Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(5):821-834. doi:10.7150/jca.82066 This issue Cite
Research Paper
AhR Promotes the Development of Non-small cell lung cancer by Inducing SLC7A11-dependent Antioxidant Function
1. Department of Thoracic Surgery, Hunan Key Laboratory of Early Diagnosis and Precision Therapy in Lung Cancer, Second Xiangya Hospital, Central South University, Changsha, Hunan,410011, China
2. NHC Key Laboratory of Carcinogenesis, Cancer Research Institute and School of Basic Medicine, Central South University, Changsha, Hunan, 410078, China
3. Department of dermatology, Second Xiangya Hospital, Central South University, Changsha,410011, China
4. Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan, 410008, China
5. Department of Neurosurgery, Xiangya Hospital, Central South University, Changsha, Hunan, 410008, China
6. Center for Tissue Engineering and Stem Cell Research, Guizhou Medical University, Guizhou, 550025, China
7. Department of Pathology, Zhuzhou Hospital Affiliated to Xiangya School of Medicine, Central South University, Zhuzhou, Hunan, 412007, China
8. Department of Pathology, School of Basic Medicine and Xiangya Hospital, Central South University, Changsha, Hunan, 410008, China
Received 2022-12-21; Accepted 2023-3-3; Published 2023-3-27
Abstract
Objective: Aryl hydrocarbon receptor (AhR) is a transcription factor. It is reported that AhR is associated with non-small cell lung cancer (NSCLC), but the mechanisms underlying this relationship remain unclear. Therefore, we investigated the role of AhR in NSCLC to elucidate the underlying mechanisms.
Methods: We collected clinical lung cancer samples and constructed AhR overexpression and knockdown cell lines to investigate the tumorigenicity of AhR in vivo and in vitro. Furthermore, we performed a ferroptosis induction experiment and chromatin immunoprecipitation experiment.
Results: AhR was highly expressed in NSCLC tissue. AhR knockdown cells showed ferroptosis related phenomenon. Furthermore, Chromatin immunoprecipitation confirmed the correlation between AhR and solute carrier family 7 member 11 (SLC7A11) and ferroptosis induction experiment confirmed that AhR affects ferroptosis via SLC7A11. Specifically, AhR regulates ferroptosis-related SLC7A11, which affects ferroptosis and promotes NSCLC progression.
Conclusions: AhR promoted NSCLC development and positively correlated with SLC7A11, affecting its actions. AhR bound to the promoter region of SLC7A11 promotes NSCLC by activating SLC7A11 expression, improving the oxidative sensitivity of cells, and inhibiting ferroptosis. Thus, AhR affects ferroptosis in NSCLC by regulating SLC7A11, providing foundational evidence for novel ferroptosis-related treatments.
Keywords: Receptors, aryl hydrocarbon, Ferroptosis, non-small-cell lung cancer, SLC7A11
Introduction
Globally and in China, lung cancer is one of the most common malignancies with the greatest fatalities. More than 80% of lung cancers are non-small cell lung cancer (NSCLC). Furthermore, air pollution is becoming increasingly worse as China enters the late stage of industrialization. Thus, the incidence of NSCLC in China will likely continue to increase over the next 30 years. Therefore, understanding the pathogenesis of and how to prevent NSCLC is crucial [1, 2].
Aryl hydrocarbon receptor (AhR) is a member of the transcription factor family that requires ligand activation, and its classic high-affinity ligand, tetrachlorodibenzo-p-dioxin, is a common industrial pollutant [3]. AhR is a multi-functional protein involved in growth, development, inflammation, tumors, the cell cycle, and cell proliferation, differentiation, and migration [4-6]. Currently, controversy exists about AhR's role in cancer and cancer cells. AhR directly and indirectly regulates the expression of various genes, such as those for vital functional proteins (e.g., interleukin-6 and -21 [cytokines], vascular endothelial growth factor, epiregulin, and amphiregulin [growth factors], cyclin D, and matrix metalloproteinase [a matrix protein]) and transcription factors (e.g., Slug) [7]. Therefore, the functions and mechanisms of AhR often vary among tissues, cells, and their molecular microenvironments [8-10].
