Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(16):2956-2963. doi:10.7150/jca.87335 This issue Cite
Review
The Safety of Immunosuppressants Used in the Treatment of Immune-Related Adverse Events due to Immune Checkpoint Inhibitors: a Systematic Review
1. Department of Internal Medicine, The University of Texas Health Science Center, Houston, TX, USA.
2. Department of Gastroenterology, Hepatology, and Nutrition, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
3. Department of Medicine, Baylor College of Medicine, Houston, TX, USA.
4. Inflammatory Bowel Disease Center, Cleveland Clinic, Cleveland, OH, USA.
5. Department of Genitourinary Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Received 2023-6-20; Accepted 2023-8-30; Published 2023-9-11
Abstract
Purpose: Immune checkpoint inhibitor (ICI) use can lead to immune-related adverse events (irAEs) that require treatment with immunosuppressive medications in moderate to severe cases. Oncology society guidelines recommend systemic steroids and immunosuppressants such as infliximab and vedolizumab for the treatment of refractory cases. Limited information is available about the safety profile and potential adverse effects of these immunosuppressants. We have investigated the safety profile of multiple immunosuppressants which are used in the treatment of ICI-related irAEs.
Methods: We performed a systematic review of studies reporting irAEs, from ICI use, and their medical management with immunosuppressants in adult cancer patients. We searched MEDLINE, EMBASE, Cochrane Library, and ClinicalTrials.gov from inception through September 1, 2022, using the following keywords or their equivalents: ICI, immunosuppressant, and irAE. We extracted observational studies and clinical trials that matched our criteria. A random effects model was used to estimate the overall incidence of infections associated with the treatment of irAEs.
Results: Among the 11 studies included in this review (1036 total patients), melanoma (548 patients, 52.9%) was the most common primary cancer, followed by lung cancer (139 patients, 13.4%) and genitourinary cancers (131 patients, 12.6%). PD-1/PD-L1 monotherapy (460 patients, 44.4%) was used most, followed by a combination of PD-1/PD-L1 and CTLA-4 therapy (350 patients, 33.8%) and CTLA-4 monotherapy (226 patients, 22%). A total of 1024 (98.8%) patients had their irAEs treated with systemic steroids with majority having colitis and hepatobiliary irAEs; 335 patients (32.3%) were also treated with infliximab (mainly for colitis). Our review found 22.3% of patients treated for irAEs developed infectious adverse events (95% CI: 15.6%-29.1%, p<0.001). Among the 3 studies reporting the types of infections (41 total patients), bacterial (80.5%), followed by fungal (36.6%), infections were most common.
Conclusions: Adverse events from irAE treatment occurred in about one-third of patients that received either steroids or a combination of steroids and other immunosuppressants. Clinicians should be aware of these immunosuppressant-related adverse effects, which can negatively impact cancer treatment and patient outcomes, when treating irAEs and consider shortening treatment duration or using alternative strategies when possible to mitigate these complications, future prospective studies should further investigate the safety of immunosuppressants in treating irAEs.
Keywords: immune checkpoint inhibitor, immune-related adverse events, immunosuppressant, complications, infection, meta-analysis
Introduction
Immune checkpoint inhibitors (ICIs) have become the standard of care in the management of several cancers [1]. With increased utilization of ICIs, clinicians have noted an increased incidence of immune-related adverse events (irAEs) during cancer treatment [2]. These irAEs affect multiple organs, including the gastrointestinal tract, lungs, thyroid, and skin. The involvement of the heart and the nervous system is rare but is associated with a higher mortality. [3]. These adverse effects are the main barrier to using ICIs in clinical care [4]. For example, in a large retrospective study, 61.8% of patients who received anti-programmed cell death protein 1/programmed death ligand 1 (PD-1/PD-L1) experienced irAEs, resulting in 46.6% of the study population to discontinue treatment [5]. Further, a large meta-analysis of 112 clinical trials reported fatality rates of these toxicities to be 0.36% for patients treated with anti PD-1 therapy and up to 0.38% for patients treated with anti PD-L1 therapy [6]. Compared with patients who received PD-1/PD-L1 inhibitors, patients who received the cytotoxic T-cell lymphocyte-associated antigen 4 (CTLA-4) inhibitor ipilimumab (as monotherapy or in combination with other ICIs) had a higher incidence of irAEs [7]. Early detection and effective management of these adverse effects can help clinicians and patients consider ICI re-challenge and improve overall patient outcomes. Notably, certain irAEs such as colitis are more common than the other organs, such as lung, heart or endocrine [8]. The clinical practice also differs among many academic or practice groups, which could lead to wide variety of medical treatment for irAEs [9].
