Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(2):456-465. doi:10.7150/jca.88148 This issue Cite
Research Paper
Patterns and Prognosis of Local Recurrence of Nasopharyngeal Carcinoma after Intensity-modulated Radiotherapy
State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Sun Yat-sen University Cancer Center, Guangzhou 510060, P. R. China.
* These authors contributed equally to this work.
Received 2023-7-16; Accepted 2023-11-12; Published 2024-1-1
Abstract
Objective: To investigate the patterns of local failure and prognosis in patients with locally recurrent nasopharyngeal carcinoma (rNPC) after primary intensity-modulated radiotherapy (IMRT).
Methods: The data of 298 patients with locally rNPC after IMRT were retrospectively analyzed. Magnetic resonance images of the initial and recurrent tumors were reviewed and, for patients with extra-nasopharyngeal local recurrence, the gross tumor volume of local recurrence was transferred to the original IMRT plan for dosimetry analysis. Significant prognostic factors for overall survival (OS) were selected by multivariate Cox regression analysis.
Results: The commonest recurrence sites were the nasopharynx (93%, 277/298) and skull base (53.7%, 160/298). Of the 21 patients with extra-nasopharyngeal recurrence (19 cases valid), 12 had in-field failures, 4 had marginal failures, and 3 had out-field failures. The ethmoid sinus (57.1%, 4/7) and nasal cavity (28.6%, 2/7) were the most frequent sites of marginal and out-field failures. After median follow-up of 37 months, the 3-year and estimated 5-year OS rates were 57.3% and 41.7%, respectively. Multivariate analysis showed that age, recurrence interval, plasma Epstein-Barr virus (EBV) DNA level, and recurrent T stage were independent prognostic factors for OS.
Conclusions: Local failure after IMRT occurs most commonly in the nasopharynx and skull base. In patients with extra-nasopharyngeal recurrence, in-field failure remains the main failure pattern, and marginal and out-field failures mainly occur in the ethmoid sinus and nasal cavity. Elder age, shorter recurrence interval, detectable plasma EBV DNA, and advanced recurrent T stage are negative predictors of OS in patients with rNPC.
Keywords: nasopharyngeal carcinoma, local recurrence, failure pattern, prognosis, IMRT
Introduction
Nasopharyngeal carcinoma (NPC) is the most common cancer of the head and neck region in Southeast Asia. It is highly sensitive to radiotherapy and chemotherapy [1]. In patients with non-metastatic NPC, intensity-modulated radiotherapy (IMRT) allows delivery of a highly conformal radiation dose to the tumor, with a sharp dose gradient outside the target volume that minimizes irradiation of adjacent organs. Several studies have shown that tumor control and survival are better, and the incidence of severe late adverse events significantly lower, with IMRT than with two-dimensional radiotherapy [2, 3].
Although the application of IMRT and systemic therapy has greatly improved local control of NPC, about 10% of patients still experience local failure [3, 4], mostly in-field failures within the primary tumor volume [5-9]. In-field recurrence is usually associated with radiation resistance, whereas marginal and out-field failures are usually due to inadequate target volume delineation and/or dose coverage. Defects in target volume delineation increase the risk of target miss and local recurrence.
In NPC treated with IMRT, local failure sites and patterns are closely related to the quality of target volume delineation. However, because of the low local recurrence rate of NPC, large study samples are difficult to assemble. To our knowledge, this is the largest sample-sized study to analyze the incidence of local recurrence at various anatomic sites and the relationship between local recurrence sites and initial tumor sites in patients with locally rNPC after IMRT. Moreover, we classified local recurrence into nasopharyngeal and extra-nasopharyngeal recurrence. Local recurrence at the nasopharynx is broadly correlated with intrinsic radioresistance, while extra-nasopharyngeal recurrence might be correlated with inadequate radiation dose and/or target volume delineation. Therefore, we focused on cases with extra-nasopharyngeal recurrence, and performed a detailed dosimetric and target volume analysis of the extra-nasopharyngeal recurrence, which has seldom been reported before. The findings of this study may help physicians improve target volume delineation in patients with NPC. We also investigated the outcomes and prognostic factors in this cohort of patients with locally recurrent NPC (rNPC) following salvage treatment to better predict prognosis and guide treatment.
Methods
Patients
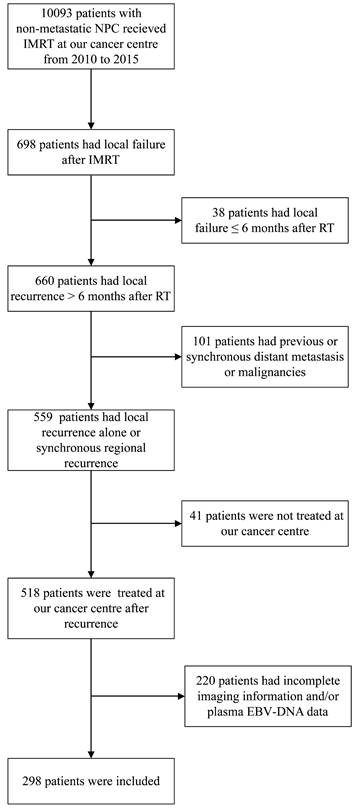
The data of patients with newly diagnosed, non-distant metastatic, histologically confirmed NPC treated with IMRT at Sun Yat-sen University Cancer Center from 2010 to 2015 were extracted from the medical records and retrospectively analyzed. The eligibility criteria for inclusion in this study were 1) local or locoregional recurrence occurring > 6 months after the end of primary IMRT and before June 30, 2020; 2) availability of complete imaging data (including magnetic resonance imaging [MRI], computed tomography [CT] and/or 18F-fluorodeoxyglucose positron emission tomography-CT [PET-CT]) of initial and recurrent tumor; 3) no previous or synchronous distant metastasis; 4) no record of partial response to the first course of IMRT; 5) salvage treatments after recurrence included reirradiation, surgery and/or systemic treatment; and 6) no other concomitant malignant tumor or severe comorbidity. The flow chart in Figure 1 shows the patient selection process.
