Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(5):835-842. doi:10.7150/jca.80803 This issue Cite
Review
Current status and progress of the development of prostate cancer vaccines
1. Department of Urology, the Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, China.
2. Jiangsu Key Laboratory of Biological Cancer Therapy, Xuzhou Medical University, Xuzhou 221002, China.
# Contributed equally
Received 2022-11-13; Accepted 2023-3-8; Published 2023-4-1
Abstract
At present, common treatments of prostate cancer mainly include surgery, radiotherapy, chemotherapy and hormone therapy. However, patients have high recurrence rate after treatment, and are prone to castration-resistant prostate cancer. Tumor vaccine is based on tumor specific antigen (TSA) and tumor associated antigen (TAA) to activate specific immune response of the body to cancer cells. With continuous maturity of tumor vaccine technology, different forms of prostate cancer vaccines have been developed, such as cellular vaccines, extracellular-based anti-tumor vaccines, polypeptide vaccines, and nucleic acid vaccines. In this review, we summarize current status and progress in the development of prostate cancer vaccines.
Keywords: prostate cancer, cancer vaccine, tumor specific antigen, tumor associated antigen
Introduction
Prostate cancer is the second most common cancer among men in the world, and there are 1,414,259 new cases and 375,304 deaths worldwide in 2020. In China, with the acceleration of population aging, the growth of economic level and the improvement of detection methods, the incidence of prostate cancer is on the rise in recent years [1].
The mechanism of anticancer vaccines is the immune activation of specific tumor-associated antigens (TAA) or tumor-specific antigens (TSA), which can actively trigger the immune response by binding to costimulatory molecules localized on immune cells of cancer patients [2,3]. Dendritic cells (DCs) are a kind of antigen presenting cells (APCs) that play an important role in antigen cross presentation [4]. The discovery of antigen cross presentation has a far-reaching impact on the development of therapeutic tumor vaccines.
Prostate cancer has a large number of highly targeted TAA and TSA, such as PCA3, PAP, PSA, PSMA [5]. Therefore, prostate cancer is an ideal candidate for cancer vaccine therapy, and most of these studies focus on metastatic castration-resistant prostate cancer (mCRPC) [6]. Recently, several promising vaccines have been developed for prostate cancer treatment, such as Prostvac, Provenge (Sipuleucel-T) and individualized PPV (polypeptide vaccine). Provenge is the only cellular active immune product licensed by FDA to treat asymptomatic or mild symptomatic CRPC [7]. Below we will provide further details of prostate cancer vaccines and summarize current status and progress in the development of prostate cancer vaccines.
Dendritic cell vaccine
Dendritic cell vaccine is made of DCs loaded with tumor antigens. DC vaccines are inoculated into cancer patients to induce efficient anti-tumor response, including DC and tumor cell fusion vaccine, tumor antigen peptide or protein extract sensitized DC vaccine, gene modified DC vaccine, tumor cell extracellular sensitized DC vaccine [6]. DCs are professional antigen presenting cells, so they can absorb, process and transmit antigen information.
Sapuleucel-T is currently the only active cellular immune product licensed by FDA to treat asymptomatic or mild symptomatic CRPC [7]. Sipuleucel-T mainly includes peripheral blood monocytes activated by PAP and GM-CSF recombinant fusion protein in vitro, including antigen presenting cells. PAP is an enzyme secreted by the prostate and is highly expressed in metastatic prostate cancer and is the target of Sapuleucel-T [6]. PA2024 antigen is a recombinant fusion protein containing PAP and GM-CSF, and GM-CSF receptors are widely expressed in APCs. The recombinant protein binds to the patient's APCs in vitro to make the APC display the antigen on its surface, and then the engineering APC is re-injected into the patient [7].
Polypeptide vaccine
In the early 1980s, Lerner proposed determining the structure of natural antigens to synthesize peptides with both antigenicity and immunogenicity, and established a method for the development of synthetic peptide vaccines [8]. TSA peptides delivered to the MHC (major histocompatibility complex) on the surface of APC (antigen presenting cell) are degraded in APC to form peptide-MHC-TCR complexes, which trigger the corresponding reaction of CTL (cytotoxic T lymphocyte). The advantage of peptide vaccine is that it is possible to combine various peptides obtained from different antigens into one carrier, and to construct corresponding synthetic antigenic peptides for complex discontinuous natural antigenic determinants [9]. Therefore, prostate cancer polypeptide vaccines with many candidate TAA peptides have good application prospect.
GV1001 is an II-like telomerase polypeptide vaccine that can activate the immune system, leading to the activation of both CD4+ and CD8+T cell responses to recognize and kill tumor cells [10]. Telomere terminal transferase activity is considered to be a common feature of all tumor cells. Therefore, GV1001 may be a universal tumor vaccine. At present, the vaccine has significantly improved the survival rate in a phase-adjusted I/II clinical trial in pancreatic cancer therapy, and a phase II clinical trial showed that the telomerase GV1001 polypeptide vaccine was well tolerated in the therapy of stage III NSCLC (non-small cell lung cancer) [11]. However, there are no clinical trials of prostate cancer.
