Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(10):2928-2939. doi:10.7150/jca.94142 This issue Cite
Research Paper
Mendelian randomization combined with multi-omics explores the relationship between heart failure and cancer
1. Hubei provincial key laboratory of diabetic cardiovascular diseases, Xianning Medical College, Hubei University of Science and Technology, Xianning 437100, Hubei, People's Republic of China.
2. School of Basic Medical Sciences, Xianning Medical College, Hubei University of Science and Technology, Hubei University of Science and Technology, Xianning 437100, Hubei, People's Republic of China.
3. School of Pharmacy, Xianning Medical College, Hubei University of Science and Technology, Xianning 437100, Hubei, People's Republic of China.
4. School of Nuclear Technology and Chemistry & Biology, Hubei University of Science and Technology, Xianning 437100, Hubei, People's Republic of China.
Received 2024-1-10; Accepted 2024-2-27; Published 2024-3-25
Abstract
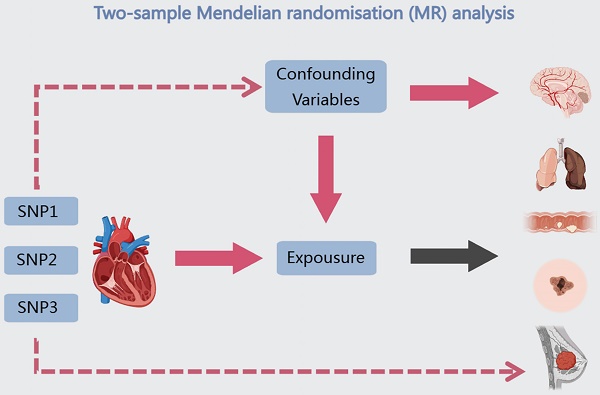
Background: Whether there is an association between HF (HF) and cancer has not been conclusively established, and it is not clear whether patients with cancer can share similar hospitalization strategies and outcomes with patients with HF.
Methods: Genome-wide association summary statistics were performed using a two-sample Mendelian randomization (MR) method for HF patients and cancer patients from the GWAS directory, with co-localization and Summary Data-Based Mendelian Randomization (SMR) analyses to identify HF-associated genes, and transcriptomic analyses to analyze the roles of these genes in the clinical diagnosis and targeted therapies of multiple cancer types.
Results: Two-sample MR analysis showed that increased risk of HF was associated with decreased risk of cervical, brain, breast, colorectal, lung, and skin cancers, and co-localization combined with SMR analysis identified ABO and SURF1 as HF-associated genes, and transcriptomic analyses showed that ABO is a risk factor for HF and a protective factor against cancer, whereas SURF1 is a protective factor against HF and a protective factor against cancer.
Conclusion: There was no causal relationship between heart failure and cancers (Cervical, brain, breast, colorectal, lung and skin cancers) risk factors, however there was a trend toward a negative causal relationship between heart failure and cancers (Cervical, brain, breast, colorectal, lung and skin cancers) occurrence.
Keywords: HF, cancer, MR, co-localisation analysis, SMR, transcriptomic analyses
1. Introduction
The International Agency for Research on Cancer (IARC), a cancer agency of the World Health Organization, has released updated estimates of the global burden of cancer as of 1 February 2024. Around 20 million new cancer cases and 9.7 million deaths are expected in 2022. With approximately 53.5 million survivors within five years of cancer diagnosis, about one fifth of people will develop cancer in their lifetime, and about one-ninth of men and one-two-twelve of women will die from cancer. An increasing number of new cases of cancer are projected to surpass 35 million globally by 2050. In this battle without smoke, cancer has become one of the world's biggest health and lifespan "killers"[1]. The worldwide prevalence of HF is estimated to range between 1 and 2 percent [2]. HF, often known as the "cardiovascular cancer," is a severe form of many cardiovascular diseases. It is characterized by high mortality and morbidity rates [3]. According to the clinical consensus released by the European Society of Cardiology HF Association (ESC-HFA) in 2023, the in-hospital mortality rate for patients with HF and reduced ejection fraction (HFrEF) was recorded at 3.4%, while those with HF and mid-range ejection fraction (HFmrEF) had a mortality rate of 2.1%. Additionally, patients with HF and preserved ejection fraction (HfpEF) exhibited an in-hospital mortality rate of 2.2%. The respective annual hospitalization rates for these groups were reported as follows: HFrEF (48%), HFmrEF (35%), and HfpEF (42%). According to the American Heart Insufficiency Association, there are almost 6.7 million adults aged 20 and above in the United States who experience this condition during their lifetime [4]. The China Cardiovascular Health and Disease Report 2020, released by the National Centre for Cardiovascular Disease, indicates that over 3.3 million individuals have been diagnosed with heart disease, while around 8.9 million individuals are suffering from severe insufficiency. The five-year mortality rate for HF exceeds that of many common malignancies, such as prostate cancer and bladder cancer, by up to 50%. Moreover, based on a survey carried out in six cities in China, the incidence of age-specific HF is 1.1 percent, impacting approximately 12.1 million urban-central persons aged 25 and above who experience cardiac insufficiency [5]. While there has been an advancement in the uniformity of treatment for patients with HF, the rates of death and readmission to the hospital continue to be significant, resulting in substantial financial strain on the healthcare system.
Although cancer and HF are distinct medical conditions, they may occasionally manifest similar symptoms including but not limited to weight loss, depressive symptoms, appetite changes, and weakness [6]. They also exhibit a number of additional risk factors in common, such as aging, obesity, smoking, hypertension, diabetes [7-9], and excessive alcohol consumption [10], genetic predisposition, compromised cardiopulmonary function, and drug toxicity [11]. In conjunction with extended periods of inactivity, excessive tension, and prolonged sleep, these elements contribute to a heightened mortality rate and an increased financial strain on the healthcare system.
