0). Over 2400 compounds were scored, with lower scores indicating stronger inhibitory effects. The 50 compounds with the lowest scores were selected as potential therapeutics for the high-signature group.
Statistics
All of the statistical analyses are described above and analyzed by R (v4.2.2).
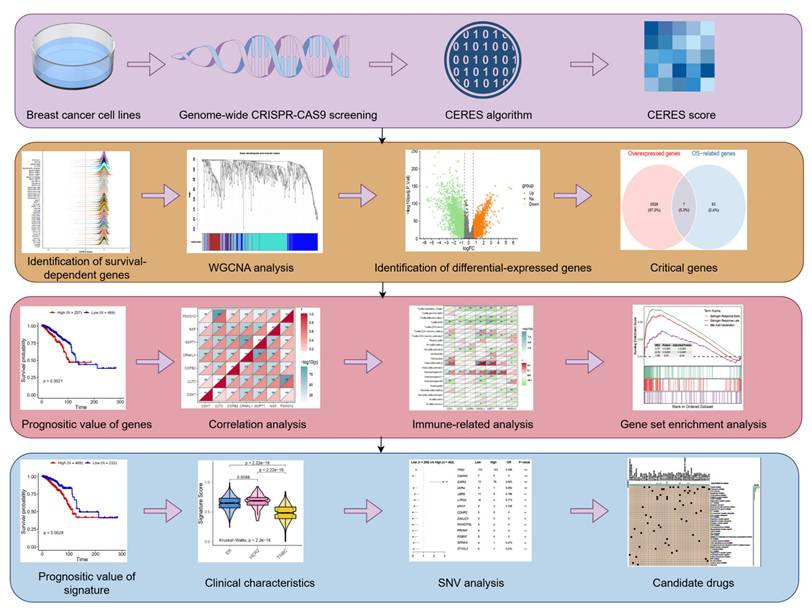
Identification of tumor dependency-related genes. (A) The peak map showing the CERES score distribution of 42 breast tumor cell lines. (B) Sample clustering. (C) Scale-free fit index for various soft-thresholding powers. Mean connectivity for various soft-thresholding powers (D) Dendrogram of all differentially expressed genes clustered based on dissimilarity measure (1-TOM). (E) The forest map showing the prognosis of the module.
Results
Identification of tumor proliferation-associated genes
Genome-wide CRISPR-Cas9 knockout data from 42 breast cancer cell lines were analyzed using the CERES algorithm, yielding a CERES matrix of 17,386 genes. Through stringent filtering (CERES score < -1 in ≥75% of cell lines), 698 tumor proliferation-associated genes were identified (Figure 2A). Weighted gene co-expression network analysis (WGCNA) was subsequently performed on 721 samples to identify genes linked to overall survival. After hierarchical clustering and removal of 17 outlier samples (Figure 2B), an unsigned topological overlap matrix was constructed with a soft thresholding power of 5 (scale-free R² = 0.9; Figure 2C). Three gene modules were defined (minimum module size: 30 genes; Figure 2D, Table S1). Univariate Cox regression revealed that the blue module (N=275) significantly correlated with poor prognosis (P<0.05; Figure 2E). Taken together, 275 genes were prioritized as tumor proliferation-associated candidates for further investigation.