KRM-20 is a new tumor vaccine composed of 20 peptides to induce CTL against 12 different tumor-associated antigens highly expressed in prostate cancer tissues. A phase II study of KRM-20 showed that there was no evident difference in OS and PFS between the experimental group and the placebo group, but KRM-20 was effective in patients with chronic prostate cancer with lymphocyte ≥ 26% or PSA < 11.2 ng/ml [12].
Individualized polypeptide vaccine (personalized peptide vaccine, PPV) refers to the preparation of anti-tumor vaccine by selecting at most four peptides that match HLA-A1 (human leukocyte antigen A1) subtype according to the difference of individual genetic background [13]. The advantage of PPV lies in the ability to bypass immune diversity and avoid immune tolerance. The results of phase I clinical trial showed that PPV was well tolerated and the level of PSA decreased [14]. The results of the first randomized controlled phase II clinical trial of PPV in patients with CRPC showed that the overall survival (OS) of the low dose EMP group was significantly better than that of the standard dose EMP group [15]. Another phase II randomized controlled clinical trial of 72 patients with CRPC showed that PFS and median OS in the PPV+ dexamethasone group were significantly higher than control group treated with dexamethasone alone [16]. The patients selected in this study were in the early stage of CRPC, indicating that early CRPC patients can significantly improve PFS and OS with PPV treatment. In addition, the phase III clinical trial of PPV is being carried out, but the results have not been reported.
DNA Vaccine
DNA vaccine is a closed circular DNA plasmid designed to encode antigens or epitopes of interest under the strong promoter of mammals [17]. The mechanism of DNA vaccine is that naked plasmid DNA can induce the production of endogenous antigens and the presentation of MHC-I molecules. By changing the sequence of plasmid DNA, DNA vaccines can induce strong anti-tumor cellular immune response against tumor antigens [18]. As a simple and effective antigen presentation method, DNA vaccine can have an impact on the treatment of prostate cancer. It is still a challenge to identify and select suitable tumor-specific antigens to cause minimal damage to normal cells.
PCaA-SEV (Prostate Canner Antigens-Synthetic Enhanced DNA Vaccine) is an enhanced DNA vaccine platform for the synthesis of multiple prostate cancer antigens. Candidate vaccines include PSCA, PAP, PCTA, STEAP1, PARM1 and PSP94. Studies have shown that cellular immunity to PCAA and versatility of antigen-specific T cells can be induced by PCAA-SEV. It is worth noting that mice immunized with PSP94 DNA vaccine showed the strongest cellular response. Similarly, candidate vaccines of PSCA, PCTA and PARM1 showed a strong cellular response against antigen. Compared with other candidate vaccines, splenocytes of mice immunized with STEAP1 and PAP-SEV showed a lower immune response. The enhanced delivery of these DNA vaccines mediated by the electroporation of plasmid (EP) can produce PCAA-specific CD8+T cells and increase their levels in tumor microenvironments, thereby improving the survival of mice carrying prostate cancer [19]. Although it is often reported that EP can increase the immunogenicity of vaccines, the conversion of this technique into human trials is slightly 2-3 times higher than that of simple injection of naked DNA [20]. The advantage of DNA vaccine is that it can combine candidate vaccines to attack multiple targets at the same time to inhibit tumor growth.
The monoclonal antibody against PSMA encoded by DNA is a new vaccine strategy that uses synthetic DNA plasmid to encode human anti-PSMA monoclonal antibody. The level of PSMA in prostate cancer cells is further increased. Studies have shown that the increased expression of PSMA is closely related to the progression of prostate cancer. PSMA is an attractive target for the development of anti-PSMA monoclonal antibodies for diagnostic and therapeutic purposes because it is a membrane protein [21]. A study has shown that delivering PSMA-DMAb plasmids through EP can guide the production of strong levels of PSMA-specific human immunoglobulin in vivo. PSMA on the surface of human tumor cells can not only be recognized but also can be specifically bound by PSMA-DMAb [22]. The synthesized PSMA-DMAb can bind to Fc receptors and mediate the ADCC effect, which is at least in part mediated by NK cells [23]. This is the first report about the involvement of DNA vector of monoclonal antibody in host NK immune clearance and the use of delivery system based on DNA plasmid to guide the production of therapeutic mAb targeting tumor antigen PSMA in vivo.
PTVG-AR, MVI-118 is a DNA vaccine encoding the androgen receptor ligand binding domain, and is mainly tested in patients with mCSPC. In the preclinical model evaluation, it was found that AR expression increased in prostate tumor cells deprived of androgen, which made tumor cells more likely to be dissolved by AR specific CD8+T cells. The combination of AR targeted vaccine and androgen deprivation delayed tumor development in ovariectomized mice [24]. An open-label, randomized, multi-agency phase 1 trial showed that 27 of 40 patients (68%) had no progress at 18 months, and there was no distinct difference in the time of castration resistance or first PSA rise between the study groups with or without GM-CSF adjuvant [25]. Surprisingly, vaccine adjuvant GM-CSF did not induce better immune response, and preclinical studies of pTVG-AR vaccines in mice and rats have demonstrated the effect without additional adjuvants [26]. In addition, it was found that immunization could stimulate T cells to respond to ARLBD, but could not cause antibody response, which was consistent with the results of preclinical studies in mice. It was not antibody response but DNA immunization promoted the production of CD4 and CD8T cells [26].
