Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(16):3139-3150. doi:10.7150/jca.87315 This issue Cite
Review
Digestive tract reconstruction after laparoscopic proximal gastrectomy for Gastric cancer: A systematic review
1. General Surgery, Cancer Center, Department of Gastrointestinal and Pancreatic Surgery, Zhejiang Provincial People's Hospital (Affiliated People's Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, China. Key Laboratory of Gastroenterology of Zhejiang Province, Hangzhou, Zhejiang, China.
2. Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China.
# These authors contributed equally to this work.
Received 2023-6-19; Accepted 2023-8-10; Published 2023-9-25
Abstract
The incidence of gastroesophageal junction adenocarcinoma has gradually increased. Proximal gastrectomy or total gastrectomy is recommended for early gastric cancer of the upper third of the stomach. Because total gastrectomy is often accompanied by body mass loss and nutrient absorption disorders, such as severe hypoproteinemia and anemia, Proximal gastrectomy is more frequently recommended by researchers for early upper gastric cancer (T1N0M0) and Siewert II gastroesophageal junction cancer less than 4 cm in length. Although some functions of the stomach are retained after proximal gastrectomy, the anatomical structure of the gastroesophageal junction can be destroyed, and the anti-reflux effect of the cardia is lost. In recent years, as various reconstruction methods for anti-reflux function have been developed, some functions of the stomach are retained, and serious reflux esophagitis is avoided after proximal gastrectomy. In this article, we summarized the indications, advantages, and disadvantages of various classic reconstruction methods and latest improved reconstruction method including esophageal and residual stomach anastomosis, tubular gastroesophageal anastomosis, muscle flap anastomosis, jejunal interposition, and double-tract reconstruction.
Keywords: gastroesophageal junction adenocarcinoma, proximal gastrectomy, adenocarcinoma, tubular gastroesophageal anastomosis, muscle flap anastomosis, jejunal interposition, double-tract reconstruction
Introduction
Gastric cancer is one of the most common malignant tumors globally. In 2018, gastric cancer ranked fifth and third among malignant tumors in global incidence and mortality, respectively. China accounted for 44.1% and 49.9% of the global incidence and mortality of gastric cancer, respectively[1]. National Cancer Center data in 2020 revealed that gastric cancer is the second and third most common cancer in men and women, respectively, and the third leading cause of cancer-related mortality[2]. Over the past 30-40 years, the incidence of gastroesophageal junction adenocarcinoma has gradually increased[3,4,5]. Proximal gastrectomy (PG) or total gastrectomy is recommended for early gastric cancer of the upper third of the stomach[6,7]. Because total gastrectomy is often accompanied by body mass loss and nutrient absorption disorders, such as severe hypoproteinemia and anemia, PG is more frequently recommended by researchers for early upper gastric cancer (T1N0M0) and Siewert II gastroesophageal junction cancer less than 4 cm in length[8,9,10,11,12]. Although some functions of the stomach are retained after PG, the anatomical structure of the gastroesophageal junction can be destroyed, and the anti-reflux effect of the cardia is lost. The retained pylorus delays gastric emptying to a certain extent[13]. Severe reflux esophagitis and anastomotic stenosis are common after PG[14]. In recent years, as various reconstruction methods for anti-reflux function have been developed, some functions of the stomach are retained, and serious reflux esophagitis is avoided after PG. Although there are many reviews on digestive tract reconstruction after proximal gastrectomy, this manuscrpit adds new research progress in the last two years. In this article, the indications, advantages, and disadvantages of various reconstruction methods are summarized.
Esophageal and residual stomach anastomosis
Esophageal and residual stomach anastomosis
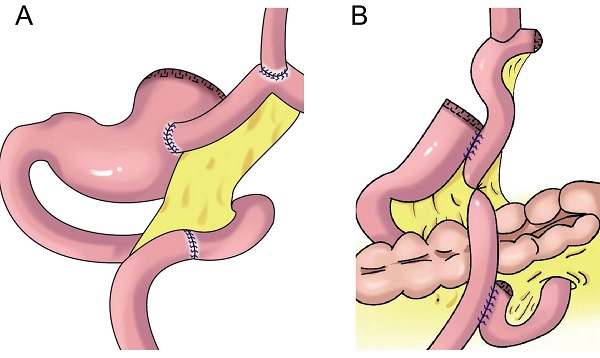
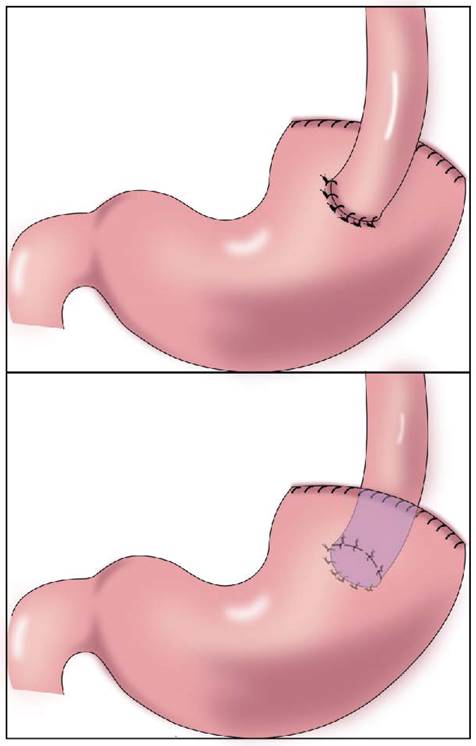
In 1897, Mikulicz performed esophageal and residual stomach anastomosis for the first time (Figure 1)[15]. This procedure resulted in a high incidence of postoperative complications (such as reflux esophagitis, abnormal gastric emptying, and malnutrition), but it was later improved to esophagogastric anterior wall anastomosis (Figure 1). The top of the retained stomach forms a structure similar to the stomach bottom, creating an angle of His, with a certain anti-reflux effect[13]. Esophageal and gastric anastomosis conforms to the physiological structure of the digestive tract, ensuring that the residual stomach has sufficient digestion and absorption function. After chyme is digested through the residual stomach and passes through the duodenum, the secretion of bile and pancreatic juice can be promoted, thereby promoting the digestion and absorption of food. Simple esophageal and residual stomach anastomosis causes a high incidence of reflux esophagitis, which seriously affects the postoperative quality of life. Reflux results in a high incidence of anastomotic stenosis, leading to reduced dietary intake and a worsened nutritional status[16]. Esophageal and residual stomach anastomosis is the simplest surgical method, but it is not the optimal technique (Table 1).
