3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(7):1202-1215. doi:10.7150/jca.83355 This issue Cite
Research Paper
Anisomycin has the potential to induce human ovarian cancer stem cell ferroptosis by influencing glutathione metabolism and autophagy signal transduction pathways
1. Department of Obstetrics and Gynecology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China.
2. Shanghai Geriatric Institute of Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 200031, China.
3. Gongli Hospital Affiliated to the Second Military Medicical University in Pudong New Area of Shanghai City, Shanghai 200135, China.
* These authors contributed equally to this work and shared the first authorship.
Abstract
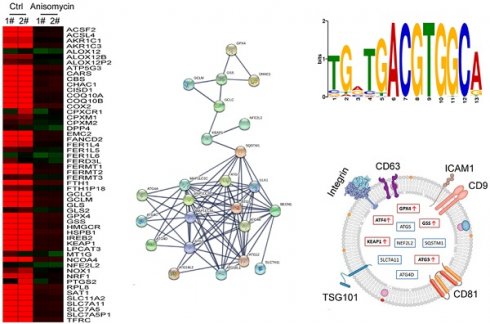
Ovarian cancer is a highly malignant gynecological tumor that seriously endangers women's health. Previously, we demonstrated that anisomycin significantly inhibited the activity of ovarian cancer stem cells (OCSCs) in vitro and in vivo. In this study, anisomycin treatment of OCSCs significantly reduced the content of adenosine triphosphate and total glutathione, increased the extent of lipid peroxidation, and increased malondialdehyde, and Fe2+ levels. The ferroptosis inhibitor Ferr-1 could significantly weaken the cytotoxicity of anisomycin. Subsequently, the cDNA microArray results suggested that anisomycin significantly reduced the transcription levels of gene clusters associated with protection from ferroptosis, such as those encoding members of the glutathione metabolism and autophagy signal transduction pathways. Bioinformatic analyses indicated that genes encoding core factors of these two pathways, and activating transcription factor 4 (ATF4), were significantly expressed in ovarian cancer tissues and correlated with poor prognosis. After overexpression or knockdown of ATF4, the ability of anisomycin to inhibit the proliferation and autophagy of OCSCs increased or decreased, respectively. Finally, analysis using a peripheral blood exosome database indicated that the contents of key factors (e.g., ATF4, GPX4, and ATG3) in peripheral blood exosomes from patients with ovarian cancer, were significantly higher than those of the healthy controls. Therefore, we hypothesized that anisomycin suppressed the expression of members of the glutathione metabolism and autophagy signal transduction pathways by downregulating the expression of ATF4. Moreover, anisomycin has the potential to induce human ovarian cancer stem cell ferroptosis. Overall, we confirmed that anisomycin has multiple targets and many mechanisms of action in inhibiting the activity of OCSCs.
Keywords: Ovarian cancer, Anisomycin, ferroptosis, glutathione, autophagy.

 Global reach, higher impact
Global reach, higher impact