RNA Vaccine
The most important RNA vaccine is the mRNA vaccine platform, which combines the immunological characteristics of live attenuated vaccine, the expression of endogenous antigens and the immunological characteristics of T cell induction and inactivated vaccine, such as determined composition and safety. RNA does not interact with the genome because of few cases of recombination between single-stranded RNA, and mRNA only contains the direct elements necessary to express the encoded protein, and mRNA does not need to cross the nuclear membrane, so it has safety advantages [27]. MRNA binds to pattern recognition receptors, and messenger ribonucleic acid vaccines may be designed to be self-regulating, a feature missing from peptide and protein based vaccines [28].
The anti-tumor vaccine CV9103 contains four self-adjuvant mRNAs, which encode PSA, STEAP1, PSMA and PSCA [29]. CV9103 encodes full-length antigens, which can induce immune responses against all epitopes contained in the target protein without being restricted by HLA. The existence of multiple antigens reduces the risk of tumor immune escape caused by the lack of expression of a single antigen [30]. In a first-person I/IIA phase study of CV9103, 44 patients with CRPC were enrolled, of whom 12 were included in the first phase of the study to determine the safe dose of IIa; 32 patients entered the second phase, and Kaplan-Meier estimated the median OS of 36 metastatic CRPC patients treated with CV9103 at 31.4 months. It was observed that immune response and survival time had no significant correlation. The median OS was 29.3 months in all 44 patients. The median OS of subgroup patients with metastatic diseases was 31.4 months [29]. Based on these results, CV9103 has good tolerance and immunogenicity. In addition, a phase IIb study of CV9104 began in 2012. CV9104 is an improved version of CV9103 vaccine that includes additional mRNA molecules that encode PAP and mucin 1 (MUC1) [31].
Adjuvant-pulsed mRNA vaccine nanoparticle (NP) includes an ovalbumin-coded mRNA, a palmitic acid-modified C16-R848 and a shell composed of lipid-polyethylene glycol. The combined transmission of C16-R848 adjuvant and OVA mRNA increases the presentation of TAA. The vaccine retains the adjuvant activity of C16-R848 and significantly increases the transfection rate of OVA messenger RNA in antigen presenting cells (> 95%) and subsequent presentation of MHC-I. Compared with the adjuvant-free mRNA vaccine NP, the mRNA vaccine NP regimen pulsed with C16-R848 adjuvant significantly improved the expansion of OVA-specific CD8+T cells in vivo and their invasion to the tumor bed, and induced a strong adaptive immune response [32]. This approach resulted in effective anti-tumor immunity against allogeneic mouse lymphoma and prostate cancer models expressing OVA, which significantly prevented tumor growth when the vaccine was given before tumor implantation (84% less than the control) and after transplantation (60% less than the control) [32]. In addition, an advantage of NP-mediated R848 over free R848 is to evade systemic cytokine responses, thereby reducing systemic toxicity [33].
MS2VLP-based PAP-GM-CSF mRNA vaccine is a recombinant phage MS2 virus-like particle (VLP) based on the interaction between 19-nucleotide RNA aptamer and MS2 phage coat protein, in which the target gene is packaged by MS2 capsid to protect the target RNA from nucleotide degradation, and then non-toxic and anti-ribonuclease MS2VLP gene vaccine can be easily produced by using recombinant protein technology [34]. The Th1/Th2 response was balanced without upregulating CD41 regulatory T cells. Ganglioside GD1a is highly expressed in CRPC cells. HVJ-E selectively binds to it, resulting in apoptosis of prostate cancer cells [35]. The capsid of MS2 can be modified by inserting peptides to target specific cells, and this vaccine is expected to be developed jointly with polypeptide vaccines.
Virus vaccine
As a new type of viral preparation for the treatment of CRPC, oncolytic adenovirus has the advantages of high selectivity and low cytotoxicity [36]. According to the oncolytic characteristics of Ad5, Ad5 is divided into two vectors: CRAd vector (conditional replication adenovirus) and RDAd vector (replication defective adenovirus). Conditional replication adenovirus vector can only proliferate and destroy cancer cells, but cannot replicate in normal cells, which is also called oncolytic adenovirus. Replication defective adenovirus vector have no intracellular replication, but can carry remedial genes [37]. The insertion of foreign genes and deletion of viral cytoskeleton genes can enhance the oncolytic ability of adenoviruses. For example, the oncolytic mutant Ad deleted without E1B19K and E1ACR2 can induce apoptosis of prostate cancer cells [38]. In addition, the addition of prostate-specific promoters / enhancers only leads to viral replication and foreign gene expression in prostate cancer cells [39]. PSA, PSMA, PB and PCA3/DD3 promoters have been used in adenovirus-mediated gene therapy of prostate cancer. Ad/PSAP-GV16- β G vector combined with prodrug DOX-GA_3 can kill LNCaP cell transplanted tumor in nude mice. Ad-PSMA (Emurp)-CD vector based on PSMA promoter drives the expression of cytosine deaminase, and effectively kills PSMA-producing CL-1 transplanted tumor with combined use of prodrug 5-flucytosine [40]. Ad vector based on PB promoter may target AR positive PCa [41]. Hao et al. designed OncoAd.mK5.DD3 vector that effectively inhibited PCa [42].