Prognostic validation of seven tumor proliferation-associated genes
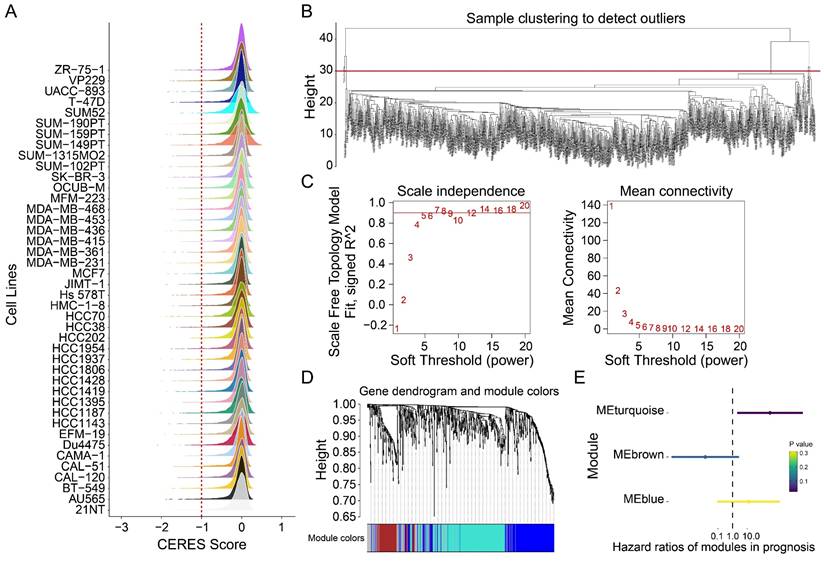
Univariate Cox regression of the 275 candidate genes identified 42 genes significantly associated with adverse outcomes (Figure 3A). Differential expression analysis between 721 tumor and 113 normal tissues revealed 2,535 upregulated genes (log2FC > 0.585, P<0.05; Figure 3B). Intersection analysis identified seven genes (CDK7, CLTC, COPB2, CRNKL1, GSPT1, NSF, PSMD12) that were both upregulated and prognostically significant (Figure 3C). Kaplan-Meier analysis confirmed their strong association with reduced overall survival (Figures 3D-J; CDK7: P=0.0013; CLTC: P=0.0019; COPB2: P=0.0021; CRNKL1: P=0.0049; GSPT1: P=0.011; NSF: P=0.0084; PSMD12: P=0.00096). Notably, these genes exhibited a strong co-expressed pattern (Figure 3K). Taken together, these findings suggest that high expression of these seven genes in tumors is associated with poor prognosis and may be potential prognostic markers and therapeutic targets for BRCA.
The prognostic value of candidate genes. (A) The forest map showing the prognosis of 42 tumor proliferation-related genes. (B) Volcanic plot exhibiting differential expressed genes (DEGs). (C) Venn plot showing the intersection of two gene sets. (D-J) Kaplan-Meiers curves of CDK7 (D), CLTC (E), COPB2 (F), CRNKL1 (G), GSPT1 (H), NSF (I), and PSMD12 (J). (K) Heatmap showing the correlations among candidate genes. **P<0.01.
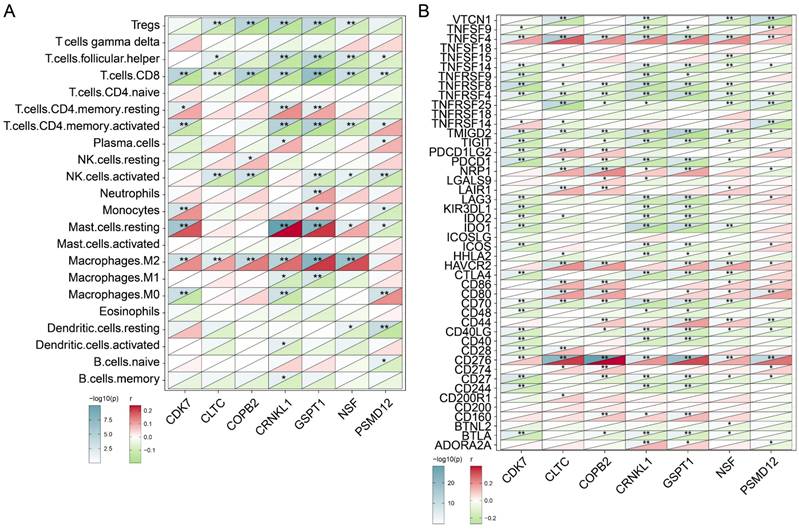
Correlation between candidate tumor proliferation-related genes and immune infiltration
The above findings have initially confirmed the prognostic role of the seven genes we identified, and we next analyzed their association with immune infiltration. CIBERSORTX was used to assess the levels of infiltration of various immune cells in BRCA samples and to analyze its correlation with the expression levels of seven genes. As shown in Figure 4A, we observed that the expression level of these seven genes was significantly negatively correlated with the infiltration level