Flow diagram of the study sample selection process (inclusion and exclusion criteria).
This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center, with waiver of the need for informed consent.
Treatment of initial tumor
All patients underwent radical IMRT with 6-MV photon beam external irradiation. Target volumes and normal tissues were delineated according to our institutional treatment protocol [10]. The prescribed doses were 66-72 Gy to the planning target volume of the gross primary tumor volume (GTVp), 60-63 Gy to the high-risk clinical target volume (CTV1), and 54-56 Gy to the low-risk clinical target volume (CTV2), delivered in 28-33 fractions. The irradiation was delivered once daily for 5 days per week. During the study period, our institutional guidelines recommended IMRT alone for stage I disease, and cisplatin-based concurrent chemoradiotherapy with or without neoadjuvant/adjuvant chemotherapy for stage II-IVA disease.
Diagnosis of local recurrence
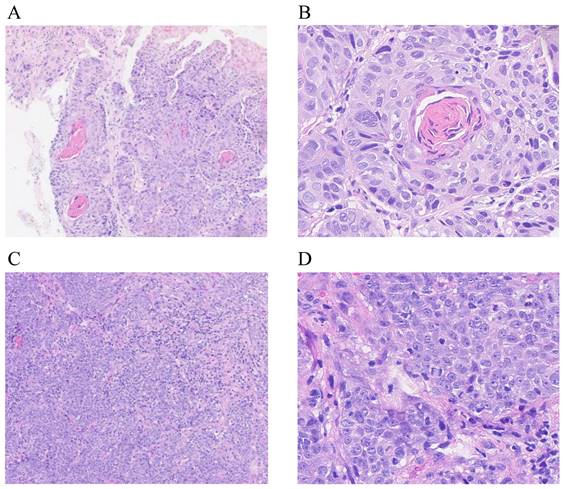
Endoscopic or surgical biopsy, or fine-needle aspiration should be used to confirm local or regional recurrence. Eligible patients had histologically confirmed keratinizing squamous, non-keratinizing, or basaloid squamous carcinoma according to the World Health Organization Classification [11] (Figure 2). However, when pathological evidence could not be obtained (e.g., in cases of skull base and intracranial involvement), diagnosis of local recurrence was based on abnormal imaging findings on PET-CT and/or MRI, and/or presence of progressive disease. All imaging-based diagnoses of local recurrence were confirmed retrospectively by two experienced radiologists. Recurrent tumors were staged according to the 8th edition of the Union for International Cancer Control and American Joint Committee on Cancer (UICC/AJCC) staging system [12]. Plasma Epstein-Barr virus (EBV) DNA concentrations were routinely measured by quantitative polymerase chain reaction [13]. For patients with local recurrence outside the nasopharynx, the gross recurrent tumor volume (GTVr) of the primary site was identified on MRI images and transferred to the original planning CT for dosimetry analysis. The recurrent lesions were evaluated by Dmax, Dmin, Dmean, and volume of recurrent tumor (Vr). The radiation dose received by Vr was calculated and analyzed using dose-volume histograms. Failure patterns were classified as [8, 9]: in-field recurrence (i.e., ≥95% Vr within the 95% isodose lines of the primary IMRT plan); 2) marginal-field recurrence (i.e., 20%-<95% Vr within the 95% isodose lines of the primary IMRT plan); or 3) out-field recurrence (i.e., <20% Vr within the 95% isodose lines of the primary IMRT plan).
Light microscopic appearance of locally recurrent nasopharyngeal carcinoma. Keratinizing squamous cell carcinoma (A: H&E stain, ×40; B: H&E stain, ×400); non-keratinizing carcinoma, undifferentiated subtype (C: H&E stain, ×40; D: H&E stain, ×400).
Treatment after local recurrence
Salvage locoregional treatments included reirradiation, endoscopic nasopharyngectomy, and neck dissection, alone or in combination. Salvage treatment was determined by the tumor size, extent, and location, as well as by the patient's preference; treatment decisions were made jointly by radiation oncologists and surgeons. The treatment approach can be briefly summarized as follows: 1) reirradiation with IMRT was considered for all locoregional recurrent NPC, but the time interval between the first and second courses of IMRT had to be ≥ 1 year. The GTVr included the primary site and neck, and the CTV included the entire nasopharynx and lymph node-positive regions. The prescribed doses were 60-70 Gy to the GTVr and 50-54 Gy to the CTV, delivered in 27-35 fractions [14, 15]; 2) endoscopic nasopharyngectomy was performed for early recurrent disease (rT1-2) or for rT3 disease confined to the floor of the sphenoid sinus. It included endoscopic resection plus microwave ablation, with or without posterior pedicle nasal mucoperiosteal flap resurfacing of the nasopharyngeal defects [16]; 3) either radical or selective neck dissection was performed for recurrent neck disease. Chemotherapy and targeted therapy were used in combination with reirradiation or surgery in selected patients.
Follow-up
After treatment completion, patients were evaluated every 3 months during the first 3 years and every 6 months thereafter until death. Time to recurrence was defined as the time from completion of the primary IMRT to the day of local recurrence. The primary endpoint was overall survival (OS), defined as the time from the day of local recurrence to the date of last follow-up or death.
