Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(17):3368-3377. doi:10.7150/jca.87901 This issue Cite
Research Paper
Development of A Nomogram for Progression-free Survival in Patients with Stage II/T3N0 Nasopharyngeal Carcinoma to Explore Different Treatment Modalities
1. Department of Radiation Oncology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, 530021, People's Republic of China.
2. Department of Oncology, Wuming Hospital of Guangxi Medical University, Nanning, Guangxi, 530199, People's Republic of China.
*These authors contributed equally to this work.
Received 2023-7-8; Accepted 2023-9-27; Published 2023-10-9
Abstract
Purpose To explore the prognostic value of clinical and serological risk factors for progression-free survival (PFS) in stage II and T3N0 nasopharyngeal carcinoma (NPC) and construct a nomogram based on these factors. Additionally, to investigate the long-term survival and short-term toxic reactions of patients in different risk stratification under different treatment modalities.
Methods The patients were randomly divided into training and validation cohorts in a 7:3 ratio. Independent prognostic factors were identified using Cox regression analysis, and a nomogram was constructed by combining these predictive factors with the TNM staging system. The nomogram was then validated in the validation cohort, and patients were classified into different risk groups based on the nomogram. The PFS, overall survival (OS), and acute toxicities were compared among different treatment modalities after balancing baseline characteristics.
Results Multivariate Cox regression analysis indicated that pathological type, alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) were independent prognostic factors(p<0.05) in this study. The nomogram showed good prognostic accuracy in both the training and validation cohorts (C-index of 0.73 and 0.70, respectively). In the different risk subgroups, there were no statistically significant differences in PFS and OS between radiotherapy and chemoradiotherapy groups(p>0.05). The treatment modality of combined chemotherapy was associated with more acute toxic reactions.
Conclusion We established and validated a nomogram for predicting PFS in patients with stage II/T3N0 NPC. Intensity-modulated radiation therapy (IMRT) combined with chemotherapy did not provide additional survival benefits for these patients and was associated with more chemotherapy-related side effects.
Keywords: nasopharyngeal carcinoma, survival, intensity-modulated radiotherapy, nomogram
Introduction
Nasopharyngeal carcinoma (NPC) is a unique head and neck tumor that commonly occurs in southeastern China[1, 2]. Currently, the tumor, node, metastasis (TNM) staging system is one of the main criteria used by clinicians to determine treatment plans. Due to its specific anatomical location and biological characteristics, radiotherapy is the primary treatment method for NPC. With the development of intensity-modulated radiation therapy (IMRT), excellent treatment outcomes have been achieved with radiotherapy alone for early-stage NPC. However, there is still controversy surrounding the treatment modalities of patients with stage II and T3N0 NPC.
Adding chemotherapy to radiotherapy can enhance the local efficacy of radiation and eliminate micro-metastases[3]. However, platinum-based chemotherapy can increase acute toxic reactions during treatment, leading to treatment interruptions, weight loss, and decreased compliance, which may compromise treatment benefits[4-6]. In the era of two-dimensional (2D) radiotherapy, the standard treatment modality for patients with stage II and T3N0 NPC was concurrent chemoradiotherapy (CCRT) [7, 8]. The National Comprehensive Cancer Network (NCCN) guidelines (2022, 2nd edition) and the Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines (2022) recommend using IMRT alone as the treatment modality for stage T2N0 patients, while other stage II patients and stage T3N0 patients are advised to undergo CCRT. Additionally, induction chemotherapy (IC) combined with CCRT and CCRT with adjuvant chemotherapy (AC) are both considered as Class II recommendations for T3N0 patients according to the CSCO guidelines. However, a multicenter phase III clinical trial evaluated patients with stage II and T3N0 NPC without adverse features and found that the 3-year failure-free survival rate with IMRT alone was not inferior to that with concurrent chemoradiotherapy, and had fewer adverse reactions during treatment[9]. In the same way, a retrospective propensity-matched cohort study suggested that adding chemotherapy concurrently to IMRT did not significantly improve survival rates for patients with stage II and T3N0 disease and resulted in more severe toxic reactions [10].
Despite the generally favorable prognosis of patients with stage II and T3N0 NPC, a small proportion of patients still experience treatment failure [11]. A reliable clinical prognostic model holds the potential to provide individualized treatment recommendations for patients with unfavorable prognoses. Currently, there is no prognostic model specifically designed for stage II and T3N0 NPC. In this study, we conducted prognostic analysis and developed a nomogram to differentiate patients' prognostic risks in this subset. Subgroup analysis further explored the long-term survival and toxicity reactions of patients with stage II and T3N0 NPC under different treatment modalities.
Materials and Methods
Study Population
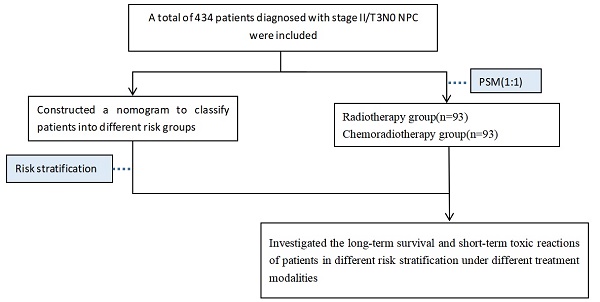
A total of 434 patients diagnosed with stage II or T3N0 NPC at Guangxi Medical University Cancer Hospital between 2011 and 2017 were included. Inclusion criteria were as follows: ① Patients pathologically confirmed nasopharyngeal carcinoma; ② Retrospective staging as stage II or T3N0 according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system; ③ General good condition (KPS ≥ 70), without severe concurrent medical or surgical diseases, and no concurrent malignancies; ④ Complete clinical data available; ⑤ Treated by IMRT with or without chemotherapy.