of CD8 T cells (P<0.01). Besides CDK6 (P<0.01), CLTC (P<0.01), COPB2 (P<0.01), CRNKL1 (P<0.01), GSPT1 (P<0.01), NSF (P<0.01) showed significant positive correlation with the abundance of M2 macrophages. The results suggested that the infiltration level of these genes may be related to the formation of immunosuppressive microenvironment of tumors. Subsequently, we analyzed the correlation of these seven genes with the level of immune checkpoint molecular expression. As shown in Figure 4B, the expression levels of TNFSF4 and CD276 were significantly positively correlated with expression levels of the above candidate genes. In contrast, the expression levels of TNFRSF4 and TMIGD2 were negatively correlated with the expression levels of these genes. These findings indicated that the immune checkpoint molecules TNFSF4 and CD276 may benefit patients with high levels of tumor proliferation genes. In a word, these analyses initially revealed subtle links between the genes we identified that are associated with tumor proliferation and immune cells and immune checkpoint molecules.
Identification of potential signaling pathways of candidate tumor proliferation genes
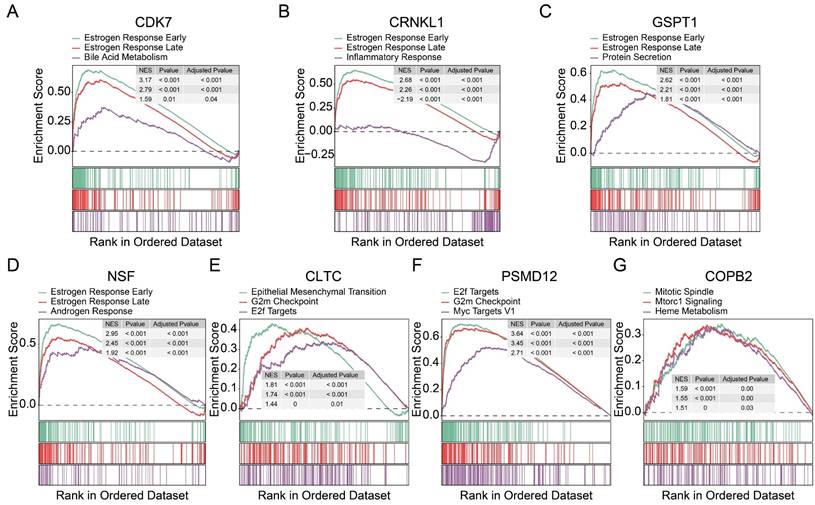
To further identify the pathways associated with these proliferation-related genes, we performed a gene set pathway analysis for each gene separately. Interestingly, we found that the expression levels of CDK7 (Figure 5A), CRNKL1 (Figure 5B), GSPT1 (Figure 5C), and NSF (Figure 5D) were significantly correlated with the estrogen pathway. The estrogen pathway has been proved to be a classic pathway to maintain the proliferation of breast cancer cells. CLTC (Figure 5E) and PSMD12 (Figure 5F) were significantly correlated with G2M checkpoint and E2F target channels. The expression level of COPB2 was also significantly correlated with the mitotic spindle (Figure 5G) pathway. These findings support that these genes are involved in tumor proliferation. At the same time, these results also preliminarily reveal the possible mechanism of action of these genes.