Statistical analysis
The McNemar test was used to assess the association between tumor invasion sites in primary NPC and recurrent NPC. Survival rates were estimated using the Kaplan-Meier method, and survival differences were compared by the log-rank test. Multivariate Cox regression analysis [17] with backward elimination was used to identify the independent predictors of survival, and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Statistical analysis was performed using SPSS 26 (IBM Corp., Armonk, NY, USA). Two-tailed P < 0.05 was considered statistically significant.
Results
Patient characteristics
Table 1 lists the characteristics of the 298 patients included in this study. The male: female ratio was 2.6:1. Median age at recurrence was 47 years (range, 22-75 years). Diagnosis was confirmed by pathological examination in 250/298 (83.9%) patients. The comparison of histopathological types between primary and recurrent tumors is shown in Table 2. Detectable plasma EBV DNA was present at recurrence in 55% (164/298) patients. The median time to recurrence was 25.8 months (range, 6.4-93.7 months). While, 222/298 (74.5%) patients had local recurrences alone, 76/298 (25.5%) patients had synchronous nodal recurrence. The T stage of recurrent tumor was rT1 in 75/298 (25.2%) patients, rT2 in 33/298 (11.1%) patients, rT3 in 102/298 (34.2%) patients, and rT4 in 88/298 (29.5%) patients. While 103/298 (34.6%) patients were classified as stage I-II, 195/298 (65.4%) were classified as stage III-IVA.
Patient characteristics (n = 298)
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 216 (72.5%) |
| Female | 82 (27.5%) |
| Age at recurrence | |
| < 60 years | 247 (82.9%) |
| ≥ 60 years | 51 (17.1%) |
| Methods for diagnosis of recurrence | |
| Pathology | 250 (83.9%) |
| MRI+ PET-CT | 36 (12.1%) |
| MRI | 12 (4%) |
| EBV DNA level at recurrence | |
| 0 copy/mL | 134 (45%) |
| > 0 - <4000 copies/mL | 119 (39.9%) |
| ≥ 4000 copies/mL | 45 (15.1%) |
| Recurrence interval | |
| ≤ 2 years after IMRT | 131 (44%) |
| > 2 years after IMRT | 167 (56%) |
| Recurrent T stage | |
| rT1 | 75 (25.2%) |
| rT2 | 33 (11.1%) |
| rT3 | 102 (34.2%) |
| rT4 | 88 (29.5%) |
| Recurrent N stage | |
| rN0 | 222 (74.5%) |
| rN1 | 63 (21.1%) |
| rN2 | 12 (4%) |
| rN3 | 1 (0.3%) |
| Recurrent clinical stage | |
| rI | 55 (18.5%) |
| rII | 48 (16.1%) |
| rIII | 106 (35.6%) |
| rIVA | 89 (29.9%) |
| Treatment after recurrence | |
| Surgery and/or reirradiation alone | 93 (31.2%) |
| Chemotherapy alone | 72 (24.2%) |
| Surgery and/or reirradiation + chemotherapy | 133 (44.6%) |
Abbreviations: EBV: Epstein-Barr virus; IMRT: intensity-modulated radiotherapy; MRI: magnetic resonance imaging; PET: positron emission tomography; CT: computed tomography.
Comparison of histopathological types between pNPC and rNPC (n = 298)
| Histopathological Types | pNPC, n (%) | rNPC, n (%) |
|---|---|---|
| Keratinizing squamous cell carcinoma | 7 (2.3%) | 5 (1.7%) |
| Non-keratinizing carcinoma | 291 (97.7%) | 245 (82.2%) |
| Unknown | 0 (0%) | 48 (16.1%) |
Abbreviations: pNPC = primary nasopharyngeal carcinoma; rNPC = recurrent nasopharyngeal carcinoma.
Patterns of local recurrence
Table 3 shows the distributions of involved sites and the comparisons of the recurrent and initial tumor invasion by McNemar test. The most common recurrence site was the nasopharynx (93%, 277/298), followed by the skull base (53.7%, 160/298), parapharyngeal space (43%, 128/298), prevertebral space (34.9%, 104/298), cavernous sinus (27.9%, 83/298), masticator space (23.2%, 69/298), and the pterygopalatine fossa (22.5%, 67/298). The invasion rates of most sites were lower in rNPC than in primary NPC, the exceptions were cavernous sinus, ethmoid sinus, and orbit.
Dosimetric analysis of primary IMRT
Among the 298 patients, 21 (7%) had extra-nasopharyngeal recurrence; for these patients, we re-located the recurrent tumor in the initial diagnostic imaging and the original IMRT plan for dosimetry analysis. Table 4 shows the clinical characteristics and patterns of local failures of these 21 patients. The original IMRT plans of two patients were lost; of the remaining 19 patients, 12 (63.2%) patients had in-field failures, 4 (21.1%) had marginal failures, and 3 (15.8%) had out-field failures. All were classified as rT3-4. The median doses to the in-field, marginal, and out-field recurrent tumors were 73.41 Gy, 66.14 Gy, and 22.83 Gy, respectively. Among the 12 patients with in-field failures, 10 (83.3%) had skull base invasion, 6 (50%) had cavernous sinus invasion, and 3 (25%) had pterygopalatine fossa invasion. Of the 7 patients with marginal or out-field failures, 4 (57.1%) had recurrence in the ethmoid sinus, 2 (28.6%) in the nasal cavity, and the other recurrent sites included the hypophyseal fossa, orbital apex, sphenoid sinus, frontal sinus, cavernous sinus, and skull base (Figure 3). We further checked whether these sites of marginal and out-field failures were invaded in the pretreatment MRI. The recurrence sites of two patients were invaded in the initial MRI and had been delineated into the GTVp to receive a radical dose; however, the large recurrent tumor volume led to classification as marginal failure. One patient had hypophyseal fossa invasion at initial MRI, but it was not completely included in GTVp; this led to marginal recurrence. The other four patients had recurrent tumors at sites uninvolved at initial imaging.
