3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(17):3275-3284. doi:10.7150/jca.86746 This issue Cite
Research Paper
Adverse Event Profile Differences between Trastuzumab Emtansine and Trastuzumab Deruxtecan: A Real-world, Pharmacovigilance Study
1. Department of Pharmacy, Hunan Cancer Hospital, the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha 410011, Hunan, China.
2. Department of Pharmacy, Yantai Hospital of Traditional Chinese Medicine, Yantai 264000, Shandong, China.
3. Department of Pharmacy, Affiliated Qingdao Central Hospital of Qingdao University, Qingdao Cancer Hospital, Qingdao, 266042, Shandong, China.
4. Precision Medicine Center of Oncology, The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao, 266003, China.
*These authors contributed equally to this work and should be considered co-first authors.
Abstract
Introduction: Trastuzumab emtansine(T-DM1) and trastuzumab deruxtecan (T-DXd, formerly DS-8201a), the human epidermal growth factor receptor 2 (HER2)-targeted antibody-drug conjugate (ADC), are commonly used in metastatic breast cancer. However, their real-world safety profile has not been adequately compared.
Objective: We aimed to investigate the adverse event (AE) profile of T-DM1 and T-DXd reported by the US Food and Drug Administration Adverse Event Reporting System (FAERS).
Methods: All indications were searched for T-DM1 and T-DXd, as primary suspected drugs, from FAERS data (January 2004 to June 2023). Disproportionality analyses were performed by reporting odds ratios (ROR) and proportional reporting ratio (PRR). The odds ratio (OR) of fatal AEs associated with T-DM1 and T-DXd under different exposure factors were performed by univariate and multivariate logistical regression analysis.
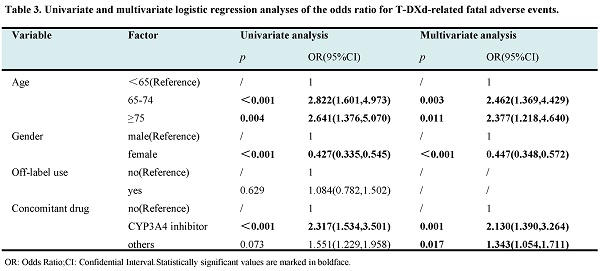
Results: 3723 and 2045 reports of T-DM1 and T-DXd were submitted to FAERS. Finally, 94 and 61 significant signals for T-DM1 and T-DXd were systematically analyzed. The valid AEs with the highest frequency and the strongest signal intensity for T-DM1 were platelet count decreased (n=108) and hepatopulmonary syndrome (ROR=680.42), respectively. Interstitial lung disease (n=262, ROR=82.55) and pneumonitis (n=89, ROR = 48.34) showed both high frequency and strong signal intensity for T-DXd. The proportion of AEs in each SOC system was different. T-DM1 had a greater proportion of valid AEs in the nervous system, musculoskeletal system, hepatobiliary system, ocular system, cardiac system and hematologic system(p<0.05). T-DXd had a greater proportion of valid AEs in the skin disorders, respiratory system, infestations, general system and gastrointestinal system(p<0.05). Furthermore, the analysis of fatal AEs in four systems revealed that T-DXd exhibited a significantly higher proportion of fatal outcomes in the hematologic and respiratory system compared to T-DM1. Conversely, T-DM1 had a significantly higher proportion of fatal outcomes in the hepatobiliary system. Neither T-DM1 nor T-DXd exhibited a high mortality ratio in the cardiac system. Logistic regression analysis indicated that advanced age (≥65 years) and male gender were identified as independent risk factors of fatal AEs for both T-DM1 and T-DXd. Additionally, the drug combination therapy, particularly with a CYP3A4 inhibitor, was found to be a risk factor for fatal AEs specifically related to T-DXd.
Conclusions: Hematological and respiratory toxicity of T-DXd and hepatobiliary toxicity of T-DM1 exhibited a high incidence of fatal outcomes. It is crucial to identify high-risk factors and enhance the monitoring of AEs during clinical application.
Keywords: trastuzumab emtansine, trastuzumab deruxtecan, FAERS, pharmacovigilance, real-world, adverse event

 Global reach, higher impact
Global reach, higher impact