Construction of a signature related to tumor proliferation
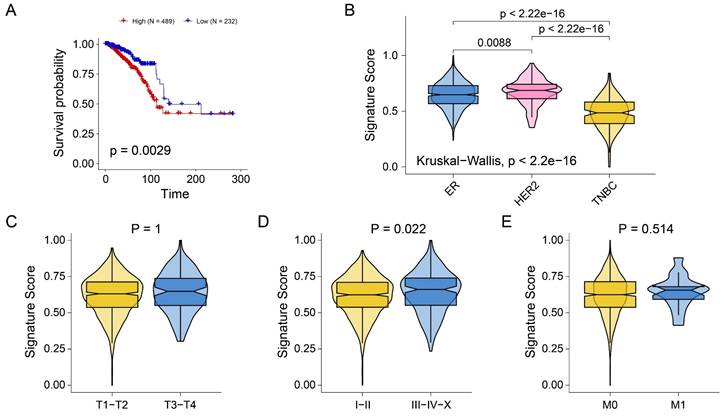
The above analysis preliminarily revealed the role of seven genes in prognosis and immune infiltration. Next, we attempted to construct a gene tag based on seven gene compositions to assess individualized tumor proliferation status. The ssGSEA algorithm was used to infer the proliferation-related scores of each BRCA sample according to the expression levels of seven genes. As shown in Figure 6A, signature scores were significantly associated with poor prognosis in BRCA patients (P=0.0029). Interestingly, we observed that signature scores were significantly higher in ER and HER2 breast cancers than in TNBC (Figure 6B). The results showed consistency with the results of previous pathway analysis. In addition, no significant difference in signature scores was observed across stage T (Figure 6C) and stage M (Figure 6E). However, the signature scores in stages I and II were significantly lower than those in stages III-IV-X (Figure 6D). The results indicated that the signature score may be primarily driven by pathways more active in hormone receptor-positive or HER2 subtypes. These findings implied that signature scores are associated with breast cancer prognosis, molecular typing, and tumor stage, and may serve as a novel clinical stratification tool.
Exploring the correlations between tumor dependency genes and immune infiltration. (A) Heatmap exhibiting the correlations between candidate genes and 22 distinct immune cell infiltration. (B) Heatmap exhibiting the correlations between candidate genes and immune checkpoints. *P< 0.05, **P<0.01.
Exploration of potential mechanism of 7 tumor dependency genes. (A-G) Gene set enrichment analyses of CDK7 (A), CRNKL1 (B), GSPT1 (C), NSF (D), CLTC (E), PSMD12 (F), and COPB2 (G).
Correlation between signature score and somatic mutations
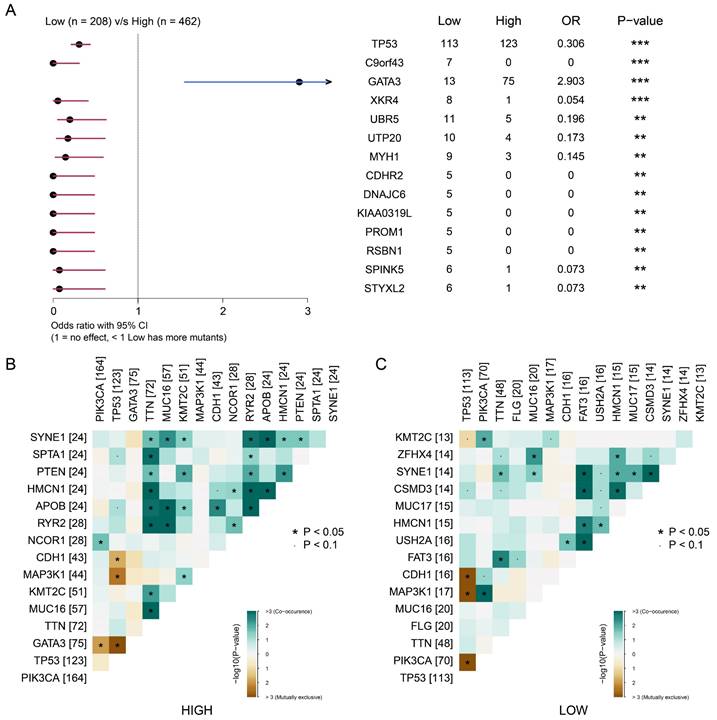
We then explored the correlation between somatic mutations and tumor proliferation. We divided the samples into high signature group and low signature group according to the signature score and the best cut-off value. As shown in Figure 7A, we observed that the high signature group was significantly associated with somatic mutations in GATA3. The low signature group was significantly associated with somatic mutations of TP53, C9orf43, XKR4 and other genes. This suggests that somatic mutations of GATA3 may be related to BRCA proliferation. Next, we analyze the relationship between signature scores and mutation patterns. As shown in Figure 7B-C, the group with a high signature score showed a more obvious co-mutation pattern than the group with a low signature score, while no significant difference was found in co-rejection between the two groups. These results reveal a potential link between tumor proliferation-associated signature and somatic cells, and suggest that mutations in GATA3, as well as co-mutation patterns, may be associated with breast cancer proliferation.