Treatment outcome
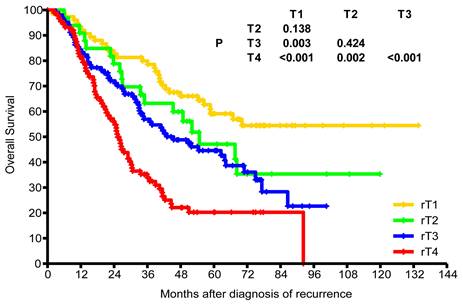
Of the 298 patients with recurrence, 65 (21.8%) received surgery alone, 155 (52%) received re-irradiation alone, and 6 (2%) received surgery plus re-irradiation. While 133 (44.6%) patients received local treatment plus chemotherapy, 72 (24.2%) received chemotherapy alone. The median dose of patients who received re-irradiation alone was 62 Gy (range, 24-70 Gy). After median follow-up of 37 months (range, 1.8-133.7 months), the 3-year and estimated 5-year OS rates were 57.3% and 41.7%, respectively. The estimated 5-year OS rates for rT1, rT2, rT3, and rT4 were 59.1%, 47.1%, 44.6%, and 20.3%, respectively (Figure 4). The estimated 5-year OS rates of recurrence stages I, II, III, and IVA were 63.5%, 50.4%, 43.5%, and 19.9%, respectively.
Univariate and multivariate analyses
Association between OS and sex, age, recurrence interval, plasma EBV DNA level at recurrence, recurrent T stage, recurrence N stage, and treatment after recurrence was examined using univariate and multivariate analyses. Only age, recurrence interval, plasma EBV DNA level, and recurrent T stage were found to be independent prognostic factors for OS (Table 5).
Comparison of tumor invasion in pNPC and rNPC (n = 298)
| Tumor invasion | rNPC, n (%) | pNPC, n (%) | pNPC and rNPC, n | Isolated invasion in pNPC, n | Isolated invasion in rNPC, n | No invasion in pNPC or rNPC, n | P |
|---|---|---|---|---|---|---|---|
| Nasopharynx | 277 (93%) | 298 (100%) | 277 | 21 | 0 | 0 | <0.001 |
| Skull base | 160 (53.7%) | 232 (77.9%) | 129 | 103 | 31 | 35 | <0.001 |
| Parapharyngeal space | 128 (43%) | 271 (90.9%) | 120 | 151 | 8 | 19 | <0.001 |
| Prevertebral space | 104 (34.9%) | 182 (61.1%) | 74 | 108 | 30 | 86 | <0.001 |
| Cavernous sinus | 83 (27.9%) | 79 (26.5%) | 40 | 39 | 43 | 176 | 0.741 |
| Masticator space | 69 (23.2%) | 91 (30.5%) | 27 | 64 | 42 | 165 | 0.041 |
| Pterygopalatine fossa | 67 (22.5%) | 90 (30.2%) | 40 | 50 | 27 | 181 | 0.012 |
| Nasal cavity | 52 (17.4%) | 107 (35.9%) | 28 | 79 | 24 | 167 | <0.001 |
| Sphenoid sinus | 52 (17.4%) | 65 (21.8%) | 29 | 36 | 23 | 210 | 0.117 |
| Ethmoid sinus | 21 (7%) | 19 (6.4%) | 6 | 13 | 15 | 264 | 0.851 |
| Oropharynx | 17 (5.7%) | 33 (11.1%) | 4 | 29 | 13 | 252 | 0.020 |
| Orbit | 12 (4%) | 12 (4%) | 5 | 7 | 7 | 279 | 1.000 |
Abbreviations: rNPC: recurrent nasopharyngeal carcinoma; pNPC: primary nasopharyngeal carcinoma.