Esophageal and residual stomach anastomosis and fundoplication
To prevent reflux esophagitis, many researchers have successively performed esophageal and residual stomach anastomosis and fundoplication. Sakuramoto et al. performed laparoscopic Toupet-like partial fundoplication in 2005 (Figure 2A). One year after surgery, 15.0% of patients had heartburn symptoms and 30.0% had reflux esophagitis, but such symptoms could be controlled by proton pump inhibition[17]. In 2013, Ichikawa et al. performed esophagogastric anastomosis with a circular stapler. The anastomotic stoma was located in the anterior wall of the stomach, forming a new gastric fundus (Figure 2B). The residual stomach and lower sarcoplasmic layer of the esophagus on both sides were saturated so that the lower esophagus was surrounded by the top of the residual stomach in a semicircular manner, forming an acute angle at the esophagogastric anastomosis to prevent reflux. It was found that an acute angle at the anastomosis did not reduce reflux esophagitis[18]. In 2017, Park et al. performed laparoscopic PG with suture anchoring between the posterior wall of the esophagus and superior wall of the stomach (SPADE, Figure 2C), finding that the incidence of reflux esophagitis was 2.9%; 14.7% and 2.9% of patients had mild and moderate reflux symptoms, respectively; and the SPADE procedure could effectively reduce reflux esophagitis[19].
Anti-reflux effect of gastroesophagostomy after proximal gastrectomy for gastric cancer
| Author | Anastomotic method | Time of first report | Disadvantage |
|---|---|---|---|
| Only Gastroesophagostomy | Esophageal and residual stomach anastomosis is the simplest surgical method, high incidence of reflux esophagitis and anastomotic stenosis was higher. | ||
| Mikulicz[15] | Gastroesophagostomy | 1897 | |
| Gastroesophagostomy with Fundoplication | Esophageal and residual stomach anastomosis with fundoplication can reduce the incidence of reflux esophagitis. Many fundoplication techniques with certain differences in anti-reflux effects are available. Only when the residual stomach is relatively large can esophageal and residual stomach anastomosis with fundoplication be completed. | ||
| Sakuramoto[17] | laparoscopy-assisted proximal gastrectomy with Toupet-like partial fundoplication | 2009 | |
| Ichikawa[18] | Esophagogastrostomy with anchoring suture created an acute angle at the anastomosis and frebuilding a new fundus | 2013 | |
| Park[19] | SPADE Operation | 2017 | |
| Ojima[20] | Gastroesophagostomy with 180 degrees Fundoplication (the remnant stomach was wrapped from the esophageal posterior wall towards the esophageal anterior wall. | 2018 | |
| Polkowski[21] | Posterior Esophago-Gastrostomy and Partial Neo-Fundoplication | 2020 | |
| Aizawa[22] | Esophagogastrostomy with Posterolateral Fundoplication | 2021 | |
| Zhu[23] | Esophagogastrostomy with “collar” fundoplication | 2022 | |
| Side overlap esophagogastrostomy | This procedure is suitable for laparoscopy, it is relatively simple with a short anastomotic time, and it reduces the incidence of reflux esophagitis and anastomotic stenosis. However, it has the disadvantage that a long abdominal esophagus and large residual stomach (more than two-thirds) should be retained. | ||
| Yamashita[24] | side overlap with fundoplication by Yamashita (SOFY) | 2017 | |
| Yamashita[26] | modified SOFY (mSOFY) | 2022 | |
| Fujii[27] | Esophagogastric Anastomosis With Stapled Pseudo-Fornix | 2022 | |
Gastroesophagostomy: The esophagus was anastomosed with the anterior wall of the residual stomach after proximal gastrectomy (above), the esophagus was anastomosed with the posterior wall of the residual stomach after proximal gastrectomy (below).
Ojima et al. performed esophagogastric side-wall anastomosis with 180° fundoplication after PG, finding no reflux esophagitis 3 months after the operation via gastroscopy[20]. Polkowski et al. performed esophageal and residual stomach anastomosis after PG and fundoplication of the gastric stump around the esophagus for 3/4 (Figure 2D), observing that partial fundoplication can reduce reflux esophagitis[21]. Aizawa et al. performed PG, esophageal and residual stomach anastomosis, and posterolateral fundoplication (Figure 2E), finding that a good anti-reflux effect was achieved with posterolateral fundoplication[22]. In 2022, Zhu et al. performed laparoscopic radical gastrectomy for proximal gastric cancer, followed by “collar” type residual stomach fundoplication after esophageal and residual stomach anastomosis (Figure 2F), achieving a good anti-reflux effect after laparoscopic radical gastrectomy for proximal gastric cancer[23].
Esophagogastrostomy with posterolateral fundoplication. A: Toupet-like partial fundoplication (TPF), the remnant stomach was wrapped around two-thirds of the esophagus, the remnant stomach was sutured to the crura of the diaphragm. B: The anchoring suture created an acute angle at the anastomosis and allowed the greater curvature near the top of the remnant stomach to rebuild a new fundus. C: SPADE Operation, both distal part of posterior wall of esophagus and proximal part of anterior wall of stomach were fixed with two interrupted sutures, one barbed continuous suture initiated at the left corner of esophagus posterior wall and stomach anterior wall, ended on the opposite right side, anterior wall anastomosis was performed in the same maneuver. D: Partial neo-fundoplication, the shortened gastric remnant is wrapped around 3-quarters of the esophagus, and sutured to the distal esophagus. E: The cut end of the stomach was fixed to both the top posterior end of the freed esophageal wall and the diaphragm, the posterior half-circumference of the esophagus was wrapped with the anterior gastric wall by placing stay sutures. F: The residual stomach was wrapped around the anterior wall of the esophagus and sutured with 3 stitches.
Esophageal and residual stomach anastomosis with fundoplication can reduce the incidence of reflux esophagitis. Many fundoplication techniques with certain differences in anti-reflux effects are available. Only when the residual stomach is relatively large can esophageal and residual stomach anastomosis with fundoplication be completed (Table 1).
Side overlap anastomosis
In 2017, Yamashita et al.[24] first reported side overlap anastomosis with fundoplication(SOFY, Figure 3A). Side overlap anastomosis generally requires that two-thirds of the abdominal esophagus and residual stomach be preserved, and the artificial gastric fundus can be reconstructed. When artificial gastric fundus pressure increases, the anastomotic stoma is closed, playing an anti-reflux role. A 60-mm linear suture device is adopted for side overlap anastomosis, resulting in a wide anastomotic orifice, thereby reducing the incidence of anastomotic stenosis[24]. The incidence of reflux esophagitis after side overlap anastomosis is 10%[25]. Yamashita et al. improved SOFY (Figure 3B) with esophageal and residual stomach anastomosis. After the operation, 17.9% of patients had reflux esophagitis in gastroscopy, but only 2.8% had reflux symptoms[26]. Fujii et al. performed laparoscopic PG and formed a “pseudo fornix” (Figure 3C) after esophageal and residual stomach anastomosis, reducing reflux esophagitis[27].
This procedure is suitable for laparoscopy, it is relatively simple with a short anastomotic time, and it reduces the incidence of reflux esophagitis and anastomotic stenosis. However, it has the disadvantage that a long abdominal esophagus and large residual stomach (more than two-thirds) should be retained (Table 1).