Systemic immunosuppression is frequently used to treat moderate to severe irAEs, but it increases the potential risk for opportunistic infections and may negatively affect anti-tumor immunity [10-11]. Patients with grade ≥ 2 irAEs or multiple concurrent irAEs often require steroids treatment [12]. Multiple oncology societies have published guidelines on the appropriate evaluation and management of irAEs [13-17]. These guidelines note that steroids and other immunosuppressants, including tumor necrosis factor-alpha inhibitors (such as infliximab), play a key role in managing irAEs for patients with steroid-refractory and steroid-dependent irAEs. Accordingly, the early introduction of infliximab or vedolizumab during disease course in patients with colitis has shown favorable clinical outcomes [18].
However, these medical treatments also have side effects of their own. The 2022 National Comprehensive Cancer Network guidelines cautioned against using infliximab in patients with reduced left ventricular ejection fraction, with the concern of potential precipitation of heart failure [15, 19]. Less commonly, infliximab was also associated with other potential toxic effect such as hepatotoxicity, and skin rash etc [13, 20, 21]. As a commonly recognized side effects, immunosuppressant use was also attributable to increased risks of hepatitis B infection, tuberculosis reactivation, and infections leading to hospitalization and possibly death [15, 21-23]. Moreover, it has been in the discussion that steroid and biologic agents may negatively impact the cancer patients' survival, although multiple confounding factors need to be taken into consideration when interpreting this data especially given the lack of high-quality prospective randomized data in this field [22, 24]. On the other hand, the use of biologic agents/steroid has been standard over the years for patients with inflammatory bowel disease (IBD) with acceptable safety profile despite the prolonged treatment duration (induction and maintenance) compared to frequent short treatment course for immune-related colitis (<3 doses) [25, 26]. However, given the different disease nature and context of malignancy, the safety of the immunosuppressant including biologics may differ and certainly warrant further investigation. In this systematic review, we investigated the safety of common immunosuppressive medications, including systemic corticosteroids and other immunosuppressants (infliximab, vedolizumab, tocilizumab, tacrolimus, alemtuzumab, leflunomide, ustekinumab, and rituximab), used to treat ICI-related irAEs with a deeper dive on the literature summary and analysis to guide clinical management which has been lacking previously.
Methods
Data sources and study selection
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [27] reporting guideline, two authors (APM and AA) independently searched the bibliographic databases MEDLINE, PMC, EMBASE, and Cochrane Library and the trial registry ClinicalTrials.gov from inception through September 1, 2022. All databases were searched for publications containing terms related to ICI agents and irAEs in their title and/or abstract (see Supplemental Methods and tables). Only studies with available full-text articles that were published in peer-reviewed journals were screened for the final analysis.
Observational (retrospective and prospective) studies and interventional trials fulfilling the following PICO criteria (Participants, Intervention, Comparison, and Outcome) were included. Participants were adult patients with any cancer who had been treated with PD-1/PD-L1 and/or CTLA-4 inhibitors. The intervention and exposure criteria were that patients were treated with an ICI and diagnosed with an irAE(s) for which they received immunosuppressants. No comparisons were made. Outcomes were complications from use of immunosuppressants.
To avoid counting the same patients multiple times, if multiple studies were suspected to share the same patient population (e.g., they were conducted at the same site with similar inclusion criteria), only the most recently published study was included. Five independent reviewers (APM, MS, AA, MHV, AK) examined all selected studies, and a mutual consensus was reached.
Data extraction and synthesis of results
Five researchers (APM, MS, AA, MHV, AK) independently extracted data from the included studies into Excel 2020 (Microsoft) using a standardized form. The types of data extracted are listed in the Supplemental Methods and tables.
Data extraction was reviewed by two independent individuals (APM, HR). Any discrepancies in individual conclusions were resolved by a joint reassessment to reach a consensus. Although we did not conduct a formal meta-analysis (due to the clinical and methodologic heterogeneity of studies identified in this review), we have summarized the studies' general characteristics and shared outcomes for descriptive purposes. In this exploratory analysis, we used random effects models to estimate the overall rates of infections among the studies reporting this information, this was done with SPSS v. 26.