Researchers are employing medical epidemiological research methodologies to investigate the population distribution and influential variables of HF and cancer, aiming to elucidate the potential correlation between the two.An exemplary example is the Golestan cohort study, which was published in BMC Medicine and has a sample size exceeding 50,000 participants. This investigation confirmed the link between ABO blood type and the vulnerability to death associated to cardiovascular disease. Furthermore, it investigated, for the initial instance, the correlation between cancer and fatality. Studies have shown that people with blood types A and B have a higher likelihood of developing gastric cancer, while non-O blood types are closely linked to increased rates of overall mortality and mortality from cardiovascular disease [12, 13]. Liang Chang and colleagues identified a notable association between blood type A and the incidence of malignant melanoma of the skin, in contrast to blood type O [14]. These findings indicate a novel association between ABO blood type and the occurrence of cancer and heart disease. Moreover, accumulating evidence supports the substantial role of glomerular dysfunction in promoting the aging process, enhancing tumor growth, and enabling the transfer of cancer cells during transplantation [15, 16]. Fibromyalgia has been identified as the main cause of the heart's inability to produce and utilize energy. This error can lead to a detrimental cycle of ventricular failure, defined by an imbalance of calcium, oxidative stress, damage caused by proteins, and death of heart muscle cells [17]. Targeting fibromyalgia directly may potentially be an effective treatment for HF [18]. SURF1 functions as a specific co-factor for phosphoric c oxidase, a protein that impedes the formation of conventional COX molecules in phosphates, hence impacting energy production. Regrettably, the current corpus of research pertaining to the correlation between SURF1 and both cancers and HF is inadequate. Therefore, additional examination and scrutiny are necessary.
Due to numerous factors, including study methodology, participant count, follow-up duration, geographic heterogeneity, demographic characteristics, and the specific types of cancer under investigation, the association between HF and the risk of developing cancer remains a topic of ongoing controversy [19]. Several studies have shown that HF is associated with an increased risk of malignancy [20-23]. Nevertheless, Irene Fernández-Ruiz and colleagues, after analyzing numerous factors, demonstrated that HF is not associated with general cancer, localized cancer or cancer-specific mortality [24, 25]. Notable advancements have been achieved in cancer and HF research in the past few decades. Nevertheless, the existing research is constrained by the drawbacks of observational studies, thus it can solely establish a correlation between HF and a heightened susceptibility to cancer. Consequently, it is not feasible to definitively elucidate whether there exists a direct causal and biological connection between the two ailments. MR is an epidemiological research method that utilizes genetic information to assess causal relationships between variables. By using genetic variation as an instrumental variable, MR avoids confounding factors and provides reliable causal relationships. Figure 1 depicts the study design, which employs a double sample MR approach to gather genetic variation data from extensive genomic databases, such as the People's Genome Programme and the British Biological Bank. The main objective of this study is to investigate the relationship between cancer and hyperlipidemia through co-localization analysis, SMR analysis and transcriptional analysis. The results are expected to deepen our understanding of the relationship between hyperlipidemia and cancer, reveal common risk factors, lay the foundation for developing preventive measures, and provide direction for the use of magnetic resonance technology in disease investigation.
2. Methods
2.1 Two-sample MR analyses
Our method of choice for examining the causal link between cancer and HF was a two-sample MR analysis. In this analysis, single nucleotide polymorphisms (SNPs) were employed as instrumental factors, cancer was the outcome variable, and HF was the exposure variable of interest. Based on the following hypotheses, two-sample MR analyses were performed: (I) the instrumental variables were highly correlated with the risk of HF; (II) the instrumental variables only increased the risk of cancer by influencing the risk of HF; and (III) the instrumental variables were unaffected by confounders. Genome-wide association summary information for 47,309 HF patients and 37,976 cancer patients (all European patients) was gathered from the GWAS catalog (https://www.ebi.ac.uk/gwas/). Summary association statistics for the exposure factors were derived using HF-specific GWAS data (ebi-a-GCST00009541), which included 47,309 HF patients and 93,0014 controls with a total of 7,773,021 SNPs (Table 1). Pooled association statistics for six different cancer types-cervical, brain, breast, colorectal, lung, and skin cancers-were included in the cancer-specific GWAS data and were utilized as pooled association statistics for the outcome variable. We used a range of MR techniques, including Egger regression, weighted median, inverse variance weighting (IVW), basic and weighted models, to conduct robust assessments of causation. The criterion of a P value <0.05 produced by at least one MR method was used to establish the significance of the etiological influence of HF on cancer. We adopted a criterion of P<1×10-8 to identify genetic variations linked to the risk of HF, based on prior research.
The investigation's design is depicted using a flow diagram.
A summary of the data for Mendelian randomization analysis
| id | trait | nsnp | ncase | sample_size | ncontrol | population | year |
|---|---|---|---|---|---|---|---|
| ebi-a-GCST009541 | HF | 7773021 | 47309 | 977323 | 930014 | European | 2020 |
| ieu-b-4954 | Lung cancer | 11078115 | 2671 | 374687 | 372016 | European | 2021 |
| ieu-b-4965 | Colorectal cancer | 11738639 | 5657 | 377673 | 372016 | European | 2021 |
| ieu-b-4876 | Cervical cancer | 8506261 | 563 | 199086 | 198523 | European | 2021 |
| ieu-a-1168 | Breast cancer (GWAS) | 13011123 | 15748 | 33832 | 18084 | European | 2015 |
| ukb-d-C_SKIN | Cancer of skin | 13586589 | 16531 | 361194 | 344663 | European | 2018 |
| ieu-b-4875 | Brain cancer | 8629116 | 606 | 372622 | 372016 | European | 2021 |
2.2 SMR analysis based on pooled data
The SMR approach serves as a robust mechanism for consolidating findings from Genome-Wide Association Studies (GWAS) and Expression Quantitative Trait Loci (eQTL) inquiries. Its primary function is to evaluate the multifaceted relationships inherent in the interplay between gene expression levels and the complex traits that are the subject of study. By utilizing this method, researchers can investigate whether SNPs exert their influence on phenotypes through the mediation of gene expression. Gene therapy targets for a wide range of complicated disorders, including diabetes and coronary heart disease, have been identified using this technique on a large scale. The SMR database (https://yanglab.westlake.edu.cn/) MR provided the "# V8 release of the GTEx eQTL/sQTL summary data" that were used in this investigation.