The latest research shows that benzopyrene, found in cigarette smoke and air pollutants, can induce programmed death-ligand 1 expression in lung epithelial cells through AhR; thus, it escapes the body's adaptive immunity, promoting tumor occurrence [11]. Our previous study found that benzopyrene activates AhR nuclear transport, leading to the malignant transformation of NSCLC [9]. Others have also reported that AhR has an important role in tumor development [7, 12] and that inhibiting its signaling pathway with AhR antagonists significantly inhibited the malignant evolution and invasion of tumors [13-15]. Our previous research also found that AhR independently activates downstream target genes [16], AhR pathway activation has been associated with stem-like peculiarities and radiation resistance in tumor cells [17] and can be stabilized by de-ubiquitination [18]. Therefore, downstream molecules are recruited for various roles after exogenous factors activate AhR, which may affect the expression of ferroptosis-related genes, such as SLC7A11, and thus the ferroptosis process.
Cell death occurs at the end of a cell's life, with essential roles in the body's survival and development. Ferroptosis is a new form of cell death, differing from apoptosis, autophagy, and necrosis. This type of cell death primarily depends on iron metabolism and is characterized by the accumulation of reactive oxygen species (ROS) [19, 20]. Intracellular signaling pathways closely regulate ferroptosis, including the iron homeostasis regulatory, RAS, and cystine transport pathways [21]. In addition, cystine transport plays an essential role in regulating ferroptosis [22]; cystine replaces xc- intracellular glutamate via the cystine-glutamate antiporter system, and excessive lipid ROS causes ferroptosis [23]. Solute carrier family 7 member 11 (SLC7A11) is a key component of system xc-, and reports indicate that oxidative and metabolic stress can induce SLC7A11 expression [24, 25]. Furthermore, the activity and stability of SLC7A11 regulate the cells' antioxidant capacity [26] and then affect ferroptosis [27]. Thus, SLC7A11 is a key regulatory molecule for ferroptosis.
To date, the mechanisms underlying the associations among AhR, NSCLC, and ferroptosis remain unclear. Therefore, this study investigated the potential effects of AhR on ferroptosis and NSCLC to elucidate the underlying mechanisms.
Materials and Methods
Cell culture, plasmids, short hairpin RNAs (shRNAs), chemicals, and antibodies
We used PC9, SPC-A-1, and A549 cell lines and followed previously described maintenance methods [28, 29]. The primary antibodies were β-actin (Cat# A5441; Sigma-Aldrich, St. Louis, MO, USA; 1:10000), AhR (Cat# sc-133088; Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:1000), and SLC7A11 (Cat# 26864-1-AP; Proteintech, Wuhan, China; 1:1000).
We used previously published shRNA sequences [17]. Plasmid transfection was performed with Lipofectamine® 3000 following the manufacturer's instructions; colonies with stable expression were screened using puromycin (1.5 μg/mL). Vigene Biosciences designed and established the AhR overexpression plasmid (Vigene Biosciences, Shandong, China).
AhR agonist V, VAF347 was purchased from Calbiochem (Cat# 182690; Calbiochem/EMD Chemicals, San Diego, CA, USA), and Erastin (Cat# S7242) and Ferrostatin-1 (Cat# S7243) were purchased from Selleck Chemicals (Houston, TX, USA).
Cell proliferation, migration and invasion, and plate colony-formation assays
We followed the previously described procedures by SY et al. and JY et al. [30, 31].
Chromatin immunoprecipitation (ChIP) assays
The ChIP assays were performed as previously described by JY et al. [31]. ChIP DNA was detected using an ABI 7500 real-time polymerase chain reaction (PCR) instrument (Applied Biosystems, Foster City, CA, USA) using SYBR Green (Bio-Rad, Hercules, CA, USA). We used the anti-AhR antibody (Cat# sc-133088; Santa Cruz Biotechnology; 1:500).