Clinical characteristics and patterns of local failures of the 21 patients with extra-nasopharyngeal recurrence
| No. of patient | Age at recurrence (years) | Sex | Stage | Recurrence site | DVH Statistics to recurrence volume | Type of relapse | |||
|---|---|---|---|---|---|---|---|---|---|
| Dmean (Gy) | Dmin (Gy) | Dmax (Gy) | Tumor volume (cm3) | ||||||
| 1 | 44 | Male | T4N0M0 IVA | Petrous apex, prepontine cistern | 73.78 | 71.81 | 76.46 | 2.90 | In-field |
| 2 | 24 | Male | T4N0M0 IVA | Parapharyngeal space, cavernous sinus | 73.91 | 70.19 | 77.37 | 23.80 | In-field |
| 3 | 47 | Male | T4N0M0 IVA | Cavernous sinus | 72.94 | 66.47 | 77.22 | 3.80 | In-field |
| 4 | 47 | Female | T4N0M0 IVA | Pterygopalatine fossa, skull base, cavernous sinus | 74.32 | 70.35 | 77.60 | 8.80 | In-field |
| 5 | 53 | Male | T4N0M0 IVA | Pterygopalatine fossa, skull base, cavernous sinus | 75.25 | 71.47 | 78.17 | 34.00 | In-field |
| 6 | 37 | Male | T3N0M0 III | Prevertebral space, occipital clivus | 70.73 | 67.25 | 75.11 | 11.80 | In-field |
| 7 | 48 | Male | T4N0M0 IVA | Foramen ovale, cavernous sinus | 72.99 | 70.63 | 75.39 | 2.90 | In-field |
| 8 | 62 | Female | T3N0M0 III | Skull base, sphenoid sinus, pterygopalatine fossa | 74.47 | 70.73 | 76.97 | 6.40 | In-field |
| 9 | 50 | Female | T3N0M0 III | Pterygoid process | 72.84 | 70.53 | 75.53 | 1.70 | In-field |
| 10 | 37 | Male | T3N0M0 III | Skull base | 71.72 | 63.42 | 77.74 | 5.51 | In-field |
| 11 | 67 | Male | T4N1M0 IVA | Skull base, cavernous sinus, prepontine cistern | 73.75 | 66.79 | 78.60 | 44.80 | In-field |
| 12 | 60 | Male | T3N0M0 III | Prevertebral muscle, skull base | 73.07 | 63.92 | 76.97 | 22.80 | In-field |
| 13 | 68 | Male | T4N0M0 IVA | Skull base, cavernous sinus | 74.67 | 44.47 | 80.65 | 32.30 | Marginal |
| 14 | 63 | Male | T3N0M0 III | Nasal cavity, ethmoid sinus | 69.97 | 51.80 | 78.02 | 40.36 | Marginal |
| 15 | 50 | Male | T4N0M0 IVA | Pituitary, hypophyseal fossa | 62.31 | 50.83 | 74.53 | 4.12 | Marginal |
| 16 | 49 | Male | T4N0M0 IVA | Sphenoid sinus, orbital apex | 61.50 | 12.13 | 79.60 | 23.86 | Marginal |
| 17 | 31 | Female | T3N0M0 III | Ethmoid sinus | 41.43 | 6.33 | 64.09 | 5.00 | Outside |
| 18 | 43 | Male | T3N0M0 III | Nasal cavity, ethmoid sinus | 22.83 | 2.95 | 63.05 | 39.20 | Outside |
| 19 | 30 | Male | T3N0M0 III | Ethmoid sinus, frontal sinus | 12.94 | 3.10 | 46.06 | 3.30 | Outside |
| 20 | 54 | Male | T4N0M0 IVA | Pterygopalatine fossa, cavernous sinus | / | / | / | / | / |
| 21 | 55 | Male | T4N0M0 IVA | Trigeminal nerves, cavernous sinus | / | / | / | / | / |
Abbreviations: DVH: dose-volume histogram.
Discussion
The incidence rates of recurrence at different anatomic sites in patients with locally rNPC have seldom been reported. In the present study, the most common sites of local recurrence were the nasopharynx (93%) and skull base (53.7%), followed by the parapharyngeal space, prevertebral space, cavernous sinus, masticator space, and the pterygopalatine fossa. This is consistent with previous reports [6, 18, 19]. In 32 patients with rNPC after IMRT, Li et al. reported recurrence rates of 78.1% and 59.4% in the nasopharynx and skull base, respectively; the authors also found significantly higher invasion rate of the ethmoid sinus in patients with rNPC than in patients with primary NPC [6]. In the present study, the invasion rates of cavernous sinus and ethmoid sinus were higher in rNPC than in primary NPC, but the difference was not statistically significant.
Previous studies have found in-field failure to be the main pattern of local recurrence [6-9, 20]. Among 710 NPC patients treated with IMRT and followed up for a median of 38 months, Li et al. observed 34 local failures (32 cases valid), of which 16 (50%) were central failures, 9 (28.1%) were marginal failures, and 7 (21.9%) were out-field failures [6]. In a retrospective study of 645 patients with new histological-confirmed NPC treated with IMRT and followed up for a median of 62 months, Chen et al. reported 60 cases of locoregional recurrence: 56 (93.3%) in-field failures, 3 (5%) marginal failures, and 1 (1.7%) out-field failure [9]. Potential reasons for local recurrence include the following: 1) radiation resistance, i.e., the existence of radio-insensitive stem cells and increase in hypoxic cells [7]; 2) large-sized tumors that cannot really be cured [6]; 3) intentional restriction of radiation dose because of need for dose limitation to critical organs; and 4) defects in target volume delineation and inaccuracy of radiation treatment plan design [18].