Side overlap esophagogastrostomy. A: After side overlap esophagogastrostomy, the linear stapler was rotated counter clockwise on its axis, suturing the gastric wall to the left side of the esophagus. B: After side overlap esophagogastrostomy with the right side of the esophageal stump and the anterior gastric wall, the esophagus was rotated counter clockwise on its axis, suturing the gastric wall to the left side of the esophagus, the left and lower side of the esophagus was sutured to the remnant stomach. C: The stapler established a connection between the esophagus and the remnant stomach without creating a common lumen to create a pseudofornix, the entry hole was closed using the laparoscopic hand-sewn suturing technique, a small V-shaped anastomosis was created.
Tubular gastroesophageal anastomosis
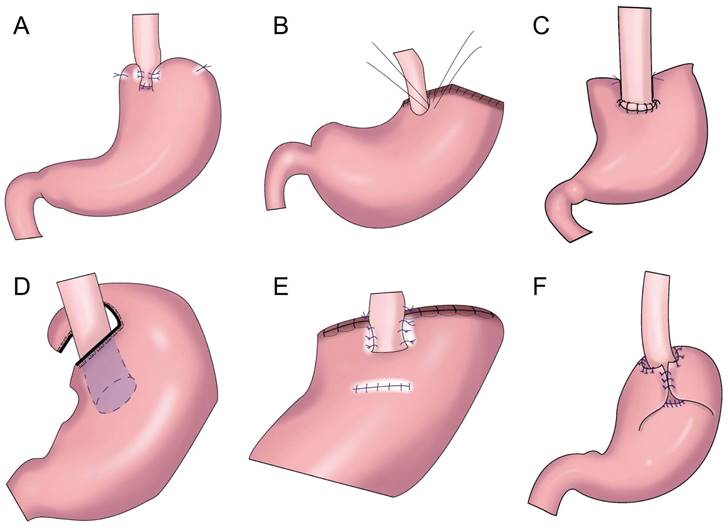
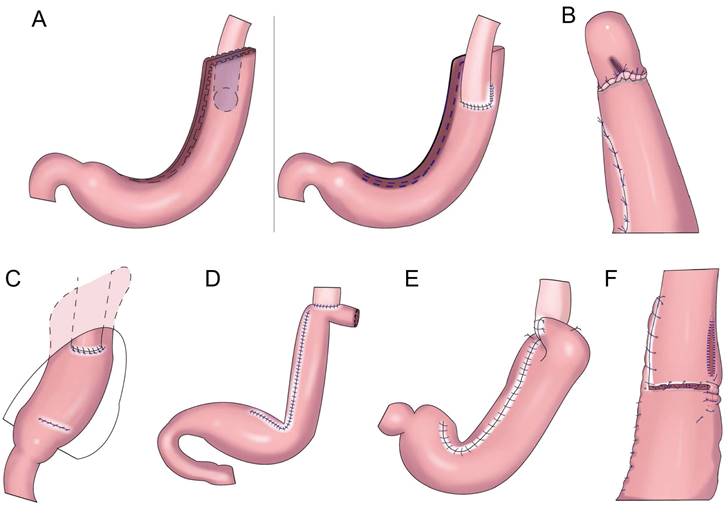
Shiraishi et al.[28] first reported tubular gastroesophageal anastomosis in 1998 (Figure 4A). This operation forms a structure similar to the gastric fundus at the top of the residual stomach. When the patient is lying flat, the reflux gastric juice is temporarily stored in the “gastric fundus” to avoid direct reflux to the lower end of the esophagus to a certain extent[29]. Chen et al.[30] found that only 14.3% of patients with tubular gastroesophageal anastomosis had postoperative reflux symptoms and that 5.7% were diagnosed with reflux esophagitis, and the degree of reflux esophagitis after tubular gastroesophageal anastomosis was lower than that after conventional esophageal and residual stomach anastomosis. Ronellenfitsch al et.[31] found that 30% of patients had reflux symptoms after tubular gastroesophageal anastomosis, albeit with mild symptoms. Kukar et al. reported six cases of tubular stomach (posterior wall) esophageal anastomosis (Figure 4A), including two cases with anastomotic stenosis and one case with severe reflux[32]. In 2014, Hosogi et al. reported tubular stomach and pseudodome anastomosis (Figure 4B), in which the incidence of reflux esophagitis on endoscopic examination 1 year after surgery was 26.7%[33]. In 2015, Yasuda et al. inserted the upper part of the tubular stoma into the mediastinum (Figure 4C) to form an angle of His, thereby exerting an anti-reflux effect[34]. In 2018, Cheng et al. reported Giraffe reconstruction (Figure 4D), this method reconstructed the angle of His and fundus of the stomach, producing good gastric motility and anti-reflux effects according to the anatomical characteristics of the stomach and the anti-reflux mechanism of the interposed jejunum and tubular stomach[35]. Subsequently, the clinical effect of Giraffe reconstruction for PG in 100 patients with gastroesophageal junction adenocarcinoma was reported in a retrospective multicenter study. In the study, 8.0% of the patients had reflux symptoms, and 11.0% had reflux esophagitis on gastroscopy. Reflux symptoms could be controlled in all patients through behavioral guidance or oral proton pump inhibitor therapy[36, 37]. Good effects on reflux and gastric emptying in digestive tract reconstruction were achieved in Giraffe anastomosis, but the risk of anastomotic leak was increased because of the long narrow tubular stomach. Toyomasu et al.[38] performed esophageal and posterior wall anastomosis and residual stomach anterior wall anastomosis with 180° esophageal folding (Figure 4E), finding that the incidence of reflux symptoms after tubular gastroesophageal anastomosis was 16.7% and that 9.8% of patients had anastomotic stenosis. Hosogi et al. revealed that the incidence of reflux esophagitis and anastomotic stricture could be reduced by esophagogastric side wall anastomosis and esophageal folding (Figure 4F)[39].
Tubular gastroesophageal anastomosis forms a structure similar to the gastric fundus at the top of the residual stomach, avoiding direct reflux to the lower end of the esophagus to a certain extent. Part of the gastric antrum is resected for the tubular stomach, thus reducing the secretion of gastrin and gastric acid, maintaining the anatomical structure of the stomach, and improving the quality of life of patients, but the incidence of reflux esophagitis remained higher (Table 2).