Data Collection and Follow-up
Baseline data collected included clinical information and serum parameters of all patients before treatment, such as gender, age, smoking history, pathological type, TNM staging, hemoglobin (HGB), albumin (ALB), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio(LMR), and treatment modalities. The primary endpoint was progression-free survival (PFS), and secondary endpoints was overall survival (OS).
Follow-up was conducted through various means, including phone calls, outpatient visits, and hospital reexaminations. Within the first 2 years after treatment, patients were followed up every 3 months. In the third year, follow-ups were conducted every 6 months, and thereafter, annually. For the local and regional recurrence of NPC, our follow-up measures included symptoms inquiries, physical examinations, nasopharyngoscopy, MRI of the nasopharynx and neck, and testing for EBV DNA. Regarding distant metastasis, our important follow-up methods consisted of whole-body CT, PET/CT, bone scintigraphy, and EBV DNA.
Treatment Methods
All patients received complete IMRT treatment with or without chemotherapy. Delineation of the target area and organs at risk followed the guidelines of International Commission on Radiation Units and Measurements Reports (ICRU) 50 and 62. The prescribed doses for target areas were as follows: GTVnx 70.06-75 Gy(30-32 fractions), GTVnd 60-73.6 Gy(30-32 fractions), CTV1 60-64 Gy(30-32 fractions), CTV2 54-57.6 Gy(30-32 fractions). Chemotherapy regimens included TP (docetaxel 75 mg/m2, day 1; cisplatin 75 mg/m2, day 1), PF (cisplatin 80 mg/m2, days 1-3; fluorouracil 750 mg/m2 continuous intravenous infusion over 120 hours), and TPF (docetaxel 60 mg/m2, day 1; cisplatin 60 mg/m2, day 1; fluorouracil 600 mg/m2 continuous intravenous infusion over 120 hours), administered every 3 weeks for 1-4 cycles for induction therapy. Concurrent chemotherapy consisted of cisplatin (100 mg/m2, days 1-3) every 3 weeks for 1-3 cycles. Adjuvant chemotherapy was administered with TPF, TP, or PF regimens, every 3 weeks for 1-4 cycles.
Statistical Analysis
X-tile software (Chicago, Rim Lab) was employed to determine the optimal cut-off values for continuous variables of laboratory data, and they were subsequently divided into binary variables. Statistical analysis was conducted using the 25th edition of SPSS for Windows (SPSS, Chicago, IL) software. Chi-square test or Fisher's exact test was performed to compare baseline characteristics and toxic reactions among different treatment groups. Patients were randomly assigned to training and validation cohorts in a 7:3 ratio. In the training cohort, univariate and multivariate Cox regression were used to identify independent prognostic factors for PFS. Kaplan-Meier method was performed for survival analysis, and survival rates were compared using the log-rank test. Independent prognostic factors were incorporated into the nomogram prediction model using R software version 4.3.0 (R project, http://www.R-project.org/). The concordance index (C-index) was calculated, and calibration curves were plotted to assess the accuracy of the model. X-tile software was used for risk stratification in the nomogram. Propensity score matching (PSM) with a caliper of 0.05 was employed in subgroup analysis to reduce selection bias between the radiotherapy and chemoradiotherapy groups. P-values of <0.05 were considered statistically significant for differences.
Results
Patient Characteristics
According to the inclusion criteria, we included 434 cases in this study. Among them, 304 patients were assigned to the training cohort, and 130 cases were assigned to the validation cohort. The baseline characteristics of the two cohorts are presented in Table 1. There were no statistically significant differences in baseline characteristics between the training and validation cohorts (p>0.05).
Baseline characteristics of patients with stage II/T3N0 NPC in training cohort and validation cohort
| Clinical factors | Total cohort | Training cohort | Validation cohort | p-value | |
|---|---|---|---|---|---|
| (N = 434) | (N =304) | (N = 130) | |||
| Age(years) | ≤50 | 284(65.4%) | 199(65.5%) | 85(65.4%) | 0.988 |
| >50 | 150(34.6%) | 105(34.5%) | 45(34.6%) | ||