2.3 MCA
MCA is a method used to investigate the interrelationships and mechanisms of several genetic variants or biomarkers involved in the progression of a shared disease. This analysis involves examining the MCA of these variants or biomarkers to get insights into their potential interactions and contributions to the disease. A comprehensive collection of information on all eQTL snp, encompassing both cis and trans snp, was achieved by integrating eQTL data and GWAS data derived from multiple tissues. When the co-localization of the GWAS signaling pathway with an eQTL is observed, it suggests that the GWAS locus may influence the expression phenotype of the target gene. The identification of the target gene at the HF risk locus is determined by the Log Bayes Factor (LBF) value. A higher LBF value indicates that the target gene is more likely to be associated with the risk locus for HF. In instances when a larger LBF is observed, it indicates a more robust correlation between the locus and the gene. In this study, we utilized GWAS data pertaining to HF, specifically the dataset labeled "ebi-a-GCST00009541." Additionally, we incorporated eQTL data from the Genotype-Tissue Expression (GTEx) project, which encompasses whole blood and whole brain samples. Specifically, we focused on GTEx data that pertained to blood and cardiac tissues, encompassing myocardial tissues, atria, ventricles, atrioventricular node, sinus node, and heart valves. The GTEx project can be accessed at https://www.gtexportal.org/home/index.html. Co-localization analysis was conducted to assess the risk of HF using the coloc R program, specifically focusing on significant MR findings.
2.4 Transcriptomics analysis
We conducted an analysis of the expression profiles of cancer risk genes using transcriptomics data obtained from The Cancer Genome Atlas (TCGA) database, specifically utilizing RSEM-standardized RNASeq gene expression profiles. The TCGA database can be accessed at https://gdc.cancer.gov/.
3. Results
3.1 MR Analysis Shows Inverse Risk Trend, But No Causal Relationship, Between Hypertension and Cancer
Two-sample MR analysis were conducted using SNPs as instrumental variables. The exposure of interest was HF, whereas the outcome was cancer. Summary association statistics for the exposure were derived from GWAS data pertaining to HF, whereas summary association statistics for the result were obtained from GWAS data specific to each cancer type.
Cervical cancer is a malignancy that arises in the vaginal portion of the uterus and the cervical canal in females. It ranks among the most prevalent malignant tumors affecting women, occupying the second position in terms of incidence among female malignancies. Globally, approximately 400,000 women receive a diagnosis of invasive cervical carcinoma annually, with an estimated 200,000 succumbing to the disease [26]. The dataset for the GWAS pertaining to cervical cancer comprised a total of 563 individuals diagnosed with cancer and 198,523 individuals serving as controls. This dataset encompassed 9,822,229 SNPs, as indicated in Table 1. The findings from Mendelian randomization analyses showed a possible negative effect of HF risk on cervical cancer risk (PMR-Egger=0.145, Pweighted-median=0.415, PIVW=0.367, Psimple-mode=0.87 and Pweighted-mode=0.244) (Table S1), and assessment of heterogeneity showed little evidence of correlation (QMR-Egger=1.01 and P=0.985, QIVW=5.21 and P=0.634) (Table S1). Furthermore, the results of the horizontal pleiotropy studies provided limited support for the notion that pleiotropy was linked to this particular connection (P=0.086) (Table S1). Brain cancer, referred to as intracranial tumors in medical terminology, is characterized by the growth of neoplastic organisms within the cranial cavity. These tumors can originate from various sources such as the brain, meninges, nerves, blood vessels, and cerebral appendages. Additionally, they can also be formed through the metastasis of malignant tumors from other tissues or organs in the body to the cranium. The prolonged presence of brain cancer can lead to several detrimental effects including increased intracranial pressure, displacement of brain tissues, visual impairments, paralysis, psychiatric disorders, cerebral hernia, cerebral hemorrhage, and potentially even death [27]. The 8,629,116 SNPs covered by the 606 cancer patients and 372,061 controls in the GWAS data for brain cancer are shown in Table 1. Mendelian randomization analyses suggest that HF risk may have a negative effect on brain cancer risk (PMR-Egger=0.461 Pweighted-median=0.063, PIVW=0.069, Psimple-mode=0.186 and Pweighted-mode=0.172) (Table S1), with assessment of heterogeneity showing little evidence of correlation (QMR-Egger=3.74 and P=0.711, QIVW=3.80 and P=0.802) (Table S1). Moreover, there was minimal indication that pleiotropy was connected to the relationship in horizontal pleiotropy studies (P=0.816) (Table S1). Breast cancer can occur in men as well as women and is a malignant tumor originating from the mammary epithelium or ductal epithelium, primarily due to abnormal mammary cell growth, and it is one of the most heterogeneous and frequently diagnosed cancers among women worldwide [28]. The breast cancer GWAS data includes 15,748 cancer patients and 18,084 controls, with 13,011,123 SNPs included (Table 1). Mendelian randomization analyses suggest that HF risk may have a negative effect on breast cancer risk (PMR-Egger=0.467, Pweighted-median=0.607, PIVW=0.605, Psimple-mode=0.699 and Pweighted-mode=0.528) (Table S1). The study of heterogeneity revealed no indication of association (QMR-Egger=72.5 and P=0, QIVW=83.1 and P=0) (Table S1). Furthermore, horizontal pleiotropy tests revealed no indication of pleiotropy being related to this connection (P=0.346; Table S1). Colorectal cancer, which accounts for 11% of all cancer diagnoses, is a malignant tumor that begins in the mucosal epithelium or glands of the large intestine. The colorectal cancer GWAS data contained 5,657 cancer patients and 372,016 controls, totaling 11,738,639 SNPs (Table 1). Mendelian randomization analyses suggest that HF risk may have a negative effect on colorectal cancer risk (PMR-Egger=0.806, Pweighted-median=0.839, PIVW=0.234, Psimple-mode=0.933, and Pweighted-mode=0.887) (Table S1). The study of heterogeneity revealed no indication of association (QMR-Egger=4.27 and P=0.748, QIVW=4.78 and P=0.781) (Table S1). The instrumental variables were not horizontally polytomous, according to horizontal polytomous analysis (P=0.5) (Table S1). Lung cancer is a neoplastic growth that manifests as a malignancy in the pulmonary region, originating from either alveolar or respiratory epithelial cells. On a global scale, lung cancer holds the highest prevalence and fatality rates among malignant tumors [29]. 11,078,115 SNPs were covered by 2,671 cancer patients and 372,016 controls in lung cancer (ieu-b-4954) (Table 1). Mendelian randomization analyses suggest that HF risk may have a negative effect on lung cancer risk (PMR-Egger=0.116, Pweighted-median=0.322, PIVW=0.251, Psimple-mode=0.207 and Pweighted-mode=0.308) (Table S1), with heterogeneity. The assessment showed little evidence of correlation (QMR-Egger=4.02 and P=0,QIVW=7 and P=0.778) (Table S1), whereas horizontal multivariate analyses showed the presence of heterogeneity, with instrumental variables possibly influencing the occurrence of the outcome through factors other than exposure factors (P=0.049) (Table S1). Skin cancer, which predominantly develops in sun-exposed regions of the skin, is a malignant neoplasm of skin cells. Its mortality and morbidity rates are progressively rising, rendering it a significant global public health concern [30]. Skin cancer (ukb-d-C_SKIN) included 16,531 cancer patients and 344,663 controls covering 13,586,589 SNPs (Table 1). Mendelian randomization analyses suggest that HF risk may have a negative effect on skin cancer risk (PMR-Egger=0.492, Pweighted-median=0.631, PIVW=0.778, Psimple-mode=0.500 and Pweighted-mode=0.589) (Table S1). Heterogeneity assessment showed little evidence of correlation (QMR-Egger=7.92 and P=0.34, QIVW=8.79 and P=0.36) (Table S1). In addition, horizontal pleiotropy analyses showed little evidence that pleiotropy was associated with this association (P=0.41) (Table S1).