Muscle flap anastomosis
Double-flap anastomosis (Kamikawa method)
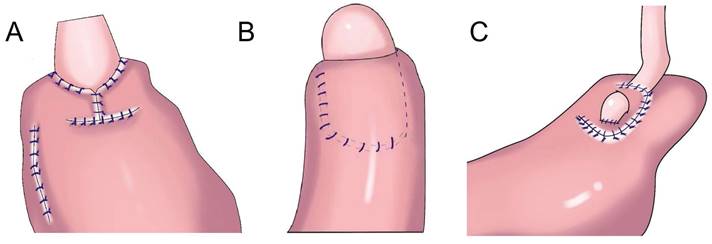
In 1998, Kamikawa designed the double-flap technique, also known as the Kamikawa method (Figure 5A), to prevent reflux[40]. In this operation, an “エ-shaped” plasma muscle flap is made below the cutting edge of the residual stomach, and then the mucosa and submucosa are cut at the lower edge of the “window.” The esophageal cutting edge is anastomosed with the mucosa and submucosa, and finally, the two plasma muscle flaps are covered at the lower segment of the esophagus and upper layer of the anastomosis. This method increases the pressure at the lower end of the esophagus, and it is conducive to reducing the incidence of reflux esophagitis. Kuroda et al. conducted a multicenter retrospective study to evaluate the effectiveness and safety of double-muscle valve anastomosis. The study included 546 patients from 18 centers, of whom 464 patients underwent endoscopic examination to evaluate reflux esophagitis 1 year after the surgery. The researchers found that the incidence of reflux esophagitis above grade B on endoscopy was 6%, and the incidence of anastomotic stenosis was 5.5%[41]. Double muscle flap anastomosis has a good anti-reflux effect, and it can improve the nutritional status and quality of life of patients after surgery[42,43]. Yamamoto et al. improved double-flap gastroesophageal reconstruction after laparoscopic PG, which can reduce reflux symptoms and the incidence of reflux esophagitis[9]. Double muscle flap anastomosis has become one of the most recommended digestive tract reconstruction methods after PG[6, 7, 41, 44]. Double-flap anastomosis is applicable to early gastric cancer of the upper third of the stomach with estimated residual gastric volume > 50%, but it is not applicable for patients with tumors invading the lower esophageal segment. Double-flap anastomosis can significantly reduce the incidence of postoperative hiccup and reflux esophagitis, but the technique is limited by complicated procedures, a long operative time, and a high incidence of postoperative anastomotic stenosis. Ensuring good blood supply of the double muscle flap, controlling tension, and avoiding anastomotic stenosis are the difficulties of this surgical method, which has high technical requirements for the operator and team[45]. Appropriate extension of plasma muscle flap can reduce the incidence of anastomotic stenosis[46].
Gastric tube reconstruction. A: Tubular gastroesophagostomy: the esophagus was anastomosed with the anterior wall of the residual stomach(Left), the esophagus was anastomosed with the posterior wall of the residual stomach(Right), B: Esophagogastric tube reconstruction with stapled pseudo-fornix, C: A newly modified esophagogastrostomy with a reliable angle of His by placing a gastric tube in the lower mediastinum, D: Cheng's Giraffe reconstruction, E: The posterior wall of esophagus was anastomosed with the anterior wall of the residual stomach, with 180 degrees Fundoplication(the wall of the gastric tube was wrapped to the anterior aspect of the esophagus and secured to the right margin of the esophagus with two or three sutures), F: Side-overlap esophagogastric tube (SO-EG) reconstruction.
Anti-reflux effect of Gastric tube reconstruction after proximal gastrectomy for gastric cancer
| Author | Anastomotic method | Time of first report | Advantages and disadvantages |
|---|---|---|---|
| Shiraishi[28] | Gastric tube reconstruction: the esophagus was anastomosed with the anterior wall of the residual stomach | 1998 | the incidence of reflux symptoms and anastomotic stenosis was higher |
| Hosogi[33] | Esophagogastric tube reconstruction with stapled pseudo-fornix | 2014 | With the advantage of a gastric tube, a tension-free anastomosis was possible even for bulky tumors that needed lower esophagectomy, but reflux esophagitis was higher |
| Yasuda[34] | A newly modified esophagogastrostomy with a reliable angle of His by placing a gastric tube in the lower mediastinum | 2015 | the formation of a pseudo-fornix and optimal angle of His appeared to reduce bile reflux, the gastric remnant in the form of a narrow gastric tube with low compliance resulted in decreased food residue. |
| Cheng[35] | Cheng's Giraffe reconstruction | 2018 | The reconstructed digestive tract is consistent with physiological characteristics and has good anti-reflux effects, but the risk of anastomotic leak was increased because of the long narrow tubular stomach. |
| Kukar[32] | Tubular gastroesophagostomy: the esophagus was anastomosed with the posterior wall of the residual stomach | 2018 | the incidence of anastomotic strictures, and significant reflux were higher. |
| Toyomasu[38] | Tubular gastroesophagostomy with 180 degrees fundoplication | 2021 | Tubular gastroesophagostomy with 180 degrees fundoplication has anti-reflux effects, the incidence of anastomotic strictures were higher. |
| Hosogi[39] | Side-overlap esophagogastric tube (SO-EG) reconstruction | 2022 | Fundoplication with a longer overlap might be better to completely prevent refux. |
Anti-reflux effect of flap anastomosis after proximal gastrectomy for gastric cancer
| Author | Anastomotic method | Time of first report | Advantages and disadvantages |
|---|---|---|---|
| Kamikawa[40] | double⁃flap anastomosis | 1998 | complicated surgical suture technique, difficult operation, strict surgical indications and high incidence of postoperative anastomotic stenosis |
| Peng[47] | right-open single-flap technique | 2022 | Compared with double-flap anastomosis, the operation of single-flap anastomosis is relatively simple, and the blood supply at the edge of the single-flap may be worse than that of the double-flap. More clinical data are still needed to verify its safety. |
| Li[48] | "arch bridge" reconstruction of esophageal remnant stomach | 2022 | |
| Yang[50] | left-open single-flap technique | 2022 |
Single-flap anastomosis
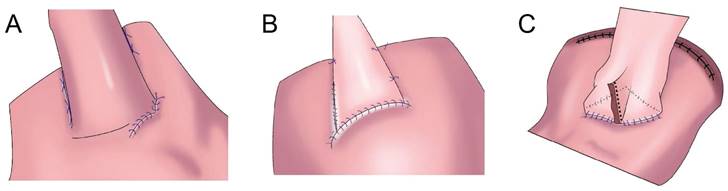
To overcome the shortcomings of complicated double-flap anastomosis, difficult surgical procedures, long operative times, and high rates of postoperative anastomotic stenosis, Peng et al. performed right open single-flap anastomosis after laparoscopic PG, finding that single-flap anastomosis had a simple procedure[47]. Li et al. performed the “arch bridge” reconstruction of the esophagus and residual stomach after PG. In vitro, the gastric wall was cut approximately 1 cm from the proximal broken end of the anterior wall of the residual stomach, and the “ㄈ”-shaped single flap with an opening toward the small curved side was made. The full layer (approximately 2 cm long) of the anterior wall of the residual stomach was cut transversely at approximately 1 cm from the lower edge of the flap, the surrounding of the opening gastric wall was sutured with 4-0 absorbable sutures, and the longitudinal opening of the single flap was closed with 4-0 absorbable sutures to complete the “arch bridge” (Figure 5B). The residual stomach was lifted up to the hole, the esophagus was pulled through the “arch bridge” of the flap of the anterior wall of the residual stomach, and the broken end of the esophagus was sutured with the opening of the anterior wall of the residual stomach. In vitro flap anchoring avoids the limitation of vision caused by laparoscopicsuture and reduces technical difficulty, and it is conducive to ensuring tunnel quality[48]. Wang et al. reported the short-term efficacy of laparoscopic PG and esophagogastrostomy with single-flap technology in seven patients, observing satisfactory short-term efficacy for laparoscopic PG with single-flap anastomosis[49]. Yang et al.[50] proposed single-flap anastomosis on the basis of double-flap anastomosis, that is, a “⊐”-shaped single flap with an opening toward the small curved side was made on the front wall of the gastric stump, the entire layer of the front wall of the gastric stump was cut at the lower edge of the muscle flap transversely, mucosal-esophageal anastomosis of the anterior wall of the stomach was performed, and the single flap and anterior wall of the stomach were continuously sutured before the anastomosis. A multicenter, prospective, randomized controlled study with a target completion date of July 31, 2027 could provide better conclusions from the research results.