| Gender | male | 315(72.6%) | 225(74.0%) | 90(69.2%) | 0.306 |
| female | 119(27.4%) | 79(26.0%) | 40(30.8%) | ||
| Smoking | No | 301(69.3%) | 215(70.7%) | 86(65.9%) | 0.319 |
| Yes | 133(30.7%) | 89(29.3%) | 44(34.1%) | ||
| pathology | K-NPC/ basaloid SCC | 46(10.6%) | 33(10.9%) | 13(10.0%) | 0.791 |
| NK-NPC | 388(89.4%) | 271(89.1%) | 117(90.0%) | ||
| T stage | T1 | 58(13.4%) | 39(12.8%) | 19(14.6%) | 0.359 |
| T2 | 357(82.2%) | 249(81.9%) | 108(83.1%) | ||
| T3 | 19(4.4%) | 16(5.3%) | 3(2.3%) | ||
| N stage | N0 | 68(15.7%) | 48(15.8%) | 20(15.4%) | 0.915 |
| N1 | 366(84.3%) | 256(84.2%) | 110(84.6%) | ||
| Clinical stage | C2 | 415(95.6%) | 288(94.7%) | 127(97.7%) | 0.168 |
| C3 | 19(4.4%) | 16(5.3%) | 3(2.3%) | ||
| Treatment | RT | 98(22.6%) | 71(23.4%) | 27(20.8%) | 0.605 |
| CCRT | 262(60.4%) | 181(59.5%) | 81(62.3%) | ||
| IC+CCRT | 26(6.0%) | 16(5.3%) | 10(7.7%) | ||
| CCRT+AC | 48(11.1%) | 36(11.8%) | 12(9.2%) | ||
| HGB(g/L) | ≤137 | 169(38.9%) | 119(39.1%) | 50(38.5%) | 0.894 |
| >137 | 265(61.1%) | 185(60.9%) | 80(61.5%) | ||
| ALB(g/L) | ≤45.1 | 283(65.2%) | 198(65.1%) | 85(65.4%) | 0.960 |
| >45.1 | 151(34.8%) | 106(34.9%) | 45(34.6%) | ||
| ALP(U/L) | ≤72 | 319(73.5%) | 222(73.0%) | 97(74.6%) | 0.731 |
| >72 | 115(26.5%) | 82(27.0%) | 33(25.4%) | ||
| LDH(U/L) | ≤222 | 386(88.9%) | 276(90.8%) | 110(84.6%) | 0.060 |
| >222 | 48(11.1%) | 28(9.2%) | 20(15.4%) | ||
| NLR | ≤2.2 | 261(60.1%) | 189(62.2%) | 72(55.4%) | 0.186 |
| >2.2 | 173(39.9%) | 115(37.8%) | 58(44.6%) | ||
| PLR | ≤114.4 | 150(34.6%) | 106(34.9%) | 44(34.1%) | 0.837 |
| >114.4 | 284(65.4%) | 198(65.1%) | 86(65.9%) | ||
| LMR | ≤4.2 | 142(32.7%) | 100(32.9%) | 42(32.3% | 0.905 |
| >4.2 | 292(67.3%) | 204(67.1%) | 88(67.7%) | ||
| Progression | No | 391(90.1%) | 274(90.1%) | 117(90.0%) | 0.966 |
| Yes | 43(9.9%) | 30(9.9%) | 13(10.0%) |
Note: NK-NPC: non-keratinizing squamous cell carcinoma; K-NPC: keratinizing squamous cell carcinoma; basaloid SCC: basaloid squamous cell carcinoma; RT: radiotherapy; CCRT: concurrent chemoradiotherapy; IC: induction chemotherapy; AC: adjuvant chemotherapy; HGB: hemoglobin; ALB: albumin; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LMR: Lymphocyte-monocyte ratio.
Univariate and multivariable cox analysis of the risk fators for PFS in the training cohort
| Characteristic | Univariate Cox regression analysis | Multivariate Cox regression analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Gender (Female, Male) | 0.900(0.386~2.098) | 0.807 | - | - |
| Age (Years; ≤50, >50) | 1.737(0.848~3.559) | 0.131 | - | - |
| Smoking (No, Yes) | 0.838(0.373~1.884) | 0.670 | - | - |
| pathology (Others, NK-NPC) | 0.376(0.161~0.877) | 0.024 | 0.324(0.138~0.759) | 0.009 |
| Treatment (radiotherapy, chemoradiotherapy) | 1.332(0.764~2.324) | 0.312 | - | - |
| HGB (g/L; ≤137, >137) | 0.709(0.346~1.454) | 0.348 | - | - |
| ALB (g/L; ≤45.1, >45.1) | 0.683(0.311~1.501) | 0.342 | - | - |
| ALP (U/L; ≤72, >72) | 3.130(1.523~6.432) | 0.002 | 3.181(1.532~6.604) | 0.002 |
| LDH (U/L; ≤222, >222) | 3.966(1.765~8.913) | <0.001 | 3.559(1.570~8.066) | 0.002 |
| NLR (≤2.2, >2.2) | 1.741(0.851~3.562) | 0.129 | - | - |
| PLR (≤114.4, >114.4) | 1.859(0.798~4.333) | 0.151 | - | - |
| LMR (≤4.2, >4.2) | 0.522(0.255~1.071) | 0.076 | - | - |
| T stage (T1, T2-3) | 2.056(0.490~8.631) | 0.325 | - | - |
| N stage (N0, N1) | 1.729(0.525~5.701) | 0.368 | - | - |
| Clinical stage (II, III) | 1.472(0.350~6.184) | 0.598 | - | - |
Note: Female, age≤50 years old, no smoking history, other pathology, radiotherpy, HGB≤137g/L, ALB≤45.1g/L, ALP≤72U/L, LDH≤222U/L, NLR≤2.2, PLR≤114.4, LMR≤4.2, T1, N0 and clinical stage of II were the reference groups. NK-NPC: non-keratinizing Squamous Cell Carcinoma; HGB: hemoglobin; ALB: albumin; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LMR: Lymphocyte-monocyte ratio.
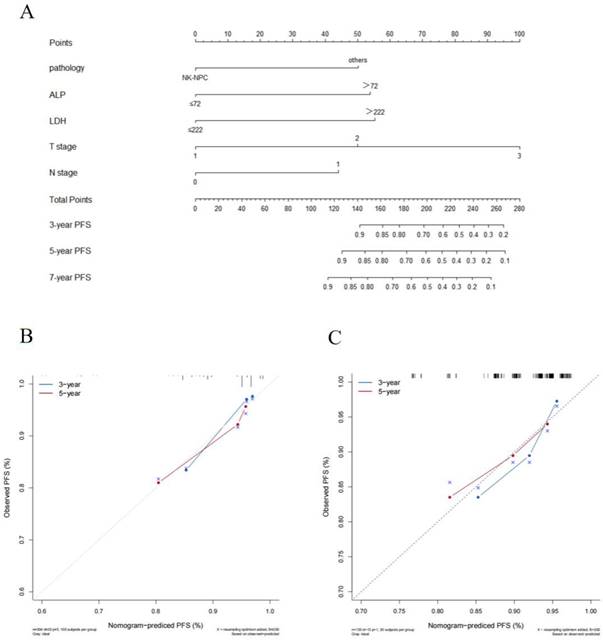
(A) Prognostic nomogram predicting NPC patients with stage II /T3N0 for PFS in the training cohort.The calibration curves of the nomogram for (B) the training cohort and (C) the validation cohort. Note: NK-NPC: non-keratinizing squamous cell carcinoma; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; PFS: progression-free survival.