In summary, when HF was used as the exposure factor of interest and cancer was the outcome, Mendelian randomization analyses did not reveal a significant causal effect of HF on lung, cervical, breast, brain, colorectal, or skin cancers, but there was a trend toward a reduced risk of lung, cervical, breast, brain, colorectal, or skin cancers from HF.
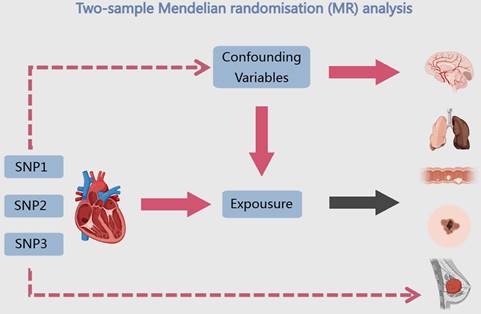
3.2 Co-localisation and SMR identify HF target genes
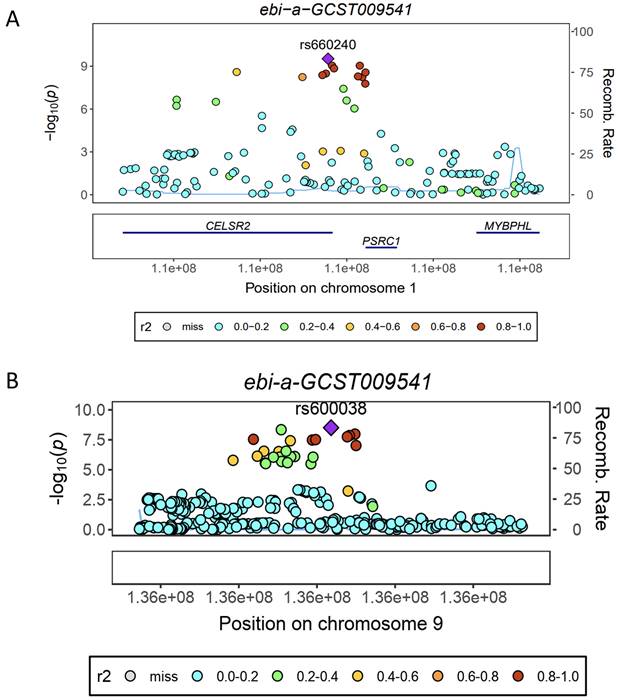
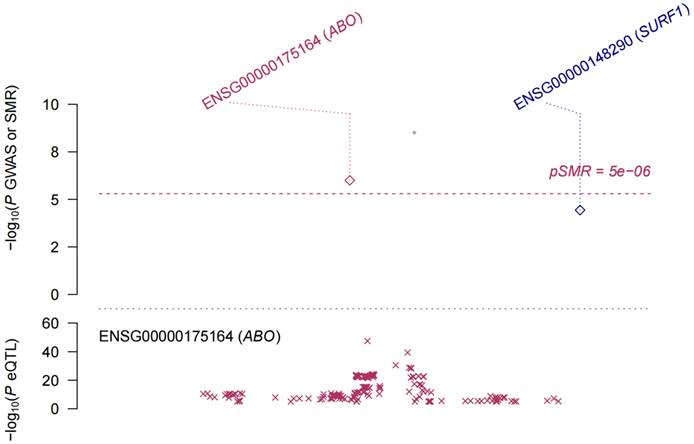
eQTL refer to genetic variations that are linked to phenotypic traits related to gene expression. In order to investigate the potential sharing of causal genetic variation between SNPs associated with HF and eQTLs, only eQTL data specifically related to HF were utilized for co-localization analysis. This analysis revealed that the occurrence of co-localization effects involving rs660240 (Figure 2A) and rs600038 (Figure 2B) significantly influenced the manifestation of HF. In the analysis of drug target genes using MR, cis-expression quantitative trait loci (cis eQTL) situated within the genomic region of drug target genes are frequently employed as proxy variables for drug target genes to exert an influence on gene expression. Subsequent investigation through co-localization analysis has revealed that a SNP identified as rs600038 has the potential to impact the ABO blood grouping system on chromosome 9 via multiple molecular pathways (Table S2). The serological Mendelian randomization study conducted for HF revealed an association with 568 SNPs, as depicted in Figure 3A. Similarly, the mitochondrial Mendelian randomization analysis for HF demonstrated an association with 8 SNPs (Figure 3B). Through the utilization of SMR analysis, the investigation identified ABO and SURF1 as genes that are associated with HF. Notably, it was observed that the effect values of ABO and SURF1 exhibited opposing directions in relation to HF (Figure 4). Additionally, Increased ABO expression was associated with an increased risk of HF (b_SMR > 0, P_HEIDI < 0.05) (Table S3). Conversely, higher levels of SURF1 gene expression were associated with a decreased risk of HF (b_SMR > 0, P_HEIDI < 0.05). These findings collectively contribute to the understanding of the initiation and progression of HF.