Drug identification based on proliferation-related signature
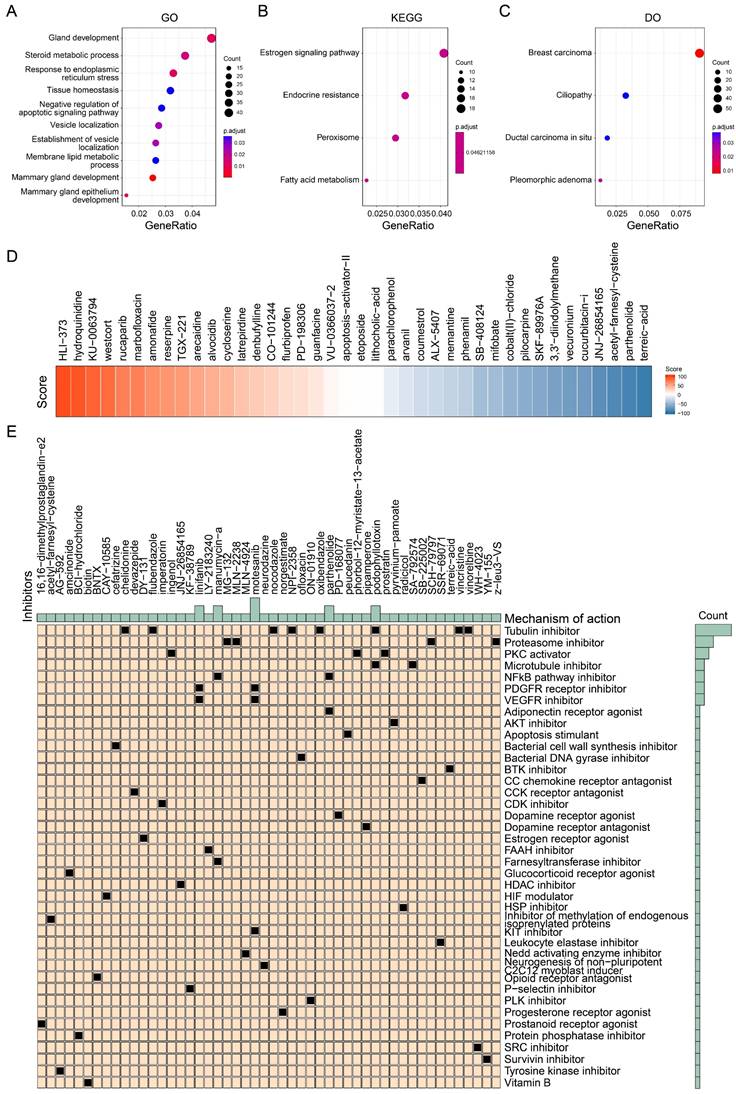
We further analyzed the pathways associated with the signature scores to screen potential drugs. We analyzed the difference between the group with a high signature score and the group with a low signature score according to the previous grouping results, and extracted the top 1500 genes in the group with a high signature score for enrichment analysis. As shown in Figure 8A, GO analysis showed that these genes were associated with the negative regulation of apoptosis signaling pathways and breast development. KEGG analysis showed that these genes were associated with the estrogen signaling pathway and endocrine resistance (Figure 8B). DO analysis showed that these genes were most associated with breast cancer (Figure 8C). These findings are consistent with the results we obtained above. Because of the poor prognosis in the group with a high signature score, we further screened drugs for patients in the group with a high signature score. More than 2400 compounds and their scores were obtained from the CMAP database. As shown in Figure 8D, some of the drugs and their scores were showed. It should be noted that the lower the score, the closer the compound's action is to that of an inhibitor. Subsequently, we screened the 50 compounds with the lowest scores as drug candidates for patients with high signature scores (Figure 8E, Table S2). In summary, we screened drugs that are potentially suitable for patients with high tumor proliferation signature scores, providing a new idea for individualized treatment of BRCA.