Establishment and Validation of the Nomogram
The cut-off values for HGB (137g/L), ALB (45.1g/L), ALP (72U/L), LDH (222U/L), NLR (2.2), PLR (114.4) and LMR (4.2) were calculated by X-tile software. In the training cohort, we explored the prognostic factors for PFS and constructed a nomogram. Univariate Cox regression analysis indicated that pathology, LDH, and ALP were significant prognostic factors for patients (p < 0.05). The factors with statistical significance in the univariate analysis were then incorporated into the multivariate Cox regression model, revealing that pathology, LDH, and ALP were independent prognostic factors (p < 0.05), as shown in Table 2. Although T stage and N stage were not independent prognostic factors in the Cox regression analysis of this study, their importance is widely recognized [12]. Therefore, we constructed the nomogram for PFS by combining the independent prognostic factors with TNM stage (Figure 1A).
The C-index of the nomogram was 0.73(95% CI: 0.63-0.83) in the training cohort and 0.70(95% CI: 0.54-0.86) in the validation cohort which shows good distinction. The calibration curves in both cohorts closely approximated the standard curve, indicating high accuracy of the nomogram (Figure 1B and C).
Risk Stratification and Survival Analysis
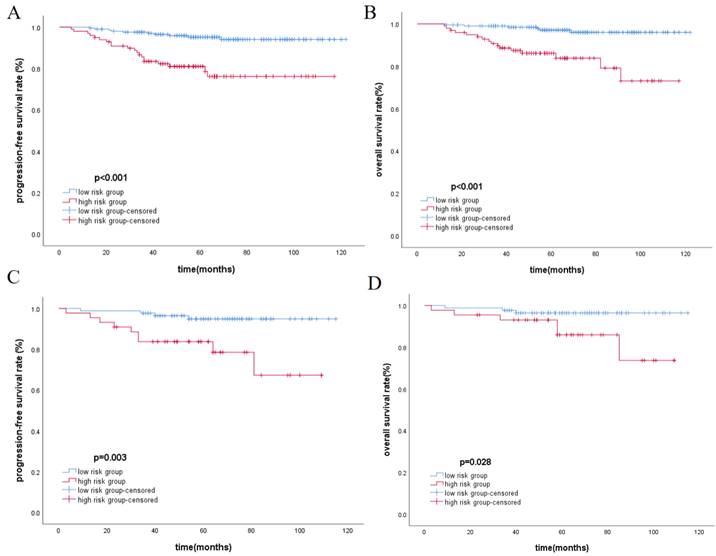
The total score of the nomogram was calculated for each patient, and the X-tile software was used to select the optimal cut-off value of 104 points. Based on this value, patients were divided into the low-risk group (≤104) and the high-risk group (>104). The Kaplan-Meier method was used to plot survival curves, indicating that in the training cohort and validation cohort, the low-risk group had significantly better PFS and OS compared to the high-risk group (p<0.05), as shown in Figure 2. In the overall cohort, the 5-year PFS for the low-risk group was 95.1%, while it was 81.9% for the high-risk group. Similarly, patients in the low-risk group achieved better OS, with 5-year OS rates of 96.9% for the low-risk group and 85.9% for the high-risk group.
Kaplan-Meier analysis for PFS and OS in training cohort(A, B) and validation cohort(C, D), respectively.
Toxicity and Subgroup Survival Analysis
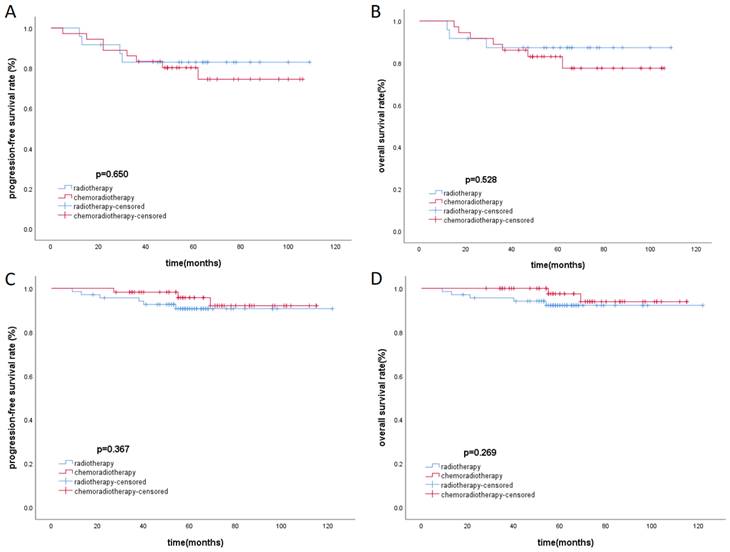
There was baseline data imbalance between the radiotherapy group and the chemoradiotherapy group. After propensity score matching (with a strict caliper of 0.05), 93 pairs of cases were selected, which balanced the baseline differences between the two cohorts (Table 3). Acute toxicities during treatment were evaluated in the PSM cohort, including the radiotherapy group and the chemoradiotherapy group. Compared to the radiotherapy group, patients in the chemoradiotherapy group had higher rates of grade 1-4 leukopenia, anemia, thrombocytopenia, and gastrointestinal reactions (p<0.05). Moreover, the chemoradiotherapy group had a higher proportion of patients experiencing severe grade 3-4 leukopenia (Table 4). In the low-risk group and the high-risk group after PSM, Kaplan-Meier survival analysis showed no statistically significant differences in PFS and OS under different treatment methods (Figure 3).