The anastomotic stoma of the esophagus and stomach with a muscle flap is wrapped into a soft valve that acts as a one-way valve with good anti-reflux effects. The anastomotic stoma was wrapped with a seromuscular layer, resulting in a low incidence of anastomotic leak. However, muscle flap anastomosis has a complicated procedure, high requirements for suture technology, and a long operative time, thus increasing the incidence of anastomotic stenosis (Table 3).
Flap anastomosis. A: An “エ-shaped” plasma muscle flap is made below the cutting edge of the residual stomach, the mucosa and submucosa are cut at the lower edge of the “window.” The esophageal cutting edge is anastomosed with the mucosa and submucosa, the two plasma muscle flaps are covered at the lower segment of the esophagus and upper layer of the anastomosis. B: "arch bridge" reconstruction of esophageal remnant stomach, the “ㄈ”-shaped single flap with an opening toward the small curved side was made, the full layer of the anterior wall of the residual stomach was cut transversely, the surrounding of the opening gastric wall was sutured, and the longitudinal opening of the single flap was closed to complete the “arch bridge”, the esophagus was pulled through the “arch bridge” of the flap of the anterior wall of the residual stomach, and the broken end of the esophagus was sutured with the opening of the anterior wall of the residual stomach. C: A“⊐”-shaped single flap with an opening toward the small curved side was made on the front wall of the gastric stump, the entire layer of the front wall of the gastric stump was cut at the lower edge of the muscle flap transversely, mucosal-esophageal anastomosis of the anterior wall of the stomach was performed, and the single flap and anterior wall of the stomach were continuously sutured before the anastomosis.
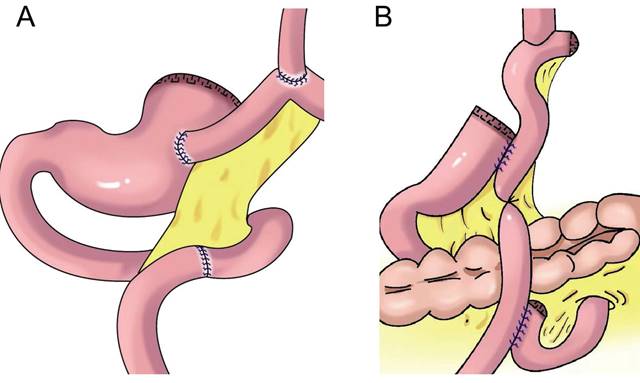
Jejunal interposition reconstruction. A: jejunal interposition reconstruction, between the residual stomach and esophagus, inserting a section of jejunum approximately 10-15 cm. B: piggyback jejunal interposition reconstmction with uncut jejunal continuity.
Jejunal interposition
Jejunal interposition aims to restore the connectivity between the esophagus and residual stomach by inserting a section of jejunum between the esophagus and residual stomach and build an anti-reflux barrier between the residual stomach and esophagus using the tolerance of the jejunum itself to acidic gastric juice and alkaline intestinal juice and the natural peristalsis of the intestine. In 1941, Zhenxin[51] first reported jejunal interposition (Figure 6A). In the 1960s, short jejunal interposition and pylorus formation were generally performed. In the 1970s, to better resist reflux, the length of the interposed jejunum was up to 30-40 cm. An excessive length of jejunal interposition was not conducive to endoscopy, and it increased the retention time of food in the intestine[52]. At present, the length of jejunal interposition tends to be approximately 10-15 cm[53,54]. Kameyama et al.[55] reported that jejunal interposition + storage bag insertion can retain the storage capacity, and it is easy to observe the curative effect through endoscopy after operation. Katai et al. found that the incidence of reflux after jejunal interposition was 5.5%, and the incidence of endoscopic reflux esophagitis was 1.7%[56]. Jejunal interposition can ensure the scope of gastrectomy, preserve pyloric function and the physiological channel of food, effectively resist reflux, and improve the quality of life of patients after surgery[57]. The upper part of the stomach is replaced by the small intestine. Compared with the stomach, the fascia of the small intestine is thinner, and its storage capacity is limited by hyphological weakness. Xu reported PG combined with jejunum interposition. Based on double-tract reconstruction, jejunal access was blocked at the distal end of the gastrojejunostomy(Figure 6B)., preserving the continuity of the interposition jejunal bowel, reducing the possibility of food emptying disorders, and improving the nutritional status of patients[58, 59, 60].
This method has low requirements concerning the size of the residual stomach, and it is suitable for reconstruction after most PG procedures. However, because of the complicated operative procedure, long operative time, and high cost, the possibility of emptying obstruction and internal hernia increases[61].