Discussion
In this study, by integrating CRISPR-CAS9 functional genomics (DEPMAP database) and TCGA multi-omics data, seven key genes (CDK7, CLTC, COPB2, CRNKL1, GSPT1, NSF and PSMD12) that are closely related to the proliferation and prognosis of breast cancer were systematically identified. A gene signature based on these genes were constructed. The signature can not only effectively distinguish the survival risk of patients, but also significantly associated with the tumor immune microenvironment, molecular typing and somatic mutation characteristics, providing a novel intervention strategy for the individualized treatment of breast cancer.
Construction of tumor proliferation-related gene signature. (A) Kaplan-Meiers curves showing the prognostic value of signature. (B-E) Boxplots showing the signature score in molecular subtypes (B), T stage (C), tumor stage (D), and M stage (E).
In this study, seven genes were found to be significantly overexpressed in breast cancer and associated with poor prognosis in patients, suggesting that they may act as oncogenic genes to drive tumor progression. For example, CDK7 is a core kinase in cell cycle regulation, which can promote tumor proliferation and maintain tumor stemness by activating CDK-RB-E2F, YAP, and other mechanisms, and has been confirmed as a therapeutic target for many cancers [16, 17]. As a proteasome subunit, PSMD12 is involved in the regulation of protein degradation, and its elevated expression inhibits apoptosis signaling pathways to maintain the survival of breast cancer cells [18]. In addition, COPB2 has been reported to promote proliferation in triple-negative breast cancer [19]. Long et al. [20] demonstrated that the absence of GSPT1 inhibits the proliferation and migration of colon cancer cells in vitro. These reports are consistent with our findings. It is worth noting that these seven genes are significantly co-expressed in breast cancer, and whether these genes form a complex network to promote breast cancer progression remains to be verified by further experiments. Immune infiltration is an important indicator of tumor response to treatment [21, 22]. Thus, we analyzed the correlations between gene expression and immune infiltration. The expression levels of the seven genes were negatively correlated with CD8+ T cell infiltration, but positively correlated with M2 macrophage infiltration, suggesting that they may promote immune escape by reshaping the immunosuppressive microenvironment. M2 macrophages inhibit T cell activity by secreting IL-10 and TGF-β, while promoting angiogenesis and metastasis [23]. In addition, the gene signature scores were significantly positively correlated with immune checkpoint molecules CD276 (B7-H3) and TNFSF4 (OX40L), further supporting the existence of an immunosuppressive microenvironment. CD276 has been reported to be significantly negatively associated with immune infiltration of CD8+ T cells in breast cancer [24]. The OX40/OX40L (TNFSF4) signaling pathway has also been reported to promote T cell activation and immune stimulation [25]. These findings provide a theoretical basis for the combination of targeting tumor proliferation genes and immune checkpoint inhibitors.
Further analysis showed that the constructed seven-gene signature was significantly overexpressed in ER+/HER2+ subtypes and correlated with advanced tumor stage, suggesting that it may be used as a potential prognostic marker for hormone receptor-positive breast cancer. The group with high signature scores was enriched in estrogen signaling pathways and G2M checkpoints, which is consistent with the classical mechanism of estrogen-dependent proliferation in ER+ breast cancer [26]. Notably, co-mutation patterns were more common in the high-signature group than in the low-signature group, suggesting a potential correlation between tumor proliferation characteristics and somatic co-mutation. These findings provide a new perspective for the comprehensive assessment of the proliferation drivers of breast cancer cells. Finally, we screened drugs suitable for patients with high signature scores, providing new ideas for individualized treatment and combination therapy for breast cancer patients.