Western blot analysis
We followed previously described instructions for the Western blot analysis [31]. We used mouse monoclonal anti-β-actin (Cat# A5441, Sigma-Aldrich, St. Louis, MO, USA; 1:10000), mouse monoclonal anti-AhR (Cat# sc-133088, Santa Cruz Biotechnology; 1:1000), and rabbit polyclonal anti-SLC7A11 (Cat# 26864-1-AP, Proteintech; 1:1000) for protein detection.
Total and lipid ROS and intracellular iron measurements
We followed previously described procedures for these measurements [23].
Flow cytometry
Cells were stained with antibodies or fluorescent dyes following standard protocols (D3861-BODIPY™ 581/591 C11; Thermo Fisher Scientific, Waltham, MA, USA) before being loaded for flow cytometry. Fluorescein-green and Texas-red were used to detect the labeled cells. The analysis was performed using FlowJo software (FloJo LLC., Ashland, OR, USA).
Reverse transcription-quantitative real-time PCR
We followed previously described procedures [23].
Histology and immunohistochemistry (IHC)
The Pathology Department of Xiangya Hospital in China provided the lung cancer samples. Details of the immunohistochemical analysis of paraffin sections of the lung cancer tissue have already been published [23]. Two pathologists at Xiangya Hospital in Changsha, China, performed the differential quantifications.
Nude mice experiment
Four-week-old female severe combined immune-deficiency mice were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Hunan, China). The Animal Protection and Use Agency Committee of Xiangya Medical College of Central South University approved all animal experiment protocols, which complied with the legal authorization and federal animal protection and conservation guidelines.
Each mouse was subcutaneously injected with AhR overexpression or knockdown cells (1 × 106 cells/animal). The control mice were injected with the same concentration of cells transfected with a control plasmid. The tumor volume and body weight were measured every three days until day 31.
Statistical analyses