Sendai virus is a kind of virus with cell fusion activity but loss of replication ability after UV inactivation. HVJ-E (HVJ envelope) retains the ability of cell fusion [43]. HVJ-E selectively binds to Ganglioside GD1a and causes the apoptosis of PCa cells. In a phase I / II trial, 7 patients were selected and 6 patients were treated with HVJ-E. The PSA effective rate of HUJ-E treatment was 16.6%, and the adverse reactions were mild. The results showed that the level of PSA decreased completely in patients with metastatic prostate cancer [44]. In another phase I dose increment study, the safety and efficacy of higher dose HVJ-E (GEN0101) were tested. The inhibitory effect on the increase of PSA with high dose was stronger than that in the low one. The NK cell activity was enhanced in 2 cases with low dose and 5 cases with high dose [45]. The results showed that intratumoral and subcutaneous GEN0101 injection was well tolerated.
Prostvac is a tumor vaccine based on recombinant poxvirus vector, which consists of two components, one is recombinant vaccinia virus, which can stimulate the initial immune response, and the other is fowlpox virus, which is used to enhance immune response [46]. Phase I clinical trials confirmed the safety and immune activity of Prostvac [47]. In a phase II clinical trial, 82 patients were enrolled in the Prostvac treatment group and 40 patients in the control group. Three years after treatment, the mortality in the Prostvac group (69.5%) was significantly lower than that in the control group (82.5%). The average survival time of metastatic CRPC patients treated with Prostvac was significantly prolonged (25.1 months vs. 16.6 months) [48].
The combination of prostate cancer vaccines and other treatments
Although prostate cancer vaccines show promise, it should be emphasized that the treatment of prostate cancer should be a combination of multiple treatment methods, such as immune checkpoint inhibitors (ICI), CART cells, prostate cancer vaccines, hormone therapy, radiotherapy and chemotherapy. Targeting the immune system may be a promising treatment for prostate cancer in the future. The combination of PD-1/PD-L1 blockers and other treatments has shown good results for prostate cancer [49]. Anti-PD-1/PD-L1 combined with other drugs showed effective anti-tumor effects, including androgen deprivation, anti-tumor vaccine, radiotherapy and chemotherapy [50].
Androgen deprivation therapy (ADT) can induce T cells to activate prostate antigen and temporarily reduce the tolerance of T cells. A synergistic relationship between ADT and immunotherapy has been proposed [51]. So far FDA has authorized the use of ipilimumab monoclonal antibody as cancer immunotherapy. Current clinical trials of mCRPC use a mixture of immune checkpoint inhibitors as an alternative. For example, CHECKMATE650, a phase II clinical study, began to examine the efficacy of a combination of ipilimumab and nivolumab in patients with mCRPC who are resistant to androgen receptor (AR) targeted therapy [52].
Recent studies showed better efficacy of combined immunotherapy and chemoradiotherapy for cancer treatment [53,54]. Several clinical trial in mCRPC patients showed the benefits of combined chemotherapy and immunotherapy for prostate cancer therapy [55,56]. After vaccination with tumor antigen-specific DNA vaccine, the expression of PD-L1 on circulating tumor cells (CTCs) increased, which was related to the development of T cell immunity and longer progression-free survival. These findings provide the support for the combination of anticancer vaccines and PD-1 blocking antibodies in the treatment of prostate cancer [57]. In TRAMP mice in advanced stage of prostate cancer, VLP vaccine alone or in combination with anti-PD1 antibody could significantly reduce the tumor burden by using novel virus-like particles (VLP) vaccine, anti-PD1 antibody or combined immunotherapy [58]. Continuous injection of anti-PD-1 and anti-TIM-3 antibodies further improved the therapeutic effect of anchored granulocyte-macrophage colony-stimulating factor (GM-CSF) vaccine. Tumor regression was found in more than 60% of mice. This triple therapy can increase specific cytotoxic activity, proliferation and secretion of CD8+TIL, and reduce the production of tumor-promoting cytokines. These results suggest that triple therapy can obtain effective anti-tumor immune response in patients with prostate cancer [59].
In addition, other chemotherapeutic drugs have been shown to improve the anticancer effect of PCa when CTLA-4 and PD1/PD-L1 are inhibited. A study reports that hip-T vaccine can increase immune penetration of prostate cancer and create a favorable environment for PD-1/PD-L1 blocking, suggesting that the combination of hip-T vaccine and PD-1/PD-L1 blocking may be a promising immunotherapy strategy [60].