Analysis of co-localization in HF. (A) HF is impacted by rs660240's co-localization. (B) HF is impacted by rs600038's co-localization.
3.3 Transcriptomic analysis
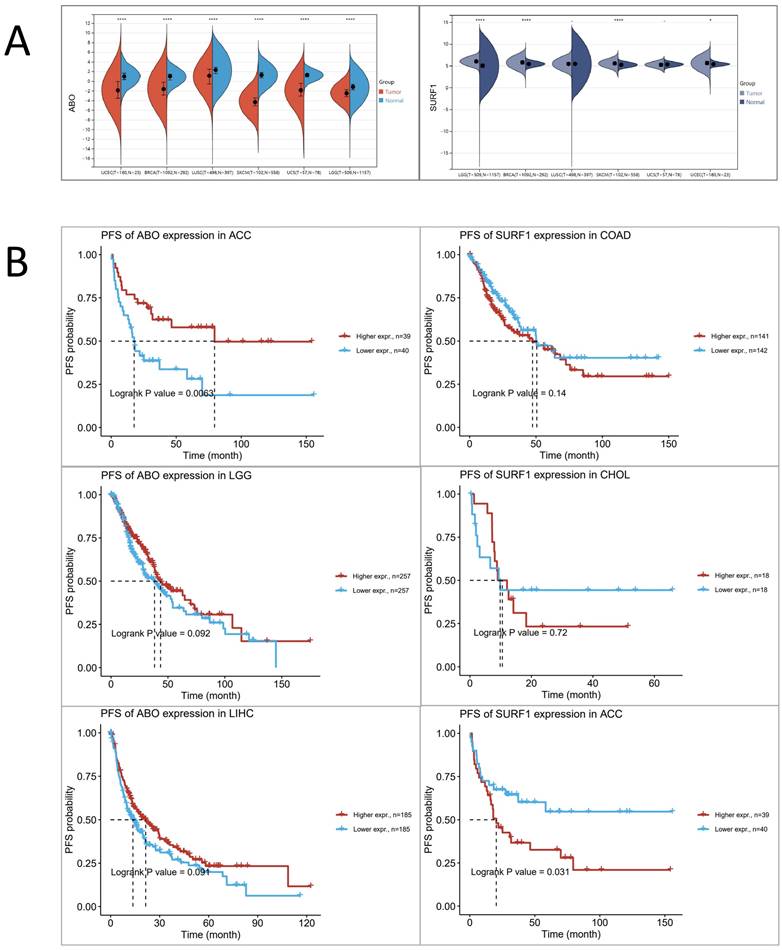
Based on the aforementioned research findings, which demonstrate a positive association between elevated levels of ABO and the susceptibility to HF, as well as a negative association between elevated levels of SURF1 and the risk of HF, we sought to explore potential associations between HF and trends in cancer risk reduction. This was accomplished by employing transcriptomics techniques to examine the functions of ABO and SURF1 in cancer. The transcriptome profiles of many tumor forms were examined, including uterine corpus endometrial carcinoma (UCEC), brain lower grade glioma (LGG), breast invasive carcinoma (BRCA), lung squamous cell carcinoma (LUSC), uterine Carcinosarcoma (UCS), and skin cutaneous melanoma (SKCM). The statistical significance of the changes in gene expression between tumor and normal samples was then assessed using unpaired Wilcoxon Rank Sum and Signed Rank Tests. The findings from the analyses of significance of difference indicate that the expression of ABO gene was considerably decreased in cases of LGG, UCEC, BRCA, LUSC, SKCM, and UCS (Figure 5A). Conversely, the expression of SURF1 gene was dramatically increased in cases of LGG, UCEC, BRCA, and SKCM. Moreover, the analysis of survival data demonstrated that elevated SURF1 expression is linked to a worse prognosis in cases with adrenocortical carcinoma (ACC). In contrast, it has been observed that low ABO expression is linked to an unfavorable prognosis in ACC (Figure 5B). A further analysis was conducted to compare the roles of SURF1 and ABO in BRCA. The results indicated that methylation levels of ABO were significantly higher in the BRCA group compared to the control group. Conversely, methylation levels of SURF1 were found to be lower in the BRCA group compared to the control group (Figure 6A). The immunoassay data indicates a favorable association between ABO and BRCA-induced activation of many immune cell types, such as Th2, Central memory, Tfh, NK, and CD4_T cells. In contrast, a negative connection was discovered between the expression of SURF1 and the activation of immune cells (Figure 6B). The results indicate that SURF1 may have a pro-oncogenic role in cancer, whereas ABO may have oncogenic properties.
4. Discussion
Cancer ranks as the second most prevalent cause of mortality globally, behind cardiovascular disease, and contributes to around 21% of all fatalities [31, 32]. In light of the shared metabolic pathways and clinical triggers between cancer and HF, a number of retrospective cohort studies, prospective cohort studies, and meta-analyses conducted in the past decade have consistently demonstrated an elevated risk of cancer and a poorer prognosis among individuals with HF, relative to the general population [33]. These findings suggest that HF may potentially serve as a carcinogenic agent and a risk factor [34, 35]. Even while cancer is notably more common in those with HF, the connection between HF and cancer is still up for discussion.
Analysis of Mendelian randomized histology. (A) Serological Mendelian randomization. (B) Mitochondrial Mendelian randomization.
The Mendelian randomization method, also known as SMR analysis, is employed in this study using pooled data. There is a positive correlation between increased levels of ABO expression and an elevated vulnerability to HF. Conversely, heightened levels of SURF1 gene expression have been discovered to be linked with a reduced risk of HF.
Based on a two-sample Mendelian randomization analysis, our results suggest that there may be a negative association between HF and a patient's susceptibility to multiple diseases, such as skin, breast, lung, brain, cervical, and colorectal cancers, but no statistically significant association was observed.