Results
Study selection
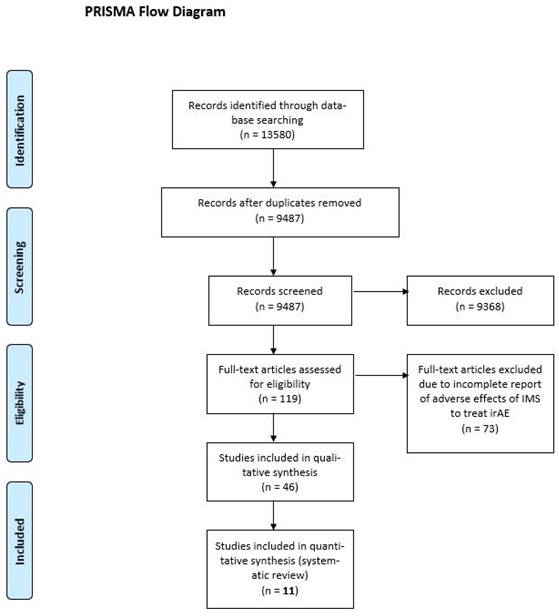
Our database search yielded 13,580 results; after duplications were excluded, 9487 articles were considered. Of the considered articles, 119 articles were identified for full-text review, of these 73 articles did not meet our inclusion criteria. Of the 46 remaining articles, 11 studies were included in the final analysis, as the others did not assess the safety of irAE treatments (Figure 1) [22, 23, 28-35].
PRISMA flow diagram. IMS, immunosuppressant; irAE, immune-related adverse event.
Study characteristics
All included articles were retrospective studies of patients who experienced irAEs related to ICI use. Most of these investigations focused on specific types of irAEs; gastrointestinal irAEs [22, 23, 30, 35], followed by hepatic irAEs [28, 32], were most common (Table 1). However, 3 studies included patients experiencing all irAE [31, 32, 36]. Treatment with systemic corticosteroids was a prerequisite for inclusion in many studies [22, 23, 29-30, 32, 33, 36]. Two studies examined steroid-refractory and/or steroid-resistant irAEs and required patients to be treated with corticosteroids and additional immunosuppressants.
Characteristics of studies included in the analysis.
| Study information | Inclusion criteria | Treatment for irAE | ||
|---|---|---|---|---|
| First author and year | Study design | ICI-related irAE | Minimum treatment | Treatment arms |
| Alexander 2021 | RCS | Colitis | CS-refractory; IFX | CS + IFX |
| Au 2018 | RCS | Hepatitis | Any | Variety* |
| Beattie 2020 | RCS | Pneumonitis | CS-refractory; IMS | CS + IFX +/- Mycophenolate |
| Johnson 2018 | RCS | Colitis | CS | CS, CS + IFX |
| Kadokawa 2021 | Case series | Any | Any | CS + IFX |
| Li 2022 | RCS | Hepatitis | CS | CS |
| Shah 2022 | RCS | Any | CS | CS + Variety* |
| Thompson 2021‡ | RCS | Rash | Any | CS |
| Wang 2018 | RCS | Colitis | Any | CS, CS + IFX |
| Williams 2019 | RCS | Any | CS | CS |
| Zou 2021 | RCS | Colitis | CS | CS, CS + IFX, CS + VDZ, CS + IFX + VDZ |
CS, corticosteroid; ICI, immune checkpoint inhibitor; IFX, infliximab; IMS, immunosuppressant; irAE, immune-related adverse event; RCS, retrospective cohort study; TNF, tumor necrosis factor; VDZ, vedolizumab.
*Includes anti-TNF, mycophenolate, and anti-thymocyte globulin.
‡Our analysis for this study was limited to those who received systemic corticosteroids.
*Includes anti-TNF, mycophenolate, and anti-thymocyte globulin.
‡Our analysis for this study was limited to those who received systemic corticosteroids.
Patient characteristics
Among the 1036 patients examined across all 11 studies, the most common primary cancer was melanoma, which was present in 52.9% of patients, followed by lung (13.4%) and genitourinary (12.6%) cancers (Table 2). Patients received a wide array of ICI treatments; PD-1/PD-L1 inhibitor monotherapy (460 patients, 44.4%) was most common, followed a combination of PD-1/PD-L1 inhibitors and CTLA-4 (350 patients, 33.8%) and CTLA-4 monotherapy (226 patients, 21.8%).