The focal somatic mutations were associated with the signature score based on tumor dependency genes. (A) Forest plot demonstrating focal somatic single nucleotide variant (SNV) and copy-number alteration (CNA) frequencies that are significantly different between patients in the high- and low- signature groups. OR>1, higher SNV frequency for patients in the high signature group. OR<1, higher SNV frequency in low signature group. **P<0.01, ***P<0.001 (B-C) Landscape of gene co-mutations in patients with high- (B) and low- (C) signature scores.
Identification of potential drugs suitable for high signature score patients. (A) Gene ontology enrichment analysis of top 1500 genes of high signature score patients. (B) KEGG enrichment analysis of top 1500 genes of high signature score patients. (C) Disease ontology analysis of top 1500 genes of high signature score patients. (D) Heatmap showing the several drugs and their score for examples. (E) The potential drugs identified for high signature score patients.
Some limitations of the study also need to be clarified. First, CRISPR-CAS9 screening data are based on only 42 breast cancer cell lines, which may not fully reflect the heterogeneity of clinical tumors. Second, the prognostic value of the gene signature needs to be validated in prospective cohorts. Third, the sample size of bulk RNA cohort is limited, and more external datasets need to be included to reduce the bias of analyses. Additionally, the oncogenic mechanisms and drug sensitivity of candidate genes still need to be confirmed by in vivo and in vitro experiments. Future studies could be combined with single-cell sequencing technology to analyze the spatial heterogeneity of the gene signature in the tumor microenvironment, and could use organoid models to verify the individualized efficacy of drugs.
Supplementary Material
Supplementary tables.
Acknowledgements
We appreciate the TCGA and Depmap databases for the availability of the data. Also, we thank 'home-for-researchers' database for creating Figure 1 (ISIYA2b225).
Funding
This work was supported by National Natural Science Foundation of China (Grant Nos. 82172835, 82304025 and 82303857) and Tianjin Science and Technology Foundation (23JCQNJC00930).
Consent for publication
All authors have agreed to publish this manuscript.
Author contributions
WGX, CXX, and WX designed the study and collected research data; CJM, ZYX, WS, and LYX were responsible for the interpretation of the results. TY analyzed data and drafted the manuscript; WGX, YY, and LYX provided the overall guidance. All authors read and approved the final Manuscript.
Availability of data and materials
The public datasets were obtained from TCGA (https://www.cancer.gov/tcga), Depmap (https://depmap.org/portal/).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Giaquinto AN, Sung H, Newman LA, Freedman RA, Smith RA, Star J. et al. Breast cancer statistics 2024. CA Cancer J Clin. 2024;74:477-95
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48
3. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
5. Nolan E, Lindeman GJ, Visvader JE. Deciphering breast cancer: from biology to the clinic. Cell. 2023;186:1708-28
6. Wang G, Shi C, He L, Li Y, Song W, Chen Z. et al. Identification of the tumor metastasis-related tumor subgroups overexpressed NENF in triple-negative breast cancer by single-cell transcriptomics. Cancer Cell Int. 2024;24:319
7. Arafeh R, Shibue T, Dempster JM, Hahn WC, Vazquez F. The present and future of the Cancer Dependency Map. Nat Rev Cancer. 2025;25:59-73
8. Xiao M, Yang J, Dong M, Mao X, Pan H, Lei Y. et al. NLRP4 renders pancreatic cancer resistant to olaparib through promotion of the DNA damage response and ROS-induced autophagy. Cell Death Dis. 2024;15:620