To enhance comprehension of the biological connection between the two, drug-targeting co-localization analysis and serological analysis of HF were employed. The co-localization of rs660240 and rs600038 was found to impact HF, and serological analysis of HF revealed that the SNP marker rs600038 could influence ABO gene expression via diverse protein levels. These findings were consistent with a prior investigation conducted by Hilde E. Groot et al. in the Netherlands, which determined that the ABO blood group was linked to eleven health and disease outcomes, the majority of which concerned cardiovascular conditions. The combined risk of HF was 10% greater among those with blood types A and B than among those with blood type O [36]. Furthermore, SMR analyses revealed that SURF1, apart from ABO, is also a gene associated with HF, and that increased expression of this gene was correlated with a decreased risk of developing HF. SURF1, a mitochondrial protein predominantly present in alveolar type II epithelial cells, has been identified to influence redox balance, electron transport, and the assembly and functionality of the mitochondrial respiratory chain complex, among other processes[37], while SURF1 does not have a direct impact on HF, its involvement in maintaining mitochondrial function, managing oxidative stress, regulating apoptosis, and facilitating mitochondrial autophagy contribute significantly to the development of HF. Additionally, studies have demonstrated a correlation between genetic variations in SURF1 and the occurrence of HF, pulmonary hypertension, and other related conditions [38].
Additional investigation into the functions of ABO and SURF1 in cancer has yielded noteworthy findings. Specifically, a considerable decrease in ABO expression has been observed in cases of LGG, UCEC, BRCA, LUSC, SKCM, and UCS. Conversely, there has been a significant increase in SURF1 expression in instances of LGG, UCEC, BRCA, and SKCM. Moreover, it has been observed that reduced ABO expression tends to be indicative of an unfavorable prognosis, while elevated SURF1 expression is associated with a poorer prognosis. Both hypomethylation events and hypermethylation events can lead to the occurrence of cancer. Hypomethylation affects the overall stability of the genome and activates proto-oncogenes, while hypermethylation leads to the silencing of tumor suppressor genes. This methylation phenomenon has been observed in the early stages of tumor development, even in populations susceptible to tumors [39]. Elevated methylation levels of ABO were discovered in individuals with BRCA, relative to the control group, suggesting a potential involvement of ABO as an oncogene in cancer development. Conversely, reduced methylation levels of SURF1 were detected in BRCA patients compared to the normal group, indicating a plausible function of SURF1 as an oncogene promoter in cancer. Tumor-derived exosomes have been observed to interact with various immune effector cells, such as macrophages, monocytes, T cells, natural killer (NK) cells, dendritic cells (DCs), γδ T lymphocytes, regulatory T cells (Treg), myeloid-derived suppressor cells (MDSCs), mast cells, and B cells, within the hypoxic tumor microenvironment (TME).
Analysing the expression profile of risk genes in cancer based on transcriptomics data. (A) Differential expression of ABO and SURF1 in normal and tumour samples. (B) Progression-Free Survival of ABO and SURF1.
Methylation of ABO and SURF1 in breast cancer and immunoexpression analysis. (A) The methylation levels of ABO were found to be higher in individuals with BRCA compared to those in the normal group. Conversely, the methylation levels of SURF1 were observed to be lower in individuals with BRCA compared to those in the normal group. (B) There was a favorable link observed between the ABO blood group and the activation of various immune cell types, including Cytotoxic, Th2, Central memory, Tfh, NK, and CD4_T cells, in individuals with BRCA. In contrast, the SURF1 gene exhibited an inverse relationship with the immune cell activation patterns previously identified in breast cancer (BRCA).
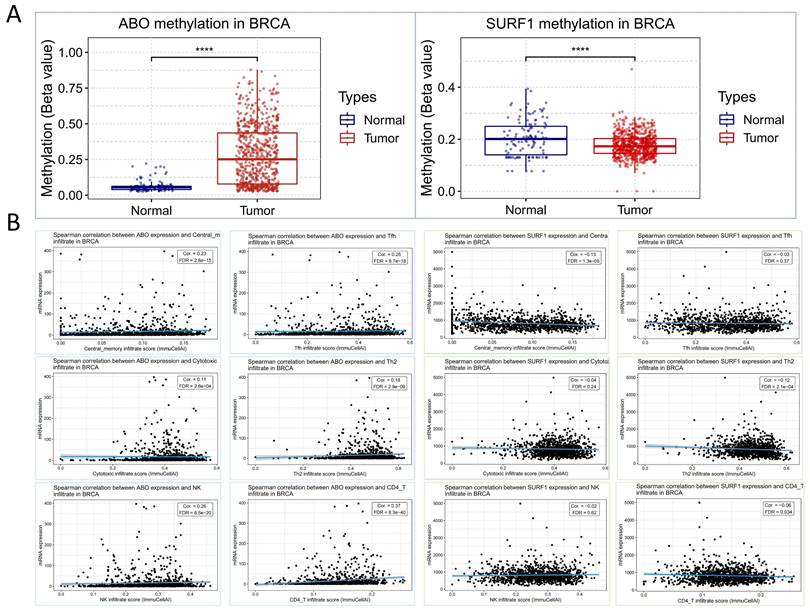
These interactions contribute to the establishment of an immunosuppressive milieu within the tumor, facilitated by the release of factors that modulate the recruitment, phenotypes, and functions of these immune cells. The tumor microenvironment is characterized by an immunosuppressive milieu [40], and the immunoassay results indicated that ABO is linked to cytotoxicity, helper T-cell 2 (Th2), central memory, follicular helper T-cells (Tfh), natural killer (NK) cells, and CD4+ T-cells in BRCA. Additionally, SURF1 exhibited a positive correlation with the activation of cytotoxic, Th2, central memory, Tfh, NK cells, and CD4+ T-cells in BRCA. These findings suggest that the distinct expressions of ABO and SURF1 in cancer might be associated with the immune evasion mechanisms of tumors.
Based on the aforementioned research findings, we propose a bold speculation that clinical evidence suggests a potential correlation between HF and accelerated tumor growth. The long-term development of tumors during HF treatment may be influenced by underlying diseases, immune system disorders, or common risk factors such as smoking, obesity, and hypertension. Additionally, lifestyle changes during treatment or clinical drug toxicity, such as the cardiotoxicity of chemotherapy drugs like anthracyclines [41], indirectly contribute to an increased risk of HF and cancer. However, it is important to note that HF itself does not elevate the risk of developing cancer.
5. Conclusion and prospect
In summary, the results of our study, which incorporate epidemiological and transcriptome analysis of genetic data, suggest that there is no significant association between heart failure and an increased risk of cancer development. Nevertheless, heart failure negatively modulates the incidence of cancer. The molecular interconnections between heart failure and cancer risk may be mediated by ABO and SURF1, whilst the inverse relationship between heart failure and cancer risk may be influenced by methylation and tumor immune escape pathways.