The experiments were repeated at least three times. The results are presented as means ± standard deviations, as indicated. All statistical analyses were performed using Prism GraphPad Software (version 8.0, GraphPad Software, San Diego, CA, USA). Student's t-tests were used to compare two groups, and an analysis of variance was used to compare three or more groups. P-values of <0.05 were considered statistically significant.
Results
AhR is upregulated in NSCLC at the protein level
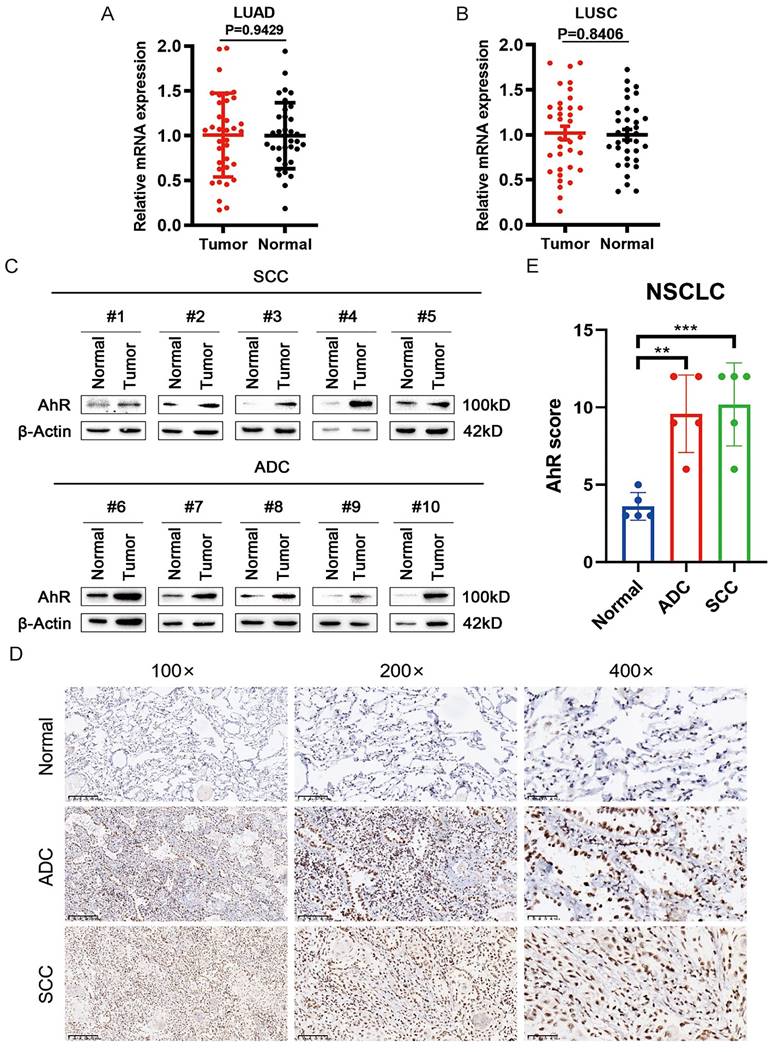
To clarify the role of AhR in NSCLC, we extracted mRNA from clinical non-small cell lung cancer samples for analysis, finding that the AhR mRNA level did not differ between lung adenocarcinoma (ADC; Fig. 1A) and squamous cell carcinoma (SCC; Fig. 1B) tissues and adjacent tissues. Subsequently, we evaluated AhR protein expression in ten NSCLC and adjacent normal tissue pairs by western blot (Fig. 1C), which showed upregulated AhR protein expression in both ADC and SCC tissues. This result suggested that AhR regulation occurred at the post-transcriptional level.
Simultaneously, we performed IHC analyses on NSCLC and adjacent normal tissue samples to confirm the AhR expression level. AhR expression was significantly higher in SCC and ADC tissues than in the normal adjacent tissue (Fig. 1D). Additionally, the AhR IHC scores in the SCC and ADC tissues were higher than those in adjacent normal tissues (Fig. 1E). These results suggested that AhR expression may increase in human NSCLC, and AhR may play a vital biological role.
AhR knockdown inhibits NSCLC progression in vitro and in vivo
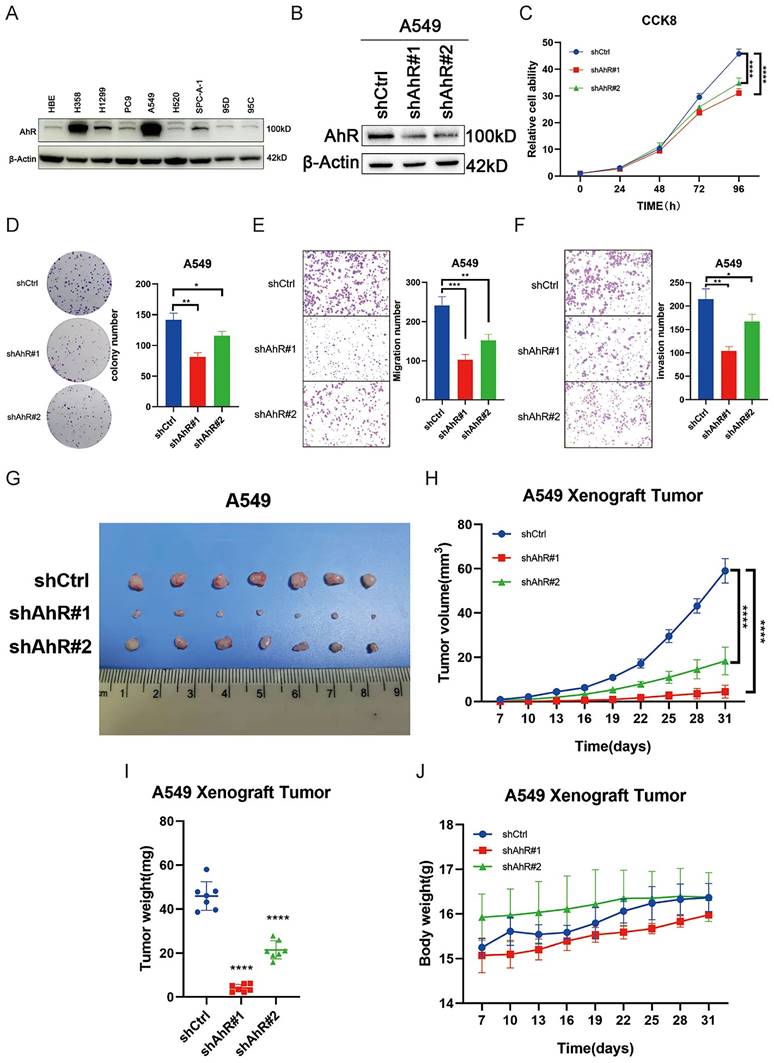
We measured AhR protein expression levels in different lung cancer cell lines (Fig. 2A). We found low AhR levels in the PC9 and SPC-A-1 cells and an increased level in the A549 cells.
Next, we stably knocked down AhR in the A549 cell line using four different AhR-targeted shRNAs; shAhR #1 and shAhR #2 had relatively high knockdown efficiencies (Fig. 2B). Therefore, all experiments were performed with these two shRNAs, unless otherwise noted. The AhR-knockdown cells grow more slowly than the control cells (Fig. 2C) and had significantly inhibited colony-forming abilities (Fig. 2D). Furthermore, the AhR-knockdown cells had lower invasion and migration capacities than the carrier cells (Fig. 2E, F).