Most irAEs involved the gastrointestinal tract and hepatobiliary tract (Table 3). Colitis was present in 448 patients (43.2%), followed by hepatic cholangiopathy in 398 patients (38.4%), dermatological irAEs such as rash in 146 patients (14.1%), and pneumonitis in 72 patients (6.9%).
The majority of patients in our review received steroids as treatment for irAEs irrespective of the type or severity (98.8%). Steroids were most frequently administered with infliximab, than alone or in combination with another immunosuppressant [22, 23, 29-31, 35]. Infliximab was used in 335 patients (32.3%); this was followed by vedolizumab [23] in 77 patients (7.4%) and mycophenolate in 53 patients (5.1%). Mycophenolate was also used to treat pneumonitis in conjunction with infliximab [29]. Methotrexate and adalimumab were used infrequently.
Adverse events from treating irAEs
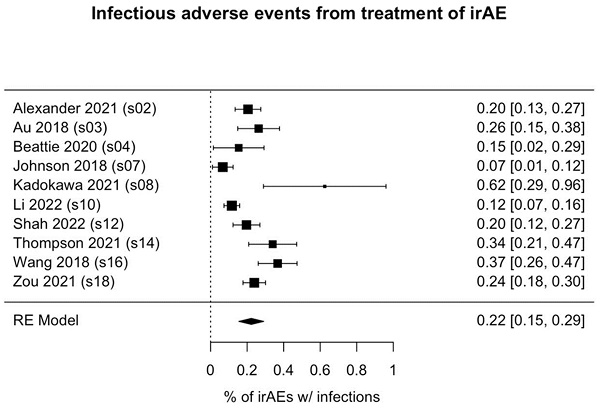
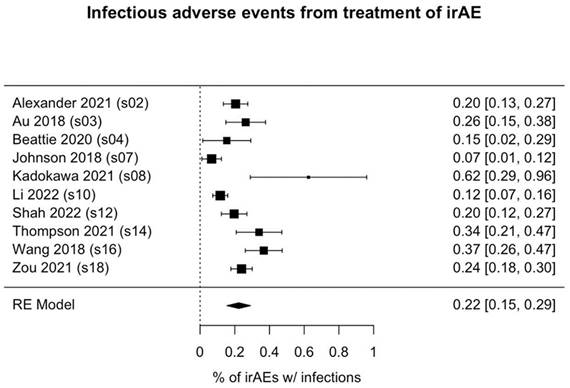
Of the 1036 patients who received immunosuppressant treatment for irAEs, 231 (22.3%) had infectious adverse events (95% CI: 15.6%-29.1%, p<0.001; Figure 2). Among the 3 studies reporting the types of infections observed (41 patients), bacterial infections (occurring in 80.5% of patients) were most common, followed by fungal infections (in 36.6% of patients). Notably, the rates of infection were highly variable among the studies, ranging from as high as 50% [31] to as low as 2.7% [30].
Cancer characteristics of and type of ICI received by patients included in the analysis.