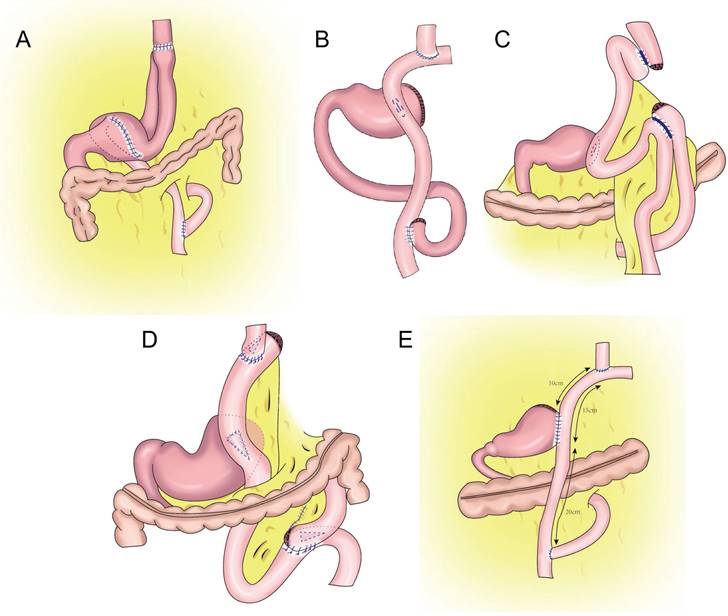
Double tract reconstruction. A: N-shaped double tract method, the residual stomach is twisted anteriorially by 180 degrees, the seromuscular sutures are inserted to reinforce the posterior wall before restoring the twist, after the gastrojejunostomy, the twisting of the residual stomach is restored to its usual portion. B: Bouble tract reconstruction of the remnant stomach anterior wall. C: Oblique jejunogastrostomy method (OJG), the jejunum is transected at a point 20-cm distant from the ligament of Treitz, a side-to-side esophagojejunostomy is performed, the stomach and jejunum are twisted posteriorly, and the posterior wall of the remnant stomach and the posterior wall of the jejunum are put together. An oblique side-to-side jejunogastrostomy from the antimesenteric wall to the posterior wall is performed, the jejunum returns the torsion of the jejunum to the counter clockwise direction and rides on the remnant stomach, a side-to-side jejunojejunostomy is made between the jejunum 20 cm below the jejunogastrostomy and the proximal jejunum. D: double tract reconstruction of colon posterior. E: double tract reconstruction of the remnant stomach side wall.
Double-tract reconstruction
In 1988, Aikou et al.[62] first reported PG with double-tract reconstruction in which the proximal stomach was severed, followed by esophagojejunal Roux⁃en⁃Y anastomosis and side overlap between the residual stomach and esophagojejunal anastomosis 10-15 cm distal to the jejunum. This strategy allowed food to enter the distal jejunum through both the residual stomach and jejunum after esophagojejunostomy. However, with double-tract reconstruction, food often cannot enter the duodenum smoothly.
Later, Aikou et al. improved the traditional double-tract reconstruction technique. Namely, after PG, esophagojejunostomy is performed behind the colon and residual stomach, and jejunostomy is performed at a distance of approximately 40 cm from the distal end of the anastomosis. The residual stomach is rotated 180° before anastomosis and restored to its normal position after anastomosis. The jejunum is “N”-shaped around the gastrojejunal anastomosis (Figure 7A), allowing food to enter the residual stomach more easily, resulting in a good anti-reflux effect[62]. In theory, double-tract reconstruction is a relatively ideal reconstruction method. Because food enters the distal digestive tract through two tracks, the residual stomach has the function of storing and digesting food, and some food can pass through the duodenum, thus extending the time food remains in the digestive tract and allowing food and digestive fluid to more fully mix for better nutrient absorption. At the same time, expansion of the residual stomach after eating can also increase the appetite of patients[63,64,65]. The retention of food in the residual stomach can induce the secretion of gastrin, facilitating the balance of gastrointestinal hormones, reducing the incidence of dumping syndrome, and increasing the absorption of iron and vitamin B[66,67]. Double-tract reconstruction can effectively reduce gastroesophageal reflux, and the incidence of reflux esophagitis is approximately 8.0%-13.8%[68,69,70,71,72]. Food sometimes cannot be evacuated according to the theoretical designed double-tract reconstruction, but most food directly enters the jejunum[73]. To avoid such a situation, domestic and foreign experts have improved the size and direction of gastrojejunal anastomosis to allow more food to enter the residual stomach[65,74] (Figure 7B-E). Xu et al. used a 60-mm linear cutting occluder to anastomose the anterior wall of the residual stomach with the jejunum to widen the anastomosis, which is theoretically more conducive to food entering the residual stomach[75]. Sato et al. performed laparoscopic PG with postcolonic double-tract reconstruction, finding that double-tract reconstruction was safe and feasible and was significantly better than total gastrectomy in terms of quality of life and nutritional status[76].
Double-tract reconstruction is applicable to reconstruction of the digestive tract after most PGs. It has low requirements concerning the volume of the residual stomach, and it is applicable to patients who have undergone an excessive number of gastrectomies and who are not suitable for esophageal and residual stomach anastomosis. However, this surgery is relatively complicated and expensive, and it results in many anastomoses, which might increase the risk of anastomotic leak.
Conclusion
Since the development of digestive tract reconstruction after PG, various improved methods have been introduced. Each reconstruction method is constantly changing and improving. Each reconstruction method has its own advantages and disadvantages. Although no uniform standard is available at present, in clinical practice, all surgeons have their own unique experience and thinking regarding the available reconstruction methods. Understanding the operating points and advantages and disadvantages of various digestive tract reconstruction can provide ideas for surgeons to make correct clinical decisions.
Acknowledgements
We thank Joe Barber Jr., PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Funding
This article was supported by Scientific research fund of national health commision of China, Key health science and technology program of Zhejiang Province (WKJ-ZJ-2201), Key Project of social welfare program of Zhejiang Science and Technology Department, “Lingyan” Program (2022C03099).
Author Contributions
Conceptualisation, Y.Y.W., and Y.P.M.; Investigation, L.L., and Z.H.L.; Writing-Original Draft Preparation, L.L., and Y.Y.W.; Writing-Review and Editing, Y.Y.W.; Drawing the figure, X.F.C.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424
2. Chen W. et al. Cancer incidence and mortality in China, 2015. JNCC. 2020;1(1):2-11
3. Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. 2013;23(1):3-9
4. Kusano C. et al. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. 2008;23(11):1662-1665
5. Liu K. et al. Changes of Esophagogastric Junctional Adenocarcinoma and Gastroesophageal Reflux Disease Among Surgical Patients During 1988-2012: A Single-institution, High-volume Experience in China. Ann Surg. 2016;263(1):88-95
6. Wang FH. et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41(8):747-795
7. Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). 2023;26(1):1-25.