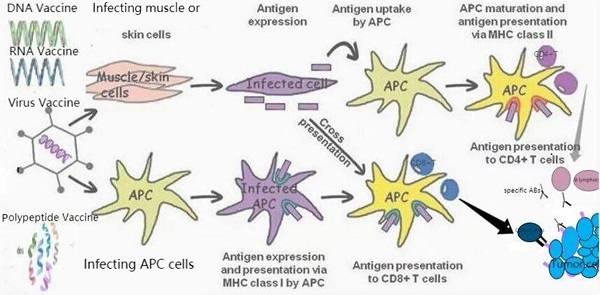
Therapeutic vaccines for prostate cancer. TAAs can be transmitted through different vaccine forms (RNA or DNA vaccines encoding TAAs, polypeptide vaccines, viral vaccines). APC induces specific cytotoxicity to tumor cells expressing TAA through the interaction of MHC class I with CD8+T cells. CD4+T cells were activated by the second type of MHC, APC. In addition, they also induce the activation of B lymphocytes to produce specific antibodies (Abs) against tumor cells expressed by TAA.
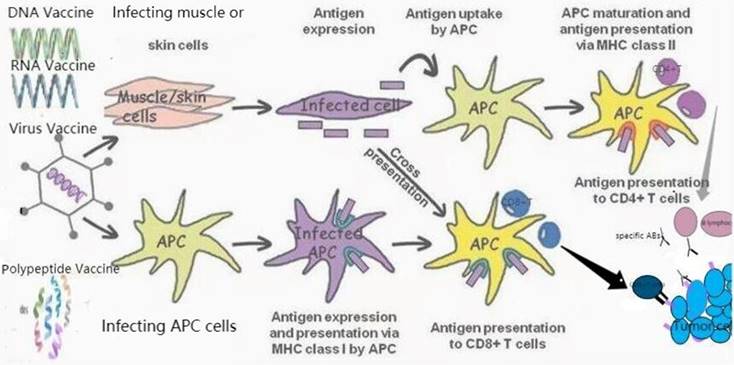
Prostate cancer vaccines under development.
| Vaccines | Specific Antigens | Immune System Effectors | Clinical Outcomes | Side Effects |
|---|---|---|---|---|
| Sapuleucel-T | PAP | Antigen presenting cells | FDA licensed cellular active immune products for asymptomatic or mild symptomatic CRPC | chills (53.1%), fever (31.3%), muscle pain (11.8%) and headache (18.1%). |
| GV1001 | Telomerase polypeptide | CD4+ and CD8+ T cells | There is no clinical trial in prostate cancer. | / |
| KRM-20 | 20 peptides | CTL | Effective for chronic prostate cancer patients with lymphocyte ≥ 26% or PSA < 11.2 ng/ml | No serious adverse reactions |
| PPvs | Up to four peptides that match human leukocyte antigen A1 (HLA-A1) are selected | CTL | Early CRPC patients can significantly improve PFS and OS | Good tolerance |
| PCaA-SEV | STEAP1, PAP, PARM1, PCTA, PSCA | T cell | Mice immunized with PSP94DNA vaccine showed the strongest cellular response. | / |
| PSMA-DMAb | PSMA | NK cells, T cells | This is the first report about the involvement of DNA vector monoclonal antibody in host NK immune clearance and the use of DNA plasmid-based delivery system to guide the production of therapeutic mAb targeting related oncology target PSMA in vivo. | No serious adverse reactions |
| pTVG-AR, MVI-118 | Androgen receptor | CD8+T cells | Patients treated with vaccine had significantly longer first PSA rise | / |
| CV9103 | PSA, PSMA, PSCA, STEAP1 | T cells | The median OS of all 44 patients was 29.3 months. In the subgroup of patients with metastatic diseases median OS was 31.4 months. | Good tolerance and immunogenicity |
| Adjuvant-pulsed mRNA vaccine nanoparticle | Adjuvant-pulsed mRNA vaccine NP | CD8+T cells | tumor growth was significantly inhibited. | Low systemic toxicity |
| MS2 VLP-based PAP-GM-CSF mRNA vaccine | TSA | B cells and T cells | The Th1/Th2 response was balanced without upregulating CD41 regulatory T cells, and protected C57BL/6 mice from prostate cancer and delayed tumor growth. | No serious adverse reactions |
| HVJ envelope (HVJ-E) | Sendai virus | T cells | In a single-arm, open-label, single-center, phase I/II HVJ-E study, the PSA response rate of HVJ-E was 16.6%. | Mild adverse reactions |
| Prostvac | PSA, Cowpox virus, Fowlpox virus | T cells | The average survival time of patients with metastatic CRPC was significantly prolonged after treatment | Mild side effects (fever and injection site reaction) |
Conclusion
In recent years, prostate cancer vaccine has received more attention (Figure 1). In this review, we summarize current status of the development of prostate cancer vaccines and highlight some emerging vaccine strategies (Table 1). The combination of dendritic cell vaccine, peptide vaccine and nucleic acid vaccine may be a promising strategy in the future. In addition, simple tumor vaccine therapy is difficult to achieve high treatment efficacy, and the combination with chemotherapy, radiotherapy and surgery may be necessary as discussed in previous section. It can be predicted that treatment strategy based on the combination of a variety of tumor vaccines and traditional treatment methods supplemented with immune adjuvants will become a major trend of anti-tumor therapy for prostate cancer.