| Study information | Primary cancer | Type of ICI used | |||||
|---|---|---|---|---|---|---|---|
| First author and year | Patientsn | Melanoma n (%) | Lungn (%) | GUn (%) | PD-1/PD-L1 n (%) | CTLA-4 n (%) | Combinationn (%) |
| Alexander 2021 | 127 | 90 (70.8) | 7 (5.5) | 9 (7.1) | 40 (31.5) | 21 (16.5) | 66 (52.0) |
| Au 2018 | 57 | NR | NR | NR | 21 (36.8) | 0 (0) | 36 (63.2) |
| Beattie 2020 | 26 | 4 (15.4) | 9 (34.6) | 6 (23.1) | 19 (73.1) | 1 (3.8) | 6 (23.1) |
| Johnson 2018 | 75 | 53 (70.7) | 2 (2.7) | 15 (20) | 17 (22.7) | 46 (61.3) | 12 (16) |
| Kadokawa 2021 | 8 | 4 (50) | 3 (37.5) | 1 (12.5) | 7 (87.5) | 0 (0) | 1 (12.5) |
| Li 2022 | 215 | 117 (54.4) | 23 (10.7) | 24 (11.2) | 94 (43.7) | 27 (12.6) | 94 (43.7) |
| Shah 2022 | 112 | 68 (60.7) | 22 (19.6) | 10 (8.9) | 50 (44.6) | 25 (22.3) | 37 (33.0) |
| Thompson 2021‡ | 50 | 13 (26) | 13 (26) | 0 (0) | 42 (84) | 2 (4) | 6 (12) |
| Wang 2018 | 79 | 79 (100) | 0 (0) | 0 (0) | 19 (24.1) | 48 (60.8) | 12 (15.2) |
| Williams 2019 | 103 | 57 (55.3) | 34 (33.0) | 5 (4.9) | 59 (57.3) | 28 (27.2) | 16 (15.5) |
| Zou 2021 | 184 | 63 (34.2) | 26 (14.1) | 61 (33.2) | 92 (50) | 28 (15.2) | 64 (34.8) |
CTLA-4, cytotoxic T-cell lymphocyte-associated antigen 4; ICI, immune checkpoint inhibitor; GU, genitourinary; NR, not reported; PD-1/PD-L1, programmed cell death protein 1/programmed death ligand 1.
‡Our analysis for this study was limited to those who received systemic corticosteroids.
Forest plot of pooled rate of infectious adverse events from immunosuppressant treatment of irAE(s). Each box's placement along the axis indicates the proportions of patients in the study who experienced infections; the horizontal lines represent 95% confidence intervals. The size of each box indicates its weight in the analysis. Results are shown for 10 of the 11 studies we analyzed; in 1 study (Williams et al.), no infections were reported. irAE, immune-related adverse event.
Patient irAE characteristics and incidence rates of infection for patients included in the analysis.
| Study information | Type of irAE | Treatment for irAE | Follow up | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First author and year | Patientsn | GIn (%) | Hepatobiliaryn (%) | Lungn (%) | Steroids n (%) | Median steroids dose (mg) | IFX n (%) | VDZn (%) | Infection rate n (%) | Duration (mons) |
| Alexander 2021 | 127 | 127 (100) | 0 (0) | 0 (0) | 127 (100) | > 5 mg | 127 (100) | 127 (100) | 26 (20.5) | 6 |
| Au 2018 | 57 | 0 (0) | 57 (100) | 0 (0) | 45 (78.9) | 1.3 mg/kg | 0 (0) | 0 (0) | 15 (26.3) | 4 |
| Beattie 2020 | 26 | 0 (0) | 0 (0) | 26 (100) | 26 (100) | 100 mg | 20 (76.9) | 0 (0) | 4 (15.4) | 2 |
| Johnson 2018 | 75 | 75 (100) | 0 (0) | 0 (0) | 75 (100) | NR | 36 (48) | 0 (0) | 2 (2.7) | 26 |
| Kadokawa 2021 | 8 | 7 (87.5) | 1 (12.5) | 0 (0) | 8 (100) | NR | 8 (100) | 0 (0) | 4 (50) | 3 |
| Li 2022 | 215 | 55 (25.6) | 215 (100) | 58 (27.0) | 215 (100) | NR | 0 (0) | 0 (0) | 25 (11.6) | NR |
| Shah 2022 | 112 | 48 (42.9) | 28 (25) | 23 (20.5) | 112 (100) | >20 mg | 15 (13.4) | 3 (2.7) | 22 (19.6) | 3 |
| Thompson 2021‡ | 50 | 0 (0) | 0 (0) | 0 (0) | 50 (100) | NR | 0 (0) | 0 (0) | 17 (34) | 3 |
| Wang 2018 | 79 | 79 (100) | 0 (0) | 0 (0) | 79 (100) | NR | 35 (44.3) | 0 (0) | 29 (36.7) | 1 |
| Williams 2019 | 103 | 0 (0) | 0 (0) | 28 (27.2) | 103 (100) | 1mg/kg | 0 (0) | 0 (0) | NR | 3 |
| Zou 2021 | 184 | 184 (100) | 0 (0) | 0 (0) | 184 (100) | NR | 94 (51.1) | 63 (34.2) | 35 (19.0) | 45 |
GI, gastrointestinal; IFX, infliximab; irAE, immune-related adverse event; NR, not reported; VDZ, vedolizumab.