8. Takiguchi N. et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18(2):407-416
9. Yamamoto M. et al. Laparoscopic Proximal Gastrectomy with Novel Valvuloplastic Esophagogastrostomy vs. Laparoscopic Total Gastrectomy for Stage I Gastric Cancer: a Propensity Score Matching Analysis. J Gastrointest Surg. 2022;26(10):2041-2049
10. Hayami M. et al. Clinical Outcomes and Evaluation of Laparoscopic Proximal Gastrectomy with Double-Flap Technique for Early Gastric Cancer in the Upper Third of the Stomach. Ann Surg Oncol. 2017;24(6):1635-1642
11. Furukawa H. et al. Short-term outcomes and nutritional status after laparoscopic subtotal gastrectomy with a very small remnant stomach for cStage I proximal gastric carcinoma. Gastric Cancer. 2018;21(3):500-507
12. Kurokawa Y. et al. Mapping of Lymph Node Metastasis From Esophagogastric Junction Tumors: A Prospective Nationwide Multicenter Study. Ann Surg. 2021;274(1):120-127
13. Writing committee of digestive tract reconstruction after proximal gastrectomy. Chinese consensus on digestive tract reconstruction after proximal gastrectomy. Chin J Gastrointest Surg. 2020;23(2):101-108
14. Yamasaki M. et al. Multicenter prospective trial of total gastrectomy versus proximal gastrectomy for upper third cT1 gastric cancer. Gastric Cancer. 2021;24(2):535-543
15. Mikulicz J. Das operieren in sterilisirten zwirnhandschuren und mit mundbinde. Zentralblarf Chir. 1897;24:713-717
16. Yoo CH. et al. Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer Res Treat. 2004;36(1):50-55
17. Sakuramoto S. et al. Clinical experience of laparoscopy-assisted proximal gastrectomy with Toupet-like partial fundoplication in early gastric cancer for preventing reflux esophagitis. J Am Coll Surg. 2009;209(3):344-351
18. Ichikawa D. et al. Evaluation of symptoms related to reflux esophagitis in patients with esophagogastrostomy after proximal gastrectomy. Langenbecks Arch Surg. 2013;398(5):697-701
19. Park SH. et al. Spade-Shaped Anastomosis after Laparoscopic Proximal Gastrectomy Using Double Suture Anchoring between the Posterior Wall of the Esophagus and the Anterior Wall of the Stomach (SPADE Operation): A Case Series. Cancers (Basel). 2022;14(2):379
20. Ojima T. et al. Fundoplication with 180-Degree Wrap During Esophagogastrostomy After Robotic Proximal Gastrectomy for Early Gastric Cancer. J Gastrointest Surg. 2018;22(8):1475-1476
21. Polkowski WP. et al. Proximal Gastric Resection with Posterior Esophago-Gastrostomy and Partial Neo-Fundoplication in the Treatment of Advanced Upper Gastric Carcinoma. Dig Surg. 2020;37(2):119-128
22. Aizawa M. et al. A Retrospective Review of a Single-Center Experience with Posterolateral Fundoplication During Esophagogastrostomy After Proximal Gastrectomy. J Gastrointest Surg. 2021;25(12):3230-3233
23. Jian-kang Z. et al. The clinical application effect of "collar" fundoplication in patients with laparoscopic proximal radical gastrectomy. Journal of Laparoscopic Surgery. 2022;27(8):561-565
24. Yamashita Y. et al. Side overlap esophagogastrostomy to prevent reflux after proximal gastrectomy. Gastric Cancer. 2017;20(4):728-735
25. Feng-yuan L. et al. Preliminary experience of Side⁃overlap in laparoscopic resection of proximal gastric cancer. Chin J Surg. 2018;56(8):623 ⁃625
26. Yamashita Y. et al. Modified side overlap esophagogastrostomy after laparoscopic proximal gastrectomy. Ann Gastroenterol Surg. 2022;6(4):594-599
27. Fujii Y. et al. Laparoscopic Esophagogastric Anastomosis With Stapled Pseudo-Fornix for Reflux Esophagitis Prevention After Proximal Gastrectomy. Cureus. 2022;14(6):e25561
28. Shiraishi N. et al. Gastric tube reconstruction prevented esophageal reflux after proximal gastrectomy. Gastric Cancer. 1998;1(1):78-79
29. Han L. Digestive tract reconstruction for carcinoma of the esophagogastric junction. Chin J Dig Surg. 2014;13(2):92 ⁃97
30. Chen XF. et al. Gastric tube reconstruction reduces postoperative gastroesophageal reflux in adenocarcinoma of esophagogastric junction. Dig Dis Sci. 2012;57(3):738-745
31. Ronellenfitsch U. et al. Functional outcomes and quality of life after proximal gastrectomy with esophagogastrostomy using a narrow gastric conduit. Ann Surg Oncol. 2015;22(3):772-779
32. Kukar M. et al. Laparoscopic proximal gastrectomy for gastric neoplasms. J Surg Oncol. 2018;118(1):95-100
33. Hosogi H. et al. Esophagogastric tube reconstruction with stapled pseudo-fornix in laparoscopic proximal gastrectomy: a novel technique proposed for Siewert type II tumors. Langenbecks Arch Surg. 2014;399(4):517-523
34. Yasuda A. et al. A newly modified esophagogastrostomy with a reliable angle of His by placing a gastric tube in the lower mediastinum in laparoscopy-assisted proximal gastrectomy. Gastric Cancer. 2015;18(4):850-858
35. Cheng XD, Xu ZY, Du YA, Hu C, Yu JF, Yang LT. et al. Preliminary efficacy analysis of Cheng's Giraffe reconstruction after proximal gastrectomy in adenocarcinoma of esophagogastric junction. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23(2):158-162
36. Xu Z. et al. Efficacy analysis of Cheng's GIRAFFE reconstruction after proximal gastrectomy for adenocarcinoma of esophagogastric junction. Chin J Cancer Res. 2022;34(3):289-297
37. Zhang YQ, Xu ZY, Du YA, Yang LT, Huang L, Yu PF. et al. Functional outcomes of 100 patients with adenocarcinoma of the esophagogastric junction undergoing Cheng's GIRAFFE(®) reconstruction after proximal gastrectomy. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25(5):447-453
38. Toyomasu Y. et al. Clinical outcomes of gastric tube reconstruction following laparoscopic proximal gastrectomy for early gastric cancer in the upper third of the stomach: experience with 100 consecutive cases. Langenbecks Arch Surg. 2021;406(3):659-666
39. Hosogi H. et al. Side-overlap esophagogastric tube (SO-EG) reconstruction after minimally invasive Ivor Lewis esophagectomy or laparoscopic proximal gastrectomy for cancer of the esophagogastric junction. Langenbecks Arch Surg. 2022;407(2):861-869
40. Kamikawa Y, Kobayashi T, Kamikawa S. A new esophagogastric anastomosis technique aimed at preventing reflux after cardiac gastrectomy. Gastrointestinal Surg. 2001;24:1053-1060
41. Kuroda S. et al. Multicenter retrospective study to evaluate the efficacy and safety of the double-flap technique as antireflux esophagogastrostomy after proximal gastrectomy (rD-FLAP Study). Ann Gastroenterol Surg. 2018;3(1):96-103
42. Kano Y, Ohashi M, Ida S. et al. Laparoscopic proximal gastrectomy with double-flap technique versus laparoscopic subtotal gastrectomy for proximal early gastric cancer. BJS Open. 2020;4(2):252-259