The utilization of genetic information in epidemiological research has the potential to mitigate the inherent biases present in conventional observational studies. This approach holds promise for investigating the relationship between ABO blood group and mitochondrial function in HF, as well as for advancing the screening, diagnosis, and treatment of HF patients with concurrent cancer diagnoses in clinical settings. Nevertheless, it is crucial to acknowledge that a significant disparity persists between genetic research and its practical implementation in clinical settings. In order to elucidate the probable underlying mechanisms linking heart failure and cancer, as well as to offer novel insights and approaches for their diagnosis, treatment, and prevention, more functional research and clinical validation of this work are needed.
Abbreviations
HF: HF; MR: Mendelian randomization; SMR: Summary Data-Based Mendelian Randomization; MCA: Mendelian randomized colocalization analysis; GWAS: genome-wide association study; IARC: the International Agency for Research on Cancer; ESC-HFA: the European Society of Cardiology HF Association; HFrEF: HF with reduced ejection fraction; HFmrEF: HF with mid-range ejection fraction; HfpEF: HF with preserved ejection fraction; IV: instrumental variable; IVW: inverse-variance weighted method; SNPs: single nucleotide polymorphisms; eQTL: expression quantitative trait loci; LBF: Log Bayes Factor; GTEx: Genotype-Tissue Expression; ACC: Adrenocortical carcinoma; BRCA: Breast invasive carcinoma; CHOL: Cholangiocarcinoma; COAD: Colon adenocarcinoma; LGG: Brain Lower Grade Glioma; LIHC: Liver hepatocellular carcinoma; LUSC: Lung squamous cell carcinoma; SKCM: Skin Cutaneous Melanoma; UCEC: Uterine Corpus Endometrial Carcinoma; UCS: Uterine Carcinosarcoma.
Supplementary Material
Supplementary tables.
Acknowledgements
Thanks to the following open databases for their selfless dedication to scientific researchers.
The open database includes SMR database, TCGA database, and GWAS database. In addition, I would like to thank Hubei Chengyu Life Technology Co., Ltd. for its financial support.
Funding
This work was supported by the Doctoral Foundation of HuBei University of Science and Technology (Grant Number BK202028 to Z.Z.), and the Horizontal research funding of Hubei University of Science and Technology (Grant Number 2023HX205 to Z.Z.), and the Foundation of Innovation Team of Hubei University of Science and Technology (Grant Number 2023T13 to S.Y.), and Natural Science Foundation of Hubei Province (Grant Number 2023AFB1027 to Z.Z.).
Author contributions
Conception and design: Z. Zhang, M. Li. Development of methodology: Z. Zhang, T. Sun. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): T. Sun, J. N. Mei, S, Shan, W Qian, and Y. Su. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Z. Zhang, M. Li. Writing, review, and revision of the manuscript: Z. Zhang. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): T. Sun, Study supervision: T. Sun.
Consent for publication
All authors consent to publication.
ORCID
https://orcid.org/0000-0001-6951-2125.
Data availability statement
The source of the raw/processed data needed to reproduce these findings is in the Methods section.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol. 2022;12:819128
2. Manzi L, Sperandeo L, Forzano I, Castiello DS, Florimonte D, Paolillo R. et al. Contemporary Evidence and Practice on Right Heart Catheterization in Patients with Acute or Chronic HF. Diagnostics (Basel). 2024;14:136
3. Nkoke C, Jingi AM, Makoge C, Teuwafeu D, Nkouonlack C, Dzudie A. Epidemiology of cardiovascular diseases related admissions in a referral hospital in the South West region of Cameroon: A cross-sectional study in sub-Saharan Africa. PLoS One. 2019;14:e0226644
4. Bozkurt B, Ahmad T, Alexander KM, Baker WL, Bosak K, Breathett K. et al. HF Epidemiology and Outcomes Statistics: A Report of the HF Society of America. J Card Fail. 2023;29:1412-51
5. Wang H, Chai K, Du M, Wang S, Cai JP, Li Y. et al. Prevalence and Incidence of HF Among Urban Patients in China: A National Population-Based Analysis. Circ Heart Fail. 2021;14:e008406
6. Lena A, Coats AJS, Anker MS. Metabolic disorders in HF and cancer. ESC Heart Fail. 2018;5:1092-8
7. Kunutsor SK, Jae SY, Makikallio TH, Laukkanen JA. High fitness levels attenuate the increased risk of HF due to low socioeconomic status: A cohort study. Eur J Clin Invest. 2022;52:e13744
8. Sun B, Wang X, Ji Z, Wang M, Liao YP, Chang CH. et al. NADPH Oxidase-Dependent NLRP3 Inflammasome Activation and its Important Role in Lung Fibrosis by Multiwalled Carbon Nanotubes. Small. 2015;11:2087-97
9. Zhu W, Gong X, Luo C, Lu J. Correlation between the levels of serum cystatin C and substance P in peripheral blood in diabetes mellitus patients complicated with hypertension. Exp Ther Med. 2018;16:1159-64
10. Moslehi J, Zhang Q, Moore KJ. Crosstalk Between the Heart and Cancer: Beyond Drug Toxicity. Circulation. 2020;142:684-7
11. Franchini M, Lippi G. The intriguing relationship between the ABO blood group, cardiovascular disease, and cancer. BMC Med. 2015;13:7
12. Etemadi A, Kamangar F, Islami F, Poustchi H, Pourshams A, Brennan P. et al. Mortality and cancer in relation to ABO blood group phenotypes in the Golestan Cohort Study. BMC Med. 2015;13:8
13. Chang L, Pei J, Li C, Zhang P, Zhou D, Du W. et al. Incidence and metastasis of cutaneous malignant melanoma with respect to ABO blood groups: a case-controlled study in northeast of China. PLoS One. 2014;9:e88096