9. Li X, Luo S, Fu W, Huang M, Huang X, Kang S. et al. Discovery of a proliferation essential gene signature and actin-like 6A as potential biomarkers for predicting prognosis and neoadjuvant chemotherapy response in triple-positive breast cancer. Cancer. 2024;130:1435-48
10. Colaprico A, Silva TC, Olsen C, Garofano L, Cava C, Garolini D. et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44:e71
11. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559
12. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47
13. Rusk N. Expanded CIBERSORTx. Nat Methods. 2019;16:577
14. Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2:100141
15. Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X. et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell. 2017;171:1437-52 e17
16. Li ZM, Liu G, Gao Y, Zhao MG. Targeting CDK7 in oncology: The avenue forward. Pharmacol Ther. 2022;240:108229
17. Lv M, Gong Y, Liu X, Wang Y, Wu Q, Chen J. et al. CDK7-YAP-LDHD axis promotes D-lactate elimination and ferroptosis defense to support cancer stem cell-like properties. Signal Transduct Target Ther. 2023;8:302
18. Du X, Shen X, Dai L, Bi F, Zhang H, Lu C. PSMD12 promotes breast cancer growth via inhibiting the expression of pro-apoptotic genes. Biochem Biophys Res Commun. 2020;526:368-74
19. Wu W, Wang C, Wang F, Wang Y, Jin Y, Luo J. et al. Silencing the COPB2 gene decreases the proliferation, migration and invasion of human triple-negative breast cancer cells. Exp Ther Med. 2021;22:792
20. Long X, Zhao L, Li G, Wang Z, Deng Z. Identification of GSPT1 as prognostic biomarker and promoter of malignant colon cancer cell phenotypes via the GSK-3beta/CyclinD1 pathway. Aging (Albany NY). 2021;13:10354-68
21. Wang Y, Song W, Li Y, Liu Z, Zhao K, Jia L. et al. Integrated analysis of tumor microenvironment features to establish a diagnostic model for papillary thyroid cancer using bulk and single-cell RNA sequencing technology. J Cancer Res Clin Oncol. 2023;149:16837-50
22. Chen Z, Yang C, Wang J, Wang B, He L, Liu Z. et al. Integrating of Radiomics and Single-cell Transcriptomics Uncovers the Crucial Role of gammadelta T Cells in Lung Adenocarcinoma. J Cancer. 2025;16:1167-80
23. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F. et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425-40
24. Kim NI, Park MH, Kweon SS, Lee JS. B7-H3 and B7-H4 Expression in Breast Cancer and Their Association with Clinicopathological Variables and T Cell Infiltration. Pathobiology. 2020;87:179-92
25. Thapa B, Kato S, Nishizaki D, Miyashita H, Lee S, Nesline MK. et al. OX40/OX40 ligand and its role in precision immune oncology. Cancer Metastasis Rev. 2024;43:1001-13
26. Miziak P, Baran M, Blaszczak E, Przybyszewska-Podstawka A, Kalafut J, Smok-Kalwat J. et al. Estrogen Receptor Signaling in Breast Cancer. Cancers (Basel). 2023;15(19):4689
Author contact
![]() Corresponding authors: Prof. Xuchen Cao, the First Department of Breast Cancer, Tianjin Medical University Cancer Institute and Hospital, Huan-Hu-Xi Road, He-Xi District, Tianjin 300060, China. Tel: 86-022-23340123 ext. 2084, E-mail: caoxuchenedu.cn. Prof. Xin Wang, the First Department of Breast Cancer, Tianjin Medical University Cancer Institute and Hospital, Huan-Hu-Xi Road, He-Xi District, Tianjin, 300060, E-mail: wangxincom.
Corresponding authors: Prof. Xuchen Cao, the First Department of Breast Cancer, Tianjin Medical University Cancer Institute and Hospital, Huan-Hu-Xi Road, He-Xi District, Tianjin 300060, China. Tel: 86-022-23340123 ext. 2084, E-mail: caoxuchenedu.cn. Prof. Xin Wang, the First Department of Breast Cancer, Tianjin Medical University Cancer Institute and Hospital, Huan-Hu-Xi Road, He-Xi District, Tianjin, 300060, E-mail: wangxincom.

 Global reach, higher impact
Global reach, higher impact