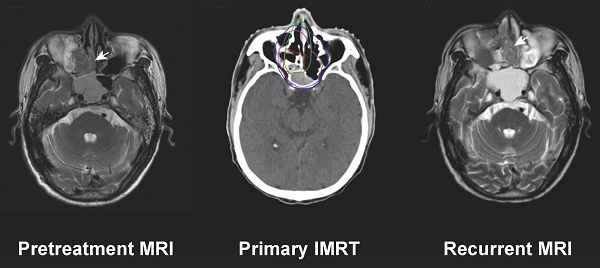
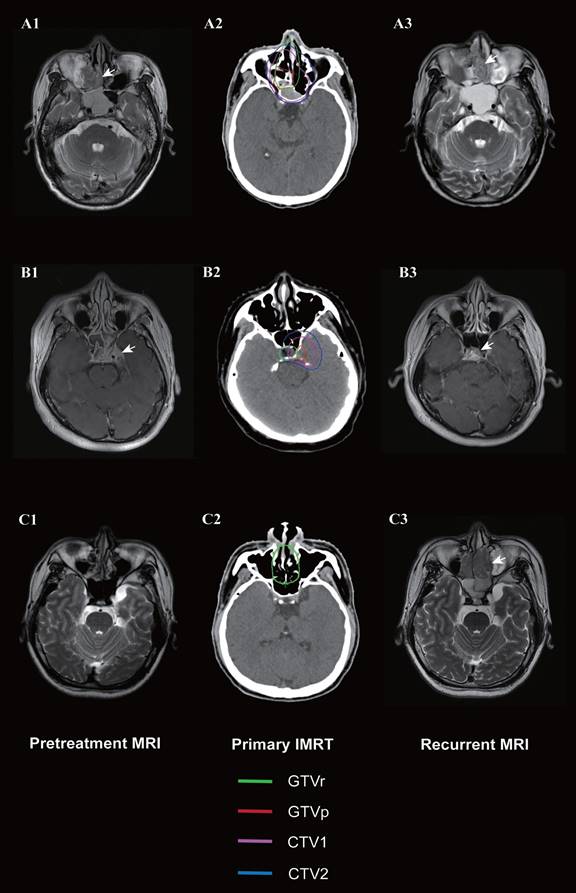
Magnetic resonance imaging (MRI) images of three patients with local recurrence outside the nasopharynx. Marginal failure in the ethmoid sinus and nasal cavity due to the large volume of the recurrent tumor (A1-A3); marginal failure at the hypophyseal fossa due to target miss (B1-B3); and out-field failure in the ethmoid sinus and nasal cavity (C1-C3). Left: Initial diagnostic MRI. Middle: Target volume delineation and dose prescription of primary intensity-modulated radiotherapy. Right: MRI at recurrence. The green line indicates the gross recurrent tumor volume (GTVr); the red line, the gross primary tumor volume (GTVp); the pink line, the high-risk clinical target volume (CTV1); and the blue line, the low-risk clinical target volume (CTV2).
Kaplan-Meier overall survival curves for patients with different recurrent T stages.
Univariate and multivariate analyses of prognostic factors for overall survival
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Sex female vs. male | 0.92 | 0.66-1.28 | 0.604 | 1.05 | 0.74-1.49 | 0.792 |
| Age at recurrence ≥ 60 vs. < 60 years | 1.83 | 1.28-2.63 | 0.001 | 1.86 | 1.29-2.68 | 0.001 |
| Recurrence interval > vs. ≤ 2 years after IMRT | 0.75 | 0.55-1.01 | 0.057 | 0.64 | 0.47-0.87 | 0.004 |
| EBV DNA level | <0.001 | 0.012 | ||||
| > 0 to < 4000 vs. 0 copies/mL | 1.64 | 1.17-2.30 | 0.004 | 1.52 | 1.07-2.15 | 0.018 |
| ≥ 4000 vs. 0 copies/mL | 2.31 | 1.53-3.50 | <0.001 | 1.79 | 1.17-2.73 | 0.007 |
| Recurrent T stage | <0.001 | <0.001 | ||||
| rT2 vs. rT1 | 1.52 | 0.85-2.72 | 0.157 | 1.77 | 0.98-3.18 | 0.058 |
| rT3 vs. rT1 | 1.91 | 1.23-2.95 | 0.004 | 1.79 | 1.15-2.79 | 0.011 |
| rT4 vs. rT1 | 3.47 | 2.25-5.37 | <0.001 | 3.28 | 2.11-5.10 | <0.001 |
| Recurrent N stage | 0.127 | 0.127 | ||||
| rN1 vs. rN0 | 1.41 | 1.00-2.01 | 0.053 | 1.47 | 1.01-2.12 | 0.042 |
| rN2-3 vs. rN0 | 1.35 | 0.69-2.66 | 0.387 | 1.04 | 0.52-2.07 | 0.908 |
| Surgery and/or reirradiation yes vs. no | 0.55 | 0.39-0.76 | <0.001 | 0.79 | 0.56-1.11 | 0.168 |
Abbreviations: HR: hazard ratio; CI: confidence interval; IMRT: intensity-modulated radiotherapy; EBV: Epstein-Barr virus.
In the present study, we classified local recurrence into two categories: nasopharyngeal and extra-nasopharyngeal recurrence. Inherent resistance to irradiation and/or large tumor size were likely the main reasons for nasopharyngeal recurrence, while inadequate radiation dose and/or target volume delineation might be the main reasons for extra-nasopharyngeal recurrence. Dosimetric analysis of patients with extra-nasopharyngeal recurrence showed that the main recurrence pattern was in-field failure. The ethmoid sinus and nasal cavity were the most common sites of marginal and out-field failures. Further, among the four patients with ethmoid sinus recurrence (all involving the anterior and middle ethmoid sinuses), three patients did not have ethmoid sinus invasion at initial imaging, while one patient had posterior ethmoid sinus involvement. However, two patients had nasal cavity invasion at initial imaging. Thus, concurrent nasal cavity and ethmoid sinus recurrence may be common. According to the international guidelines for CTV delineation for NPC [21], only the posterior-inferior part of the ethmoid sinus is routinely included in the CTV. We suggest that elective coverage of the anterior and middle ethmoid sinuses in patients with nasal cavity involvement might reduce the risk of local recurrence. In our cohort, one patient had hypophyseal fossa invasion at initial MRI, but as this was not completely included in GTVp, the patient developed marginal recurrence. Inaccuracy in GTV delineation that may lead to target miss must be avoided.