43. Ri M. et al. Key Factors for Maintaining Postoperative Skeletal Muscle Mass After Laparoscopic Proximal Gastrectomy with Double-Flap Technique Reconstruction for Early Gastric Cancer. J Gastrointest Surg. 2021;25(6):1569-1572
44. Kuroda S. et al. Double-Flap Technique as an Antireflux Procedure in Esophagogastrostomy after Proximal Gastrectomy. J Am Coll Surg. 2016;223(2):e7-e13
45. Yang L, Wu JZ. et al. A multicenter retrospective study on the efficacy of different anti-reflux reconstruction methods after proximal gastrectomy for gastric cancer. Zhonghua Wai Ke Za Zhi. 2022;60(9):838-845
46. Li Y. et al. Preliminary experience of esophagogastric anastomosis muscle flap arthroplasty (Kamikawa anastomosis) for laparoscopic proximal gastrectomy. Chin J Gastrointest Surg. 2017;20(2):227 ⁃230
47. Wei P. et al. Initial exploration of right-opened single flap plasty in the reconstruction following laparoscopic proximal gastrectomy. Chinese Journal of Practical Surgery. 2021;41(10):1173-1175
48. Ziyu L. et al. Effect analysis of "arch bridge" reconstruction of esophageal remnant stomach after proximal gastrectomy in 3 cases. Chin J Surg. 2022;60(3):261-264
49. Wang WD. et al. Preliminary experience of laparoscopic proximal gastrectomy with esophagogastrostomy single flap technique. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25(5):462-465
50. Yang QC. et al. Study protocol for comparing the efficacy of left-open single-flap technique versus double-flap technique after proximal gastrectomy: A multicenter randomized controlled trial. Front Oncol. 2022;12:973810
51. 瀬尾貞信. 空腸移植による胃切除法. 日外会誌. 1941 42, 1004-1005
52. Takagawa R. et al. A pilot study comparing jejunal pouch and jejunal interposition reconstruction after proximal gastrectomy. Dig Surg. 2010;27(6):502-508
53. Nomura E. et al. Functional outcomes by reconstruction technique following laparoscopic proximal gastrectomy for gastric cancer: double tract versus jejunal interposition. World J Surg Oncol. 2014;12:20
54. Kumamoto T. et al. Clinical outcomes of proximal gastrectomy for gastric cancer: A comparison between the double-flap technique and jejunal interposition. PLoS One. 2021;16(2):e0247636
55. Kameyama J. et al. Proximal gastrectomy reconstructed by interposition of a jejunal pouch. Surgical technique. Eur J Surg. 1993;159(9):491-493
56. Katai H. et al. Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br J Surg. 2010;97(4):558-562
57. Nomura E. et al. Postoperative evaluation of function-preserving gastrectomy for early gastric cancer. Hepatogastroenterology. 2003;50(54):2246-2250
58. Xu N. et al. Clinical study on two reconstruction methods of proximal gastrectomy and piggyback jejunal interposition for Siewert Ⅱ or Ⅲ adenocarcinoma of esophagogastric junction. Zhonghua Wai Ke Za Zhi. 2019;57(2):114-118
59. Li Z. et al. Comparison of three digestive tract reconstruction methods for the treatment of Siewert II and III adenocarcinoma of esophagogastric junction: a prospective, randomized controlled study. World J Surg Oncol. 2019;17(1):209
60. Tao K, Jianhong Dong, Songbing He, Yingying Xu, Fan Yang, Guolin Han. et al. Surgical Strategies for Siewert Type II Esophagogastric Junction Carcinomas: A Randomized Controlled Trial. Front Oncol. 2022 12, 852594
61. Takayama Y. et al. Internal hernia after proximal gastrectomy with jejunal interposition. Updates Surg. 2018;70(1):85-90
62. Aikou T. et al. Antrum preserving double tract method for reconstruction following proximal gastrectomy. Jpn J Surg. 1988;18(1):114-115
63. Nakajima K. et al. Dual-radionuclide simultaneous gastric emptying and bile transit study after gastric surgery with double-tract reconstruction. Ann Nucl Med. 2005;19(3):185-191
64. Li S. et al. A meta-analysis of comparison of proximal gastrectomy with double-tract reconstruction and total gastrectomy for proximal early gastric cancer. BMC Surg. 2019;19(1):117
65. Aburatani T. et al. Double-tract reconstruction after laparoscopic proximal gastrectomy using detachable ENDO-PSD. Surg Endosc. 2017;31(11):4848-4856
66. Kim DJ, Kim W. Laparoscopy-assisted Proximal Gastrectomy with Double Tract Anastomosis Is Beneficial for Vitamin B12 and Iron Absorption. Anticancer Res. 2016;36(9):4753-4758
67. Jung DH. et al. Laparoscopic proximal gastrectomy with double tract reconstruction is superior to laparoscopic total gastrectomy for proximal early gastric cancer. Surg Endosc. 2017;31(10):3961-3969
68. Ji X. et al. Double Tract Reconstruction Reduces Reflux Esophagitis and Improves Quality of Life after Radical Proximal Gastrectomy for Patients with Upper Gastric or Esophagogastric Adenocarcinoma. Cancer Res Treat. 2021;53(3):784-794
69. Choi NR. et al. Totally laparoscopic proximal gastrectomy with double tract reconstruction: outcomes of 37 consecutive cases. Wideochir Inne Tech Maloinwazyjne. 2020;15(3):446-454
70. Saze Z. et al. Functional benefits of the double flap technique after proximal gastrectomy for gastric cancer. BMC Surg. 2021;21(1):392
71. Yu B. et al. Double tract reconstruction versus double flap technique: short-term clinical outcomes after laparoscopic proximal gastrectomy for early gastric cancer. Surg Endosc. 2022;36(7):5243-5256
72. Xiaona W. et al. Clinical efficacy of radical proximal gastrectomy with esophagogastrostomy and double-tract anastomosis for upper gastric cancer. Chin J Dig Sing. 2021;20(6):689-694
73. Tanaka K. et al. Laparoscopic proximal gastrectomy with oblique jejunogastrostomy. Langenbecks Arch Surg. 2017;402(6):995-1002
74. Kimura K. et al. Initial Results of Laparoscopic Proximal Gastrectomy With Double-tract Reconstruction Using Oblique Jejunogastrostomy Method on the Long-term Outcome of Postoperative Nutritional Status: A Propensity Score-matched Study. Surg Laparosc Endosc Percutan Tech. 2021;31(5):603-607
75. Wang L. et al. Short-Term Surgical Outcomes of Laparoscopic Proximal Gastrectomy With Double-Tract Reconstruction Versus Laparoscopic Total Gastrectomy for Adenocarcinoma of Esophagogastric Junction: A Matched-Cohort Study. J Surg Res. 2020;246:292-299
76. Sato R. et al. Feasibility and quality of life assessment of laparoscopic proximal gastrectomy using double-tract reconstruction. Langenbecks Arch Surg. 2021;406(2):479-489
Author contact
![]() Corresponding author: YuanYu Wang, MD, PhD, Phone: +86-0571-85893408, Email: lywyy1979com.
Corresponding author: YuanYu Wang, MD, PhD, Phone: +86-0571-85893408, Email: lywyy1979com.

 Global reach, higher impact
Global reach, higher impact