Patients characteristics in unmatched cohort and matched cohort
| Clinical Factors | Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|---|
| Radiotherapy | Chemoradiotherapy | p-value | Radiotherapy | Chemoradiotherapy | p-value | ||
| (N=98) | (N=336) | (N=93) | (N=93) | ||||
| Age(years) | ≤50 | 51(52.0%) | 233(69.3%) | 0.002 | 50(53.8%) | 53(57.0%) | 0.658 |
| >50 | 47(48.0%) | 103(30.7%) | 43(46.2%) | 40(43.0%) | |||
| Gender | Male | 70(71.4%) | 245(72.9%) | 0.771 | 66(71.0%) | 72(77.4%) | 0.315 |
| Female | 28(28.6%) | 91(27.1%) | 27(29.0%) | 21(22.6%) | |||
| Smoking | No | 69(70.4%) | 232(69.0%) | 0.797 | 64(68.8%) | 53(57.0%) | 0.095 |
| Yes | 29(29.6%) | 104(31.0%) | 29(31.2%) | 40(43.0%) | |||
| pathology | K-NPC/basaloid SCC | 12(12.2%) | 34(10.1%) | 0.547 | 11(11.8%) | 12(12.9%) | 0.824 |
| NK-NPC | 86(87.8%) | 302(89.9%) | 82(88.2%) | 81(87.1%) | |||
| T stage | T1 | 15(15.3%) | 43(12.8%) | 0.521 | 14(15.1%) | 9(9.7%) | 0.265 |
| T2-3 | 83(84.7%) | 293(87.2%) | 79(84.9%) | 84(90.3%) | |||
| N stage | N0 | 23(23.%) | 45(13.4%) | 0.016 | 18(19.4%) | 20(21.5%) | 0.716 |
| N1 | 75(76.5%) | 291(86.6%) | 75(80.6%) | 73(78.5%) | |||
| HGB(g/L) | ≤137 | 45(45.9%) | 124(36.9%) | 0.107 | 41(44.1%) | 42(45.2%) | 0.883 |
| >137 | 53(54.1%) | 212(63.1%) | 52(55.9%) | 51(54.8%) | |||
| ALB(g/L) | ≤45.1 | 69(70.4%) | 214(63.7%) | 0.219 | 65(69.9%) | 66(71.0%) | 0.872 |
| >45.1 | 29(29.6%) | 122(36.3%) | 28(30.1%) | 27(29.0%) | |||
| ALP(U/L) | ≤72 | 77(78.6%) | 242(72.0%) | 0.196 | 74(79.6%) | 66(71.0%) | 0.174 |
| >72 | 21(21.4%) | 94(28.0%) | 19(20.4%) | 27(29.0%) | |||
| LDH(U/L) | ≤222 | 87(88.8%) | 299(89.0%) | 0.935 | 84(90.3%) | 81(87.1%) | 0.487 |
| >222 | 11(11.2%) | 37(11.0%) | 9(9.7%) | 12(12.9%) | |||
| NLR | ≤2.2 | 67(68.4%) | 261(60.1%) | 0.059 | 63(67.7%) | 52(55.9%) | 0.097 |
| >2.2 | 31(31.6%) | 173(39.9%) | 30(32.3%) | 41(44.1%) | |||
| PLR | ≤114.4 | 33(33.7%) | 117(34.8%) | 0.833 | 32(34.4%) | 30(32.3%) | 0.756 |
| >114.4 | 65(66.3%) | 219(65.2%) | 61(65.6%) | 63(67.7%) | |||
| LMR | ≤4.2 | 30(30.6%) | 112(33.3%) | 0.613 | 29(31.2%) | 34(36.6%) | 0.439 |
| >4.2 | 68(69.4%) | 224(66.7%) | 64(68.8%) | 59(63.4%) | |||
Note: K-NPC: keratinizing squamous cell carcinoma; basaloid SCC: basaloid squamous cell carcinoma; NK-NPC: non-keratinizing squamous cell carcinoma; HGB: hemoglobin; ALB: albumin; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LMR: Lymphocyte-monocyte ratio.
Acute toxic reactions of patients in the radiotherapy group and chemoradiotherapy group
| Adverse Event | Radiotherapy | Chemoradiotherapy | p-value | |
|---|---|---|---|---|
| (N=93) | (N=93) | |||
| Leucopenia | All | 46(49.5%) | 77(82.8%) | <0.001 |
| Grade 3-4 | 0(0.0%) | 23(24.7%) | <0.001 | |
| Anemia | All | 0(0.0%) | 31(33.3%) | <0.001 |
| Grade 3-4 | 0(0.0%) | 2(2.2%) | 0.497 | |
| Thrombocytopenia | All | 0(0.0%) | 8(8.6%) | 0.007 |
| Grade 3-4 | 0(0.0%) | 1(1.1%) | 1.000 | |
| Liver Dysfunction | All | 11(11.8%) | 3(3.2%) | 0.050 |
| Grade 3-4 | 0(0.0%) | 0(0.0%) | - | |
| Gastrointestinal reaction | All | 8(8.6%) | 52(55.9%) | <0.001 |
| Grade 3-4 | 0(0.0%) | 5(5.4%) | 0.059 |
Kaplan-Meier survival curves of PFS and OS in high-risk (A, B) group and low-risk group (C, D) respectively.