Current evidence suggests that surgery should be the treatment of first choice for rNPC, with re-irradiation considered for unresectable disease, and chemotherapy an option to improve long-term survival in patients with advanced rNPC. When formulating individualized treatment for rNPC, the physician should take into consideration the recurrent T stage, tumor size, and condition of patient [22, 23]. In this study, the proportion of patients in rT1-2 and rT3-4 were 36.2% and 63.8%, respectively. While 75.8% patients received surgery and/or re-radiotherapy, 24.2% received chemotherapy alone. Most patients receiving chemotherapy alone had larger tumors and/or radiation-related side effects such as radiation encephalopathy, which made re-irradiation inadvisable. The 3-year and estimated 5-year OS rates were 57.3% and 41.7%, respectively. This result is similar to the 3-year and 5-year OS rates of 53.2% and 41.1%, respectively, reported by Tian et al. in a retrospective analysis of 251 patients with local rNPC [14]. The poor survival of patients with local rNPC may be attributed to severe irradiation-related complications and/or treatment resistance. New modalities such as hyperfractionated IMRT [24], proton and heavy ion therapy [25, 26], and immunotherapy [27, 28] may improve outcomes in rNPC.
In the present study, multivariate analysis showed that age ≥ 60 years, recurrence interval ≤ 2 years, plasma EBV DNA level > 0 copy/mL, and advanced recurrent T stage were independent predictors of mortality. This result is consistent with previous studies [14, 29-31]. Sun et al. found age, hypertension, relapsed T stage, and EBV DNA level to be independent prognostic factors [32]. Age-related poor performance status will result in poor tolerance to disease and treatment-related toxicity. Some authors have found worse prognosis in patients with positive pre-retreatment EBV DNA than those with negative EBV DNA [32, 33]. Others have shown that longer time to recurrence (>30 months) is associated with better outcomes, probably due to longer tissue recovery time [29]. In our cohort, because choice of treatment was related to recurrent T stage, local treatment was significantly associated with better OS in univariate analysis but it was not an independent prognostic factor in multivariate analysis.
This study has several limitations. First, this was a single-center retrospective analysis, and so bias is inevitable. Second, the study included patients treated over a long-time span; there were technological advances and changes in treatment strategies over the study period. Therefore, further research is needed to determine the effect of different treatment regimens on the prognosis of rNPC.
Conclusion
In patients with nasopharyngeal carcinoma developing local failure after intensity-modulated radiotherapy, the nasopharynx and skull base are the most common sites of recurrence. Among patients with extra-nasopharyngeal local recurrence, in-field failure remains the main failure pattern, and marginal and out-field failures mainly occur in the ethmoid sinus and nasal cavity. Independent negative predictors of overall survival include elder age, shorter recurrence interval, detectable plasma Epstein-Barr virus DNA, and advanced recurrent T stage.
Abbreviations
CI: confidence interval; CT: computed tomography; CTV1: high-risk clinical target volume; CTV2: low-risk clinical target volume; EBV: Epstein-Barr virus; GTVp: gross primary tumor volume; GTVr: gross recurrent tumor volume; HR: hazard ratio; IMRT: intensity-modulated radiotherapy; MRI: magnetic resonance imaging; NPC: nasopharyngeal carcinoma; OS: overall survival; PET-CT: positron emission tomography-computed tomography; rNPC: recurrent nasopharyngeal carcinoma; Vr: volume of recurrent tumor.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China [grant number 81930072]; the Natural Science Foundation of Guangdong Province [grant number 2017A030312003]; and the Innovation Team Development Plan of the Ministry of Education [grant number IRT_17R110].
Ethical approval statement
The study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (No. B2021-330-01) and performed according to the institutional policy for protecting patients' confidential information. The need for informed consent was waived.
Author contributions
X.T.X, S.Q.Z and W.F.L contributed to study design and conception. X.T.X, W.F.L, Y.P.C, R.G and L.L.T contributed to data acquisition. X.T.X, S.Q.Z and W.F.L analyzed and interpreted the data. X.T.X and W.F.L contributed to manuscript preparation and editing. J.M, Y.S and W.F.L contributed to quality control and review of the data and manuscript. All authors read and approved the final version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80
2. Zhang B, Mo Z, Du W, Wang Y, Liu L, Wei Y. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. Oral Oncol. 2015;51(11):1041-6
3. Chen L, Zhang Y, Lai SZ, Li WF, Hu WH, Sun R. et al. 10-Year Results of Therapeutic Ratio by Intensity-Modulated Radiotherapy Versus Two-Dimensional Radiotherapy in Patients with Nasopharyngeal Carcinoma. Oncologist. 2019;24(1):e38-e45
4. Au KH, Ngan RKC, Ng AWY, Poon DMC, Ng WT, Yuen KT. et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study). Oral Oncol. 2018;77:16-21
5. Ng WT, Lee MC, Hung WM, Choi CW, Lee KC, Chan OS. et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;79(2):420-8
6. Li JX, Huang SM, Jiang XH, Ouyang B, Han F, Liu S. et al. Local failure patterns for patients with nasopharyngeal carcinoma after intensity-modulated radiotherapy. Radiat Oncol. 2014;9:87
7. Kong F, Ying H, Du C, Huang S, Zhou J, Chen J. et al. Patterns of local-regional failure after primary intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiat Oncol. 2014;9:60
8. Yang X, Ren H, Yu W, Zhang X, Sun Y, Shao Y. et al. Analysis of Clinical Target Volume Delineation in Local-regional Failure of Nasopharyngeal Carcinoma after Intensity-modulated Radiotherapy. J Cancer. 2020;11(7):1968-75
9. Chen S, Yang D, Liao X, Lu Y, Yu B, Xu M. et al. Failure Patterns of Recurrence and Metastasis After Intensity-Modulated Radiotherapy in Patients With Nasopharyngeal Carcinoma: Results of a Multicentric Clinical Study. Front Oncol. 2021;11:693199
10. Xiao WW, Huang SM, Han F, Wu SX, Lu LX, Lin CG. et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117(9):1874-83
11. Barnes L, Eveson JW, Reichart P, Sidransky D. et al. World health organization classification of tumors. In: Pathology and Genetics of Head and Neck Tumors. Lyon, France: IARC Press. 2005
12. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK. et al. AJCC Cancer Staging Manual. 8th ed2017.