Acknowledgements
This study was supported by grants from Jiangsu Province Science Foundation of China (No BK20151166), the Project of Invigorating Health Care Through Science, Technology and Education (No CXTDA2017034), and Jiangsu Provincial Medical Youth Talent (No QNRC2016794).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. Journal of the National Cancer Center. 2022;2(1):1-9
2. Sadde F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev. Vaccines. 2012;11:189-209
3. You L, Na F, Zhou J, Jiao L, Zhou Y, Ying B. Expression and prognosis analyses of Dectin-1 cluster genes in patients with lung adenocarcinoma (LUAD) and the association with immune checkpoint molecules. Biocell. 2021;45(3):649-663
4. Jin JY. Prospect of radiotherapy technology development in the era of immunotherapy. Journal of the National Cancer Center. 2022;2(2):106-112
5. Liu J, Li Y, Yang D, Yang C, Mao L. Current state of biomarkers for the diagnosis and assessment of treatment efficacy of prostate cancer. Discov Med. 2019;27(150):235-243
6. Maiorano BA, Schinzari G, Ciardiello D, Rodriquenz MG, Cisternino A, Tortora G, Maiello E. Cancer Vaccines for Genitourinary Tumors: Recent Progresses and Future Possibilities. Vaccines (Basel). 2021;9(6):623
7. Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520-6
8. Lerner RA, Green N, Alexander H. et al. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proceedings of the National Academy of Sciences. 1981;78:3403-3407
9. Arslan E, Koyuncu I. Comparison of amino acid metabolisms in mormal prostate (PNT-1A) and cancer cells (PC-3). Oncologie. 2021;23(1):105-117
10. Meloen RH, Langeveld JPM, Schaaper WMM. et al. Synthetic Peptide Vaccines: Unexpected Fulfillment of Discarded Hope? Biologicals. 2001;29:233-236
11. Bernhardt SL, Gjertsen MK, Trachsel S. et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. British Journal of Cancer. 2006;95:1474-1482
12. Noguchi M, Arai G, Egawa S, Ohyama C, Naito S, Matsumoto K, Uemura H, Nakagawa M, Nasu Y, Eto M, Suekane S, Sasada T, Shichijo S, Yamada A, Kakuma T, Itoh K. Mixed 20-peptide cancer vaccine in combination with docetaxel and dexamethasone for castration-resistant prostate cancer: a randomized phase II trial. Cancer Immunol Immunother. 2020;69(5):847-857
13. Yoshitomi M, Yutani S, Matsueda S. et al. Personalized peptide vaccination for advanced biliary tract cancer: IL-6, nutritional status and pre-existing antigen-specific immunity as possible biomarkers for patient prognosis. Exp Ther Med. 2012;3(3):463-469
14. Noguchi M, Uemura H, Naito S. et al. A phase I study of personalized peptide vaccination using 14 kinds of vaccine in combination with low-dose estramustine in HLA-A24-positive patients with castration-resistant prostate cancer. Prostate. 2011;71(5):470-479
15. Noguchi M, Kakuma T, Uemura H. et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother. 2010;59(7):1001-1009
16. Yoshimura K, Minami T, NOZAWA M. et al. A phase 2 randomized controlled trial of personalized peptide vaccine immunotherapy with low-dose dexamethasone versus dexamethasone alone in chemotherapy-naive castration-resistant prostate cancer. Eur Urol. 2016;70(1):35-41
17. Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239:62-84
18. Yan J, Tingey C, Lyde R, Gorham TC, Choo DK, Muthumani A, Myles D, Weiner LP, Kraynyak KA, Reuschel EL, Finkel TH, Kim JJ, Sardesai NY. et al. Novel and enhanced anti-melanoma DNA vaccine targeting the tyrosinase protein inhibits myeloid-derived suppressor cells and tumor growth in a syngeneic prophylactic and therapeutic murine model. Cancer Gene Ther. 2014;21:507-517
19. Bordoloi D, Xiao P, Choi H, Ho M, Perales-Puchalt A, Khoshnejad M, Kim JJ, Humeau L, Srinivasan A, Weiner DB, Muthumani K. Immunotherapy of prostate cancer using novel synthetic DNA vaccines targeting multiple tumor antigens. Genes Cancer. 2021;12:51-64
20. Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines. 2012;11:189-209
21. Santoro SP, Kim S, Motz GT, Alatzoglou D, Li C, Irving M, Powell DJ Jr, Coukos G (2015) T cells bearing a chimeric antigen receptor against prostate-specific membrane antigen mediate vascular disruption and result in tumor regression. Cancer Immunol Res 3(1):68-84
22. Muthumani K, Marnin L, Kudchodkar SB, Perales-Puchalt A, Choi H, Agarwal S, Scott VL, Reuschel EL, Zaidi FI, Duperret EK, Wise MC, Kraynyak KA, Ugen KE, Sardesai NY, Joseph Kim J, Weiner DB. Novel prostate cancer immunotherapy with a DNA-encoded anti-prostate-specific membrane antigen monoclonal antibody. Cancer Immunol Immunother. 2017;66(12):1577-1588
23. Overdijk MB, Verploegen S, Ortiz Buijsse A. et al. Crosstalk between human IgG isotypes and murine effector cells. J Immunol. 2012;189(7):3430-3438
24. Olson BM, Gamat M, Seliski J, Sawicki T, Jeffery J, Ellis L. et al. Prostate Cancer Cells Express More Androgen Receptor (AR) Following Androgen Deprivation, Improving Recognition by AR-Specific T Cells. Cancer immunology research. 2017;5:1074-1085
25. Olson BM, Johnson LE, McNeel DG. The androgen receptor: a biologically relevant vaccine target for the treatment of prostate cancer. Cancer Immunol Immunother. 2013;62:585-596
26. Olson BM, Bradley ES, Sawicki T, Zhong W, Ranheim EA, Bloom JE. et al. Safety and Immunological Efficacy of a DNA V accine Encoding the Androgen Receptor Ligand-Binding Domain (AR-LBD). The Prostate; 77(7): 812-821.