Discussion
This study explored the clinical independent prognostic factors for stage II and T3N0 NPC and successfully constructed a nomogram incorporating the TNM staging system. The nomogram we developed successfully stratified patients with stage II and T3N0 NPC into high-risk and low-risk groups. However, in different risk groups, the addition of chemotherapy to radiotherapy did not improve patients' PFS and OS and increased more chemotherapy-related toxicities.
The TNM staging system is currently the most reliable staging system for NPC and is commonly used in clinical practice to assist doctors in making treatment decisions[13]. However, there is still controversy regarding the treatment approach for patients with stage II and T3N0 NPC. These patients have similar T and N stages, making it difficult to distinguish patients with different prognoses. A reliable prognostic model is expected to provide a basis for individualized treatment strategies in clinical practice.
We explored the independent prognostic factors for PFS in this staging, including pathological type, ALP, and LDH. Previously, researchers believed that adverse prognostic factors for patients with stage II and T3N0 included extracapsular extension of cervical lymph nodes[14], lymph node cross-sectional diameter ≥3 cm, positive lymph nodes in the IV/Vb region[15], and pre-treatment plasma EB viral DNA copy number ≥4000 copies/ml[16]. Our study is expected to provide additional insights into adverse prognostic factors for patients in this staging of nasopharyngeal carcinoma. According to the 5th edition of the WHO classification of head and neck tumors, NPC can be classified into three subtypes:(i) non-keratinizing squamous cell carcinoma (NK-NPC), (ii) keratinizing squamous cell carcinoma(K-NPC), and (iii) basaloid squamous cell carcinoma (basaloid SCC)[17]. The histological subtypes of nasopharyngeal carcinoma exhibit significant geographical and racial distribution[18]. In our study, NK-NPC patients had a better prognosis, which is consistent with previous research findings[19, 20]. Although undifferentiated tumors are generally considered more invasive, NK-NPC has been shown to have higher radiosensitivity, which may be a contributing factor to the different prognosis[21].
LDH is an enzyme in the glycolytic pathway, and its levels increase with the release of anaerobic metabolism in malignant tumors[22]. Elevated LDH has been widely reported to indicate poor prognosis in various types of tumors[23]. The reason for the association between high LDH levels and poor tumor prognosis may be related to the hypoxic environment associated with high tumor burden, leading to increased LDH production[24]. Additionally, elevated LDH levels may lead to upregulation of the HIF pathway, increased expression of vascular endothelial growth factor, and weakened immune function[25-27], all of which could contribute to adverse tumor outcomes. In our study, LDH>222U/L was identified as an independent risk factor for disease progression, which aligns with these previous research findings. ALP is a phosphomonoesterase related to human bone metabolism[28]. Although elevated ALP has been shown to be a poor prognostic factor in various tumors[29-31], the exact reasons for its impact on NPC prognosis remain unclear. A study found that elevated ALP levels were common in patients with T3-T4 stage NPC and speculated a connection with skull base invasion in these patients[32]. In our study, we found that elevated ALP levels were also associated with poor prognosis in stage II and T3N0 patients, suggesting that ALP might be involved in other mechanisms contributing to adverse NPC prognosis. Previous research has shown that elevated ALP is closely related to lymph node involvement[33]. A fundamental study also suggested that ALP might promote proliferation at the cellular level[34]. Furthermore, high levels of ALP have been linked to occult metastasis, which cannot be detected by imaging[35]. Therefore, we believe that elevated ALP levels can be used for risk assessment of poor prognosis in stage II and T3N0 patients.
While HGB, ALB, NLR, and other indicators in this study have been shown to be associated with NPC prognosis[36-38], they were not independent prognostic factors in this study. This may be attributed to the differences in tumor staging and cutoff value selection in different studies. T and N staging, although representing important prognostic indicators, were not independent prognostic factors in this study, possibly due to the early staging and similar staging of this subgroup of patients. Considering that TNM staging is one of the most reliable prognostic indicators in clinical practice, we included it along with other independent prognostic factors in our nomogram. Our prognostic model incorporates commonly available clinical and serological parameters that are easily obtained and analyzed in clinical practice.
Through the nomogram, we divided patients with stage II and T3N0 NPC into high and low-risk subgroups, showing significant differences in PFS and OS (p<0.05). In subgroup survival analysis, the addition of chemotherapy did not provide additional survival benefits to different risk groups or the overall population. On the contrary, the use of chemotherapy increased the occurrence of more acute toxic reactions and severe grade 3-4 acute reactions. Recently, immunotherapy drugs such as pembrolizumab and camrelizumab have made breakthrough progress in the treatment of recurrent or metastatic NPC[39, 40]. Additionally, a large-scale retrospective study with long-term follow-up suggested that the addition of cetuximab and nimotuzumab to chemoradiotherapy may effectively maximize the survival of patients with stage II-IVb nasopharyngeal carcinoma[41]. Whether high-risk patients in stage II and T3N0 can benefit from immunotherapy or targeted therapy, or whether optimizing low-toxicity chemotherapy regimens, is worth considering and exploring in clinical trials. Our study provides evidence for evidence-based medicine in this regard. Compared with the TNM staging system, the nomogram we built contains a border type of clinical and characteristics, which could reflect the biological differences of different patients. The study is expected to provide a basis for clinicians to develop individualized treatment strategies for NPC patients in stage II and T3N0.