13. Shao JY, Li YH, Gao HY, Wu QL, Cui NJ, Zhang L. et al. Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. 2004;100(6):1162-70
14. Tian YM, Tian YH, Zeng L, Liu S, Guan Y, Lu TX. et al. Prognostic model for survival of local recurrent nasopharyngeal carcinoma with intensity-modulated radiotherapy. Br J Cancer. 2014;110(2):297-303
15. Tian YM, Zhao C, Guo Y, Huang Y, Huang SM, Deng XW. et al. Effect of total dose and fraction size on survival of patients with locally recurrent nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: a phase 2, single-center, randomized controlled trial. Cancer. 2014;120(22):3502-9
16. Liu YP, Wen YH, Tang J, Wei Y, You R, Zhu XL. et al. Endoscopic surgery compared with intensity-modulated radiotherapy in resectable locally recurrent nasopharyngeal carcinoma: a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(3):381-90
17. Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187-220
18. Zhao W, Lei H, Zhu X, Li L, Qu S, Liang X. Investigation of long-term survival outcomes and failure patterns of patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: a retrospective analysis. Oncotarget. 2016;7(52):86914-25
19. Ou X, Yan W, Huang Y, He X, Ying H, Lu X. et al. Unraveling the patterns and pathways of local recurrence of nasopharyngeal carcinoma: evidence for individualized clinical target volume delineation. Radiat Oncol. 2023;18(1):55
20. Wang L, Guo Y, Xu J, Chen Z, Jiang X, Zhang L. et al. Clinical Analysis of Recurrence Patterns in Patients With Nasopharyngeal Carcinoma Treated With Intensity-Modulated Radiotherapy. Ann Otol Rhinol Laryngol. 2017;126(12):789-97
21. Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC, AlHussain H. et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol. 2018;126(1):25-36
22. Lee AWM, Ng WT, Chan JYW, Corry J, Makitie A, Mendenhall WM. et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev. 2019;79:101890
23. Perri F, Della Vittoria Scarpati G, Caponigro F, Ionna F, Longo F, Buonopane S. et al. Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther. 2019;12:1583-91
24. You R, Liu YP, Xie YL, Lin C, Duan CY, Chen DP. et al. Hyperfractionation compared with standard fractionation in intensity-modulated radiotherapy for patients with locally advanced recurrent nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2023;401(10380):917-27
25. Romesser PB, Cahlon O, Scher ED, Hug EB, Sine K, DeSelm C. et al. Proton Beam Reirradiation for Recurrent Head and Neck Cancer: Multi-institutional Report on Feasibility and Early Outcomes. Int J Radiat Oncol Biol Phys. 2016;95(1):386-95
26. Hu J, Huang Q, Gao J, Guan X, Hu W, Yang J. et al. Clinical outcomes of carbon-ion radiotherapy for patients with locoregionally recurrent nasopharyngeal carcinoma. Cancer. 2020;126(23):5173-83
27. Yang Y, Qu S, Li J, Hu C, Xu M, Li W. et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2021;22(8):1162-74
28. Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC. et al. Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02). J Clin Oncol. 2021;39(7):704-12
29. Guan Y, Liu S, Wang HY, Guo Y, Xiao WW, Chen CY. et al. Long-term outcomes of a phase II randomized controlled trial comparing intensity-modulated radiotherapy with or without weekly cisplatin for the treatment of locally recurrent nasopharyngeal carcinoma. Chin J Cancer. 2016;35:20
30. Li YQ, Tian YM, Tan SH, Liu MZ, Kusumawidjaja G, Ong EHW. et al. Prognostic Model for Stratification of Radioresistant Nasopharynx Carcinoma to Curative Salvage Radiotherapy. J Clin Oncol. 2018;36(9):891-9
31. Sun XS, Zhu MY, Wen DX, Luo DH, Sun R, Chen QY. et al. Establishment and validation of a recursive partitioning analysis based prognostic model for guiding re-radiotherapy in locally recurrent nasopharyngeal carcinoma patients. Radiother Oncol. 2022;168:61-8
32. Sun XS, Liang YJ, Jia GD, Liu SL, Liu LT, Guo SS. et al. Establishment of a prognostic nomogram to identify optimal candidates for local treatment among patients with local recurrent nasopharyngeal carcinoma. Oral Oncol. 2020;106:104711
33. Liu MZ, Fang SG, Huang W, Wang HY, Tian YM, Huang RD. et al. Clinical characteristics and prognostic value of pre-retreatment plasma epstein-barr virus DNA in locoregional recurrent nasopharyngeal carcinoma. Cancer Med. 2019;8(10):4633-43
Author contact
![]() Corresponding author: Wen-Fei Li M.D., Ph.D., State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Sun Yat-sen University Cancer Center, Guangzhou 510060, P. R. China. Telephone: +86-20-87343581; Fax: +86-20-87343295; E-mail: liwforg.cn.
Corresponding author: Wen-Fei Li M.D., Ph.D., State Key Laboratory of Oncology in South China, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Sun Yat-sen University Cancer Center, Guangzhou 510060, P. R. China. Telephone: +86-20-87343581; Fax: +86-20-87343295; E-mail: liwforg.cn.

 Global reach, higher impact
Global reach, higher impact