14. Moro L. Mitochondrial Dysfunction in Aging and Cancer. J Clin Med. 2019;8:1983
15. Luo Y, Ma J, Lu W. The Significance of Mitochondrial Dysfunction in Cancer. Int J Mol Sci. 2020;21:5598
16. Liu M, Lv J, Pan Z, Wang D, Zhao L, Guo X. Mitochondrial dysfunction in HF and its therapeutic implications. Front Cardiovasc Med. 2022;9:945142
17. Wu C, Zhang Z, Zhang W, Liu X. Mitochondrial dysfunction and mitochondrial therapies in HF. Pharmacol Res. 2022;175:106038
18. Bertero E, Ameri P. Reply: Perspectives and Limitations of the Studies of the Association Between HF and Cancer. JACC CardioOncol. 2022;4:284-5
19. O'Rourke K. HF linked with an increased risk of cancer. Cancer. 2022;128:646
20. Roderburg C, Loosen SH, Jahn JK, Gansbacher J, Luedde T, Kostev K. et al. HF is associated with an increased incidence of cancer diagnoses. ESC Heart Fail. 2021;8:3628-33
21. Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM. et al. HF After Myocardial Infarction Is Associated With Increased Risk of Cancer. J Am Coll Cardiol. 2016;68:265-71
22. Sagastagoitia-Fornie M, Barge-Caballero E, Barge-Caballero G, Couto-Mallon D, Paniagua-Martin MJ, Enriquez-Vazquez D. et al. Cancer in patients with HF: Incidence, risk factors and prognostic impact. Eur J Intern Med. 2022;105:89-96
23. Banke A, Schou M, Videbaek L, Moller JE, Torp-Pedersen C, Gustafsson F. et al. Incidence of cancer in patients with chronic HF: a long-term follow-up study. Eur J Heart Fail. 2016;18:260-6
24. Selvaraj S, Bhatt DL, Claggett B, Djousse L, Shah SJ, Chen J. et al. Lack of Association Between HF and Incident Cancer. J Am Coll Cardiol. 2018;71:1501-10
25. Fernandez-Ruiz I. No association between HF and cancer. Nat Rev Cardiol. 2018;15:318
26. Parham GP. Comparison of cell collection and direct visualization cervical cancer screening adjuncts. Am J Obstet Gynecol. 2003;188:S13-20
27. Sorribes IC, Moore MNJ, Byrne HM, Jain HV. A Biomechanical Model of Tumor-Induced Intracranial Pressure and Edema in Brain Tissue. Biophys J. 2019;116:1560-74
28. Godbole MS, Singh V. Deciphering the impacts of gut and tissue microbiome on human cancers. Cancer Rep (Hoboken). 2023;6:e1924
29. Feng Q, Xiao K. Nanoparticle-Mediated Delivery of STAT3 Inhibitors in the Treatment of Lung Cancer. Pharmaceutics. 2022;14:2787
30. Force USPST, Mangione CM, Barry MJ, Nicholson WK, Chelmow D, Coker TR. et al. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2023;329:1290-5
31. Wu H, Huang D, Zhou H, Sima X, Wu Z, Sun Y. et al. Metformin: A promising drug for human cancers. Oncol Lett. 2022;24:204
32. Manickasamy MK, Jayaprakash S, Girisa S, Kumar A, Lam HY, Okina E. et al. Delineating the role of nuclear receptors in colorectal cancer, a focused review. Discov Oncol. 2024;15:41
33. Min Q, Bai Y, Zhang Y, Yu W, Zhang M, Liu D. et al. Hawthorn Leaf Flavonoids Protect against Diabetes-Induced Cardiomyopathy in Rats via PKC-alpha Signaling Pathway. Evid Based Complement Alternat Med. 2017;2017:2071952
34. Kitsis RN, Riquelme JA, Lavandero S. Heart Disease and Cancer: Are the Two Killers Colluding? Circulation. 2018;138:692-5
35. Ameri P, Vaduganathan M. When and how?. Two simple questions to determine cancer status and inform therapeutic decisions and trial design in HF. Eur J Heart Fail. 2023;25:1868-70
36. Groot HE, Villegas Sierra LE, Said MA, Lipsic E, Karper JC, van der Harst P. Genetically Determined ABO Blood Group and its Associations With Health and Disease. Arterioscler Thromb Vasc Biol. 2020;40:830-8
37. Kovarova N, Pecina P, Nuskova H, Vrbacky M, Zeviani M, Mracek T. et al. Tissue- and species-specific differences in cytochrome c oxidase assembly induced by SURF1 defects. Biochim Biophys Acta. 2016;1862:705-15
38. Wang X, Song C, Zhou X, Han X, Li J, Wang Z. et al. Mitochondria Associated MicroRNA Expression Profiling of HF. Biomed Res Int. 2017;2017:4042509
39. Flausino CS, Daniel FI, Modolo F. DNA methylation in oral squamous cell carcinoma: from its role in carcinogenesis to potential inhibitor drugs. Crit Rev Oncol Hematol. 2021;164:103399
40. Shao X, Hua S, Feng T, Ocansey DKW, Yin L. Hypoxia-Regulated Tumor-Derived Exosomes and Tumor Progression: A Focus on Immune Evasion. Int J Mol Sci. 2022;23:11789
41. Bozza WP, Takeda K, Alterovitz WL, Chou CK, Shen RF, Zhang B. Anthracycline-Induced Cardiotoxicity: Molecular Insights Obtained from Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs). AAPS J. 2021;23:44
Author contact
![]() Corresponding authors: These authors contributed equally to this work. Ph.D., Zhang Zhenwang, Hubei Key Laboratory of Diabetes and Angiopathy, Xianning Medical College, Hubei University of Science and Technology, Xianning 437100, Hubei, China. Email: zhenwangzhangcom; Tel.: +86 0715-8236051. Mengxi Li, School of Nuclear Technology and Chemistry & Biology, Hubei University of Science and Technology, Xianning 437100, Hubei, PR China. Email: limengxi66com.
Corresponding authors: These authors contributed equally to this work. Ph.D., Zhang Zhenwang, Hubei Key Laboratory of Diabetes and Angiopathy, Xianning Medical College, Hubei University of Science and Technology, Xianning 437100, Hubei, China. Email: zhenwangzhangcom; Tel.: +86 0715-8236051. Mengxi Li, School of Nuclear Technology and Chemistry & Biology, Hubei University of Science and Technology, Xianning 437100, Hubei, PR China. Email: limengxi66com.

 Global reach, higher impact
Global reach, higher impact