27. Pascolo S. Messenger RNA-based vaccines. Expert Opin Biol Ther. 2004;4:1285-94
28. Fotin-Mleczek M, Duchardt KM, Lorenz C, Pfeiffer R, Ojki?c-Zrna S, Probst J, Kallen KJ. Messenger RNAbased vaccines with dual activity induce balanced TLR7 dependent adaptive immune responses and provide antitumor activity. J Immunother. 2011;34:1-15
29. Kübler H, Scheel B, Gnad-Vogt U, Miller K, Schultze-Seemann W, Vom Dorp F, Parmiani G, Hampel C, Wedel S, Trojan L, Jocham D, Maurer T, Rippin G, Fotin-Mleczek M, von der Mülbe F, Probst J, Hoerr I, Kallen KJ, Lander T, Stenzl A. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J Immunother Cancer. 2015;3:26
30. Ferraro B, Cisper NJ, Talbott KT, Philipson-Weiner L, Lucke CE, Khan AS. et al. Co-delivery of PSA and PSMA DNA vaccines with electroporation induces potent immune responses. Hum Vaccin. 2011;7(Suppl):120-127
31. Rausch S, Schwentner C, Stenzl A, Bedke J. mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum Vaccin Immunother. 2014;10(11):3146-3152
32. Islam MA, Rice J, Reesor E, Zope H, Tao W, Lim M, Ding J, Chen Y, Aduluso D, Zetter BR, Farokhzad OC, Shi J. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials. 2021;266:120431
33. Zhan S, Li J, Xu R. et al. Armored long RNA controls or standards for branched DNA assay for detection of human immunodeficiency virus type 1. J Clin Microbiol. 2009;47:2571-2576
34. Caldeira JC, Peabody DS. Thermal stability of RNA phage virus-like particles displaying foreign peptides. J Nanobiotechnology. 2011;9:22
35. Li J, Sun Y, Jia T, Zhang R, Zhang K, Wang L. Messenger RNA vaccine based on recombinant MS2 virus-like particles against prostate cancer. Int J Cancer. 2014;134(7):1683-1694
36. Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Béthune M. et al. Replication-Deficient Human Adenovirus Type 35 V ectors for Gene Transfer and V accination: Efficient Human Cell Infection and Bypass of Preexisting Adenovirus Immunity. Journal of Virology. 2003; 77: 8263-8271.
37. Wei F, Wang H, Chen X, Li C, Huang Q. Dissecting the roles of E1a and E1B in adenoviral replication and RCAd-enhanced RDAd transduction efficacy on tumor cells. Cancer Biology Therapy. 2014; 15: 1358-1366.
38. Öberg D, Yanover E, Adam V, Sweeney K, Costas C, Lemoine NR. et al. Improved Potency and Selectivity of an Oncolytic E1ACR2 and E1B19K Deleted Adenoviral Mutant in Prostate and Pancreatic Cancers. Clinical Cancer Research. 2010; 16: 541-553.
39. Piya S, White EJ, Klein SR, Jiang H, McDonnell TJ, GomezManzano C. et al. The E1B19K oncoprotein complexes with Beclin 1 to regulate autophagy in adenovirus-infected cells. PLoS One. 2011; 6: e29467.
40. Zeng H, Wei Q, Huang R, Chen N, Dong Q, Yang Y. et al. Recombinant adenovirus mediated prostate-specific enzyme prodrug gene therapy regulated by prostate-specific membrane antigen (PSMA) enhancer/promoter. Journal of Andrology. 2007; 28: 827-835.
41. Lowe SL, Rubinchik S, Honda T, McDonnell TJ, Dong JY, Norris JS. Prostate-specific expression of Bax delivered by an adenoviral vector induces apoptosis in LNCaP prostate cancer cells. Gene Therapy. 2001; 8: 1363-1371.
42. Hao J, Xie W, Li H, Li R. Prostate Cancer-Specific of DD3driven Oncolytic Virus-harboring mK5 Gene. Open Medicine. 2019; 14: 1-9.