‡Our analysis for this study was limited to those who received systemic corticosteroids.
Discussion
ICIs have been widely employed over the last decade due to their highly efficacious anti-tumor activity. However, their association with irAEs has led to significant morbidity and mortality. In this systematic review, we investigated the safety of common immunosuppressants used to treat ICI-related irAEs. Our analysis showed that patients who received immunosuppressants to treat gastrointestinal and hepatobiliary irAEs experienced additional adverse events, namely, an overall infection rate of 22.3%, that emphasize the need for thorough scrutiny and early recognition of these complications.
Given the wide array of irAEs in different body systems, it is possible that those irAEs could predispose patients to secondary infections regardless of immunosuppressant treatment. This hypothesis has been proposed and evaluated in several investigations. Previous evidence suggests that ICI-related colitis increases susceptibility to gastrointestinal tract infections such as Clostridioides difficile [37], non-C. diff. bacterial, and viral infections [38]. Infections, including Cytomegalovirus infections, have also been reported in the extra-gastrointestinal tract after ICI exposure, some of which could bear fatal outcomes such as pneumonitis [39]. However, it is still unclear if the underlying inflammatory adverse event predisposes patients to a higher risk of infection and colonization or if the pathogen itself contributes to a worse inflammatory process, leading to impaired outcomes. Regardless, it is imperative for clinicians to identify the infection early during irAE evaluation and management to ensure prompt therapy [40].
Certain immunosuppressant treatments can predispose patients to a reactivation of varicella-zoster infection, latent tuberculosis, or hepatitis infections. Our analysis did not encounter any patients with these complications, which might be explained by the annual and/or frequent screening of cancer patients for these types of infections [41].
Whether immunosuppressant treatment has an impact on cancer outcome and survival remains unclear due to conflicting evidence. A recent meta-analysis [42] reported that exposure to corticosteroids for any indication prior to ICI therapy did not jeopardize survival outcomes or treatment response [43]. However, Burdett et al. showed that patients receiving steroids to treat irAEs had heterogeneous results regarding their cancer outcomes [44]. Different groups have reported that patients with irAEs treated with systemic immunosuppressants and patients without irAEs have similar cancer outcomes [45-47]. Yet, there is also evidence to suggest that treatment with a steroid plus anti-tumor necrosis factor for steroid-refractory irAEs led to a significantly lower survival rate than steroid use alone did [24]. Unfortunately, high-quality data in this area are scarce; further study is needed.
A key limitation of our systematic review is the heterogeneity of the identified studies. The studies varied greatly in their aims and objectives, inclusion criteria, ICI exposure, and safety reporting. We were unable to review cancer-related outcomes, including cancer progression, progression-free survival, overall survival, and mortality, in detail due to inconsistencies in reported key safety outcomes related to immunosuppressant use. Secondly, most studies did not report key factors that may influence a patient's predisposition to infections (e.g., use of prophylactic antimicrobials, other comorbidities, recent surgeries, time of presentation, characteristics of the host, steroid dosage and treatment duration) and other confounding factors, which may account for the high variability of infectious complications observed in these studies. Thirdly, given the low incidence of certain irAEs and the less requirement for immunosuppressant treatment, the majority of the articles we extracted was focusing on GI and hepatobiliary AEs, which potentially put limitation on the generalizability of its findings. These are common limitations of systematic reviews and meta-analyses. It is imperative to establish a systematic approach for clinicians to routinely report adverse events from management of irAEs given the rapidly emerging use of the immunosuppressive medications.
In conclusion, complications from therapy for irAEs occurred in about less than one-third of patients that required treatment with either steroids or a combination of steroids and other immunosuppressants. Clinicians should therefore be aware of this increased risk and consider minimizing immunosuppressant treatment or using alternative therapy when available to avoid interruptions or negative impact in cancer care. Future prospective studies are needed to further investigate the safety of immunosuppressants in treating irAEs.
Supplementary Material
Supplementary information.
Acknowledgements
Medical editing of this paper was provided by Madison Semro, Associate Scientific Editor, and Sunita Patterson, Senior Scientific Editor, Research Medical Library, The University of Texas MD Anderson Cancer Center.