This study has certain limitations. Firstly, it is a single-center retrospective study, which may introduce selection bias in patient selection. Secondly, EBV-DNA, as a potential prognostic factor[42], was not included in our study. This is because EBV-DNA was not a routine examination in our center, and there was currently no unified method for detecting EBV-DNA among different laboratories, leading to significant variations in results. Thirdly, this study is retrospective, and we did not completely standardize the diagnostic and treatment approaches for different patients, which may have influenced the results to some extent. Furthermore, we did not stratify the different chemotherapy methods, primarily due to the limited sample size in this staging group. These limitations need to be addressed in future studies.
Conclusion
We explored the independent prognostic factors of stage II and T3N0 NPC and validated them by constructing a nomogram. The nomogram successfully classified patients in this stage into high-risk and low-risk groups. The addition of chemotherapy to radiotherapy did not provide survival benefits in different risk groups and led to more chemotherapy-related adverse reactions. Further prospective exploration of treatment strategies for high-risk patients in this stage is needed.
Abbreviations
NPC: nasopharyngeal carcinoma; PFS: progression-free survival; OS: overall survival; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; IMRT: intensity-modulated radiation therapy; NCCN: The National Comprehensive Cancer Network; AJCC staging system: the American Joint Committee on Cancer staging system; CSCO: the Chinese Society of Clinical Oncology; CCRT: concurrent chemoradiotherapy; IC: induction chemotherapy; AC: adjuvant chemotherapy; ICRU: International Commission on Radiation Units and Measurements Reports; C-index: The concordance index; PSM: Propensity score matching; HGB: hemoglobin; ALB: albumin; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LMR: Lymphocyte-monocyte ratio; NK-NPC: non-keratinizing Squamous Cell Carcinoma; K-NPC: keratinizing Squamous Cell Carcinoma; basaloid SCC: basaloid Squamous Cell Carcinoma.
Acknowledgements
Funding
This work was sponsored by Guangxi Key R&D Program (No. GuikeAB18221007) and the National Natural Science Foundation Program (No. 81760544).
Author contributions
All authors made significant contribution to the study. CY and XDZ and SQ contributed to the conception of the study CY, RZ, XC, WWM and LL collected the data. CY, HKC, CYZ, BYC anlayzed the data. CY wrote the manuscript. All authors reviewed the manuscript.
Data sharing statement
The datasets analyzed during the study are available from the corresponding author on reasonable request.
Ethics Approval
This study was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital, in compliance with the Declaration of Helsinki. Patients identity remained anonymous, and the requirement for the informed consent was waived due to the retrospective nature of the study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wei KR, Zheng RS, Zhang SW, Liang ZH, Li ZM, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chinese journal of cancer. 2017;36(1):90
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65(2):87-108
3. Li S, Deng YQ, Zhu ZL, Hua HL, Tao ZZ. A Comprehensive Review on Radiomics and Deep Learning for Nasopharyngeal Carcinoma Imaging. Diagnostics(Basel, Switzerland). 2021 11(9)
4. Du XJ, Tang LL, Mao YP. et al. Value of the prognostic nutritional index and weight loss in predicting metastasis and long-term mortality in nasopharyngeal carcinoma. Journal of translational medicine. 2015;13:364
5. Zhao F, Yang D, Li X. Effect of radiotherapy interruption on nasopharyngeal cancer. Frontiers in oncology. 2023;13:1114652
6. Du CR, Ying HM, Kong FF, Zhai RP, Hu CS. Concurrent chemoradiotherapy was associated with a higher severe late toxicity rate in nasopharyngeal carcinoma patients compared with radiotherapy alone: a meta-analysis based on randomized controlled trials. Radiation oncology(London, England). 2015;10:70
7. Chan AT, Grégoire V, Lefebvre JL. et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology. 2012;23(Suppl 7):vii83-85
8. Aftab O, Liao S, Zhang R. et al. Efficacy and safety of intensity-modulated radiotherapy alone versus intensity-modulated radiotherapy plus chemotherapy for treatment of intermediate-risk nasopharyngeal carcinoma. Radiation oncology(London, England). 2020;15(1):66
9. Tang LL, Guo R, Zhang N. et al. Effect of Radiotherapy Alone vs Radiotherapy With Concurrent Chemoradiotherapy on Survival Without Disease Relapse in Patients With Low-risk Nasopharyngeal Carcinoma: A Randomized Clinical Trial. Jama. 2022;328(8):728-736
10. Zhang AM, Fan Y, Wang XX. et al. Increased treatment-related mortality with additional cisplatin-based chemotherapy in patients with nasopharyngeal carcinoma treated with standard radiotherapy. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;104(3):279-285
11. Luo S, Zhao L, Wang J. et al. Clinical outcomes for early-stage nasopharyngeal carcinoma with predominantly WHO II histology treated by intensity-modulated radiation therapy with or without chemotherapy in nonendemic region of China. Head & neck. 2014;36(6):841-847
12. Chiang CL, Guo Q, Ng WT. et al. Prognostic Factors for Overall Survival in Nasopharyngeal Cancer and Implication for TNM Staging by UICC: A Systematic Review of the Literature. Frontiers in oncology. 2021;11:703995
13. Pan JJ, Ng WT, Zong JF. et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122(4):546-558