43. Kaneda Y. Update on non-viral delivery methods for cancer therapy: possibilities of a drug delivery system with anticancer activities beyond delivery as a new therapeutic tool. Expert Opin Drug Deliv. 2010;7:1079-1093
44. Fujita K, Nakai Y, Kawashima A, Ujike T, Nagahara A, Nakajima T, Inoue T, Lee CM, Uemura M, Miyagawa Y, Kaneda Y, Nonomura N. Phase I/II clinical trial to assess safety and efficacy of intratumoral and subcutaneous injection of HVJ-E in castration-resistant prostate cancer patients. Cancer Gene Ther. 2017;24(7):277-281
45. Fujita K, Kato T, Hatano K, Kawashima A, Ujike T, Uemura M, Imamura R, Okihara K, Ukimura O, Miki T, Nakajima T, Kaneda Y, Nonomura N. Intratumoral and s.c. injection of inactivated hemagglutinating virus of Japan envelope (GEN0101) in metastatic castration-resistant prostate cancer. Cancer Sci. 2020May;111(5):1692-1698
46. Singh P, Pal SK, Alex A, Agarwal N. Development of PROSTVAC immunotherapy in prostate cancer. Future Oncol. 2015;11(15):2137-2148
47. Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, Tsang KY, Panicali D, Schlom J, Kufe DW. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6(5):1632-1638
48. Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099-1105
49. El-Houseini M-E, Arafat M-S, El-Husseiny A-M, Kasem I-M, Kamel M-M, El-Habashy A-H. et al. Biological and molecular studies on specific immune cells treated with checkpoint inhibitors for the thera-personal approach of breast cancer patients (ex-vivo study). Oncol Res. 2021;29(5):319-329
50. Chang WL, Hsu LC, Leu WJ, Chen CS, Guh JH. Repurposing of nitroxoline as a potential anticancer agent against human prostate cancer: a crucial role on AMPK/mTOR signaling pathway and the interplay with Chk2 activation. Oncotarget. 2015;6:39806-39820
51. Varisli L, Tolan V, Cen Jh, Vlahopoulos S, Cen O. Dissecting the effects of androgen deprivation therapy on cadherin switching in advanced prostate cancer: A molecular perspective. Oncol Res. 2022;30(3):137-155
52. Marshall CH, Antonarakis ES. Emerging treatments for metastatic castration-resistant prostate cancer: Immunotherapy, PARP inhibitors, and PSMA-targeted approaches. Cancer Treat Res Commun. 2020 23,:100164
53. Zhao H, Ma W, Fragoso RC, Iv GRH, Ashok A, Li T. Durable clinical response to the multidisciplinary management of neurosurgery, radiation and chemoimmunotherapy in a patient with PD-L1/PD-L2/JAK2 (PDJ)-amplified, refractory triple-negative breast cancer. Journal of the National Cancer Center. 2021;1(3):115-121
54. Zhang T, Wang J, Wang D, Xu K, Wu L, Wang X, Wang W, Deng L, Liang J, Lv J, Hui Z, Zhou Z, Feng Q, Xiao Z, Chen D, Wang J, Wang L, Bi N. The time-series behavior of systemic inflammation-immune status in predicting survival of locally advanced non-small cell lung cancer treated with chemoradiotherapy. Journal of the National Cancer Center. 2022;2(1):33-40
55. McNeel DG, Eickhoff JC, Wargowski E, Zahm C, Staab MJ, Straus J, Liu G. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. OncoTargets Ther. 2018;9:25586
56. McNeel DG, Eickhoff JC, Wargowski E, Johnson LE, Kyriakopoulos CE, Emamekhoo H, Lang JM, Brennan MJ, Liu G. Phase 2 trial of T-cell activation using MVI-816 and pembrolizumab in patients with metastatic, castration-resistant prostate cancer (mCRPC). J Immunother Cancer. 2022;10:e004198
57. Rekoske BT, Olson BM, McNeel DG. Antitumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. Oncoimmunology. 2016;5(6):e1165377
58. Simons BW, Cannella F, Rowley DT, Viscidi RP. Bovine papillomavirus prostate cancer antigen virus-like particle vaccines are efficacious in advanced cancers in the TRAMP mouse spontaneous prostate cancer model. Cancer Immunol Immunother. 2020;69(4):641-651
59. Zhang X, Chen H, Li G, Zhou X, Shi Y, Zou F, Chen Y, Gao J, Yang S, Wu S, Long Z. Increased Tim-3 expression on TILs during treatment with the Anchored GM-CSF vaccine and anti-PD-1 antibodies is inversely correlated with response in prostate cancer. J Cancer. 2020;11(3):648-656
60. Fong L, Carroll P, Weinberg V, Chan S, Lewis J, Corman J, Amling CL, Stephenson RA, Simko J, Sheikh NA, Sims RB, Frohlich MW, Small EJ. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst. 2014;106(11):dju268
Author contact
![]() Corresponding author: Lijun Mao, E-mail: mljmlj05edu.cn
Corresponding author: Lijun Mao, E-mail: mljmlj05edu.cn

 Global reach, higher impact
Global reach, higher impact