Author contributions
Yinghong Wang, the senior author of this article, developed the concept, designed the study, interpreted the results, ensured the preservation of data accuracy and integrity at all stages, agreed to be accountable for all aspects of the study, was in charge of the overall direction and planning of the study, and contributed to the writing of the manuscript. Antonio Pizuorno Machado, Malek Shatila, Ahmed Abdelwahab, Muhammad H. Vohra, and Andrew Kuang screened the articles for the study. Hunter Ratliff ran the analysis. Antonio Pizuorno Machado drafted the manuscript. All authors critically revised the final version of the manuscript and approved the final manuscript.
Ethics approval and consent to participate
The ethics approval for this study was granted by the institutional review board at The University of Texas MD Anderson Cancer Center (PA18-0472). Patient consent was waived for this study.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33(17):1974-82
2. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-5
3. Li C, Bhatti SA, Ying J. Immune Checkpoint Inhibitors-Associated Cardiotoxicity. Cancers (Basel). 2022;14(5):1145
4. Pauken KE, Dougan M, Rose NR, Lichtman AH, Sharpe AH. Adverse Events Following Cancer Immunotherapy: Obstacles and Opportunities. Trends Immunol. 2019;40(6):511-23
5. Brown VT, Antol DD, Racsa PN, Ward MA, Naidoo J. Real-World Incidence and Management of Immune-Related Adverse Events from Immune Checkpoint Inhibitors: Retrospective Claims-Based Analysis. Cancer Invest. 2021;39(10):789-96
6. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F. et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4(12):1721-8
7. Abu-Sbeih H, Tang T, Ali FS, Johnson DH, Qiao W, Diab A. et al. The Impact of Immune Checkpoint Inhibitor-Related Adverse Events and Their Immunosuppressive Treatment on Patients' Outcomes. 2018;1:7-18.
8. Som A, Mandaliya R, Alsaadi D. et al. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J Clin Cases. 2019;7(4):405-418
9. Powell N, Ibraheim H, Raine T. et al. British Society of Gastroenterology endorsed guidance for the management of immune checkpoint inhibitor-induced enterocolitis. Lancet Gastroenterol Hepatol. 2020;5(7):679-697
10. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK. et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277-90
11. Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE. et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-9
12. Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK. et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-8
13. Dougan M, Wang Y, Rubio-Tapia A, Lim JK. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology. 2021;160(4):1384-93
14. Haanen J, Obeid M, Spain L. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(12):1217-1238
15. Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK. et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(4):387-405
16. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M. et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol. 2021;39(36):4073-126
17. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6):002435
18. Abu-Sbeih H, Ali FS, Alsaadi D, Jennings J, Luo W, Gong Z. et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J Immunother Cancer. 2018;6(1):142
19. Page RL, O'Bryant CL, Cheng D, Dow TJ, Ky B, Stein CM. et al. Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2016;134(6):e32-69
20. Zhang HC, Luo W, Wang Y. Acute liver injury in the context of immune checkpoint inhibitor-related colitis treated with infliximab. J Immunother Cancer. 2019;7(1):47
21. Alexander JL, Ibraheim H, Sheth B, Little J, Khan MS, Richards C. et al. Clinical outcomes of patients with corticosteroid refractory immune checkpoint inhibitor-induced enterocolitis treated with infliximab. J Immunother Cancer. 2021;9(7):002742
22. Zou F, Faleck D, Thomas A, Harris J, Satish D, Wang X. et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J Immunother Cancer. 2021;9(11):003277
23. Asscher VER, van der Vliet Q, van der Aalst K, van der Aalst A, Brand EC, van der Meulen-de Jong AE. et al. Anti-tumor necrosis factor therapy in patients with inflammatory bowel disease; comorbidity, not patient age, is a predictor of severe adverse events. Int J Colorectal Dis. 2020;35(12):2331-8
24. Verheijden RJ, May AM, Blank CU, Aarts MJB, van den Berkmortel FWPJ, van den Eertwegh AJM. et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin Cancer Res. 2020;26(9):2268-74
25. Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D'Haens G. et al. The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut. 2017;66(5):839-51