14. Pan J, Xu Y, Qiu S. et al. A comparison between the Chinese 2008 and the 7th edition AJCC staging systems for nasopharyngeal carcinoma. American journal of clinical oncology. 2015;38(2):189-196
15. Huang CL, Chen Y, Guo R. et al. Prognostic value of MRI-determined cervical lymph node size in nasopharyngeal carcinoma. Cancer medicine. 2020;9(19):7100-7106
16. Zhang F, Zhang Y, Li WF. et al. Efficacy of Concurrent Chemotherapy for Intermediate Risk NPC in the Intensity-Modulated Radiotherapy Era: a Propensity-Matched Analysis. Scientific reports. 2015;5:17378
17. Badoual C. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Oropharynx and Nasopharynx. Head and neck pathology. 2022;16(1):19-30
18. Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer cell. 2004;5(5):423-8
19. Li Z, Li C, Yang D, Zhou Z, Kang M. Nomogram to Predict Long-Term Overall Survival and Cancer-Specific Survival of Radiotherapy Patients with Nasopharyngeal Carcinoma. BioMed research international. 2023;2023:7126881
20. Pan XX, Liu YJ, Yang W, Chen YF, Tang WB, Li CR. Histological subtype remains a prognostic factor for survival in nasopharyngeal carcinoma patients. The Laryngoscope. 2020;130(3):E83-e88
21. Stepan KO, Mazul AL, Skillington SA. et al. The prognostic significance of race in nasopharyngeal carcinoma by histological subtype. Head & neck. 2021;43(6):1797-1811
22. Schwartz MK. Lactic dehydrogenase. An old enzyme reborn as a cancer marker? American journal of clinical pathology. 1991;96(4):441-443
23. Deme D, Telekes A. [Prognostic importance of lactate dehydrogenase(LDH) in oncology]. Orvosi hetilap. 2017;158(50):1977-1988
24. Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. The Journal of biological chemistry. 1995;270(36):21021-7
25. Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase-5(LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor(HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Annals of surgical oncology. 2008;15(8):2336-44
26. Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL. Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway-a report of the Tumour Angiogenesis Research Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(26):4301-8
27. Zhang M, Wei S, Su L, Lv W, Hong J. Prognostic significance of pretreated serum lactate dehydrogenase level in nasopharyngeal carcinoma among Chinese population: A meta-analysis. Medicine. 2016;95(35):e4494
28. Harris H. The human alkaline phosphatases: what we know and what we don't know. Clinica chimica acta; international journal of clinical chemistry. 1990;186(2):133-150
29. Sun P, Chen S, Li Y. The association between pretreatment serum alkaline phosphatase and prognosis in hepatocellular carcinoma: A meta-analysis. Medicine. 2020;99(11):e19438
30. Wei XL, Zhang DS, He MM. et al. The predictive value of alkaline phosphatase and lactate dehydrogenase for overall survival in patients with esophageal squamous cell carcinoma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37(2):1879-1887
31. Chen L, Zeng H, Yang J. et al. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC cancer. 2018;18(1):816
32. Li G, Gao J, Tao YL. et al. Increased pretreatment levels of serum LDH and ALP as poor prognostic factors for nasopharyngeal carcinoma. Chinese journal of cancer. 2012;31(4):197-206
33. Aminian A, Karimian F, Mirsharifi R. et al. Correlation of serum alkaline phosphatase with clinicopathological characteristics of patients with oesophageal cancer. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2011;17(11):862-6
34. Han J, Yong B, Luo C, Tan P, Peng T, Shen J. High serum alkaline phosphatase cooperating with MMP-9 predicts metastasis and poor prognosis in patients with primary osteosarcoma in Southern China. World journal of surgical oncology. 2012;10:37
35. Xie Y, Wei ZB, Duan XW. Prognostic value of pretreatment serum alkaline phosphatase in nasopharyngeal carcinoma. Asian Pacific journal of cancer prevention: APJCP. 2014;15(8):3547-53
36. Zhang LN, Tang J, Lan XW, OuYang PY, Xie FY. Pretreatment anemia and survival in nasopharyngeal carcinoma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37(2):2225-2231
37. Guo SS, Tang LQ, Chen QY. et al. Is Hemoglobin Level in Patients with Nasopharyngeal Carcinoma Still a Significant Prognostic Factor in the Era of Intensity-Modulated Radiotherapy Technology? PloS one. 2015;10(8):e0136033
38. Jiang W, Chen Y, Huang J. et al. Systemic immune-inflammation index predicts the clinical outcome in patients with nasopharyngeal carcinoma: a propensity score-matched analysis. Oncotarget. 2017;8(39):66075-66086
39. Yang Y, Qu S, Li J. et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma(CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncology. 2021;22(8):1162-1174
40. Mai HQ, Chen QY, Chen D. et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nature medicine. 2021;27(9):1536-1543
41. You R, Hua YJ, Liu YP. et al. Concurrent Chemoradiotherapy with or without Anti-EGFR-Targeted Treatment for Stage II-IVb Nasopharyngeal Carcinoma: Retrospective Analysis with a Large Cohort and Long Follow-up. Theranostics. 2017;7(8):2314-2324
42. You R, Liu YP, Lin M. et al. Relationship of circulating tumor cells and Epstein-Barr virus DNA to progression-free survival and overall survival in metastatic nasopharyngeal carcinoma patients. International journal of cancer. 2019;145(10):2873-2883
Author contact
![]() Corresponding author: Xiao-Dong Zhu, zhuxiaodongedu.cn.
Corresponding author: Xiao-Dong Zhu, zhuxiaodongedu.cn.

 Global reach, higher impact
Global reach, higher impact