26. Peyrin-Biroulet L, Arkkila P, Armuzzi A. et al. Comparative efficacy and safety of infliximab and vedolizumab therapy in patients with inflammatory bowel disease: a systematic review and meta-analysis. BMC Gastroenterol. 2022;22(1):291
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89
28. Au L, R N, Possamai L, Barlow C, Tillett T, Bowen R. et al. Pathogenesis, clinical evolution and outcomes of patients with immune checkpoint inhibitor induced acute liver injury: A multicentre study. Annals of Oncology. 2018;29(8):430
29. Beattie J, Rizvi H, Fuentes P, Luo J, Schoenfeld A, Lin IH. et al. Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade. J Immunother Cancer. 2021;9(2):001884
30. Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett RL, Abdel-Wahab N. et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer. 2018;6(1):103
31. Kadokawa Y, Takagi M, Yoshida T, Tatsumi A, Fujita K, Inoue T. et al. Efficacy and safety of Infliximab for steroid-resistant immune-related adverse events: A retrospective study. Mol Clin Oncol. 2021;14(4):65
32. Li C, Bhatti SA, Ying J. Immune Checkpoint Inhibitors-Associated Cardiotoxicity. Cancers (Basel). 2022;14(5):1145
33. Shah NJ, Cook MR, Wu T, Lev-Ari S, Blackburn MJ, Serzan MT. et al. The Risk of Opportunistic Infections and the Role of Antibiotic Prophylaxis in Patients on Checkpoint Inhibitors Requiring Steroids. J Natl Compr Canc Netw. 2022;20(7):800-7.e1
34. Thompson LL, Yoon J, Krasnow NA, Chang MS, Li EB, McMahon DE. et al. Association Between Systemic Corticosteroid Treatment for Cutaneous Immune-Related Adverse Events and Survival Outcomes in Patients With Advanced Cancer. JAMA Dermatol. 2021;157(5):599-602
35. Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W. et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6(1):37
36. Williams KJ, Grauer DW, Henry DW, Rockey ML. Corticosteroids for the management of immune-related adverse events in patients receiving checkpoint inhibitors. J Oncol Pharm Pract. 2019;25(3):544-50
37. Vasavada S, Panneerselvam K, Amin R, Varatharajalu K, Okhuysen PC, Oliva ICG. et al. infection in cancer patients receiving immune checkpoint inhibitors. Ann Gastroenterol. 2022;35(4):393-9
38. McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious Colitis Associated With Ipilimumab Therapy. Gastroenterology Res. 2014;7(1):28-31
39. Panneerselvam K, Szafron D, Amin RN, Wei D, Tan D, Altan M. et al. Cytomegalovirus infection among patients with cancer receiving immune checkpoint inhibitors. Ann Gastroenterol. 2022;35(5):522-31
40. Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95
41. Del Castillo M, Romero FA, Argüello E, Kyi C, Postow MA, Redelman-Sidi G. The Spectrum of Serious Infections Among Patients Receiving Immune Checkpoint Blockade for the Treatment of Melanoma. Clin Infect Dis. 2016;63(11):1490-3
42. Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo EG, Ruggieri L. et al. Association of Steroids use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers (Basel). 2020;12(3):546
43. Shatila M, Ma W, Cui Y, Naz S, S Thomas A, N De Toni E. et al. Effects of immunosuppressive treatment on patient outcomes after immune checkpoint inhibitor-related gastrointestinal toxicity. J Cancer Res Clin Oncol. 2023 [online ahead of print]
44. Burdett N, Hsu K, Xiong L, Tapia-Rico G, Beckmann K, Karapetis C. et al. Cancer outcomes in patients requiring immunosuppression in addition to corticosteroids for immune-related adverse events after immune checkpoint inhibitor therapy. Asia Pac J Clin Oncol. 2020;16(2):e139-e45
45. Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK. et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-8
46. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J. et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol. 2017;35(7):785-92
47. Arriola E, Wheater M, Karydis I, Thomas G, Ottensmeier C. Infliximab for IPILIMUMAB-Related Colitis-Letter. Clin Cancer Res. 2015;21(24):5642-3
Author contact
![]() Corresponding author: Dr. Yinghong Wang, Department of Gastroenterology, Hepatology, and Nutrition, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1466, Houston, TX 77030. Tel.: (713) 794-5073. Fax: (713) 794-5398. Email: ywang59org.
Corresponding author: Dr. Yinghong Wang, Department of Gastroenterology, Hepatology, and Nutrition, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1466, Houston, TX 77030. Tel.: (713) 794-5073. Fax: (713) 794-5398. Email: ywang59org.

 Global reach, higher impact
Global reach, higher impact