AhR is upregulated at the protein level in non-small cell lung cancer (NSCLC). Clinical Samples analysis of aryl hydrocarbon receptor (AhR) mRNA expression in A) Lung adenocarcinoma (LUAD) (Normal, n = 35; Tumor, n = 35) and B) Lung squamous cell carcinoma (LUSC) (Normal, n = 35; Tumor, n = 35). C) AhR protein expression is higher in lung cancer (adenocarcinoma [ADC] and squamous cell carcinoma [SCC]) tissue than in paired adjacent normal lung tissue (n = 10; western blot). D) Immunohistochemistry (IHC) analyses showed increased AhR expression levels in lung cancer tissues (100× magnification, scale bar = 200 μm; 200× magnification, scale bar = 100 μm; 400× magnification, scale bar = 50 μm). E) IHC scores showing AhR expression levels in lung cancer versus normal tissues (Normal: n = 8; SCC: n = 10; ADC: n = 10). Data are presented as means ± standard deviations; **p <0.01, ***p <0.01.
Aryl hydrocarbon receptor (AhR) knockdown inhibits NSCLC progression in vitro and in vivo. A) AhR protein expression levels in lung cancer cell lines. B) AhR protein expression after AhR knockdown in A549 cells (western blot). C) Cell viability in A549 cells with stable AhR knockdown (n = 5; MTS assay). Data are shown as means ± standard deviations (SDs); ****p <0.0001. D) Colony formation abilities of A549 cells with stable AhR knockdown (n = 3; colony formation assay in plates). Data are presented as means ± SDs; **p <0.01. Representative images of E) AhR-knockdown A549 cell migration (n = 3) and F) AhR-knockdown A549 cell invasion (n = 3). G-J) A xenograft nude mouse model was established using AhR-knockdown A549 or control cells, and then the tumor volume (G, H), weight (I) and mouse body weight (J) were measured over time.
Finally, we injected A549 cells into nude mice to investigate tumor formation in vivo. AhR knockdown significantly reduced the tumor size, volume, and weight compared to the control mice (Fig. 2G-I), but body weight did not differ between the two groups (Fig. 2J). These results indicated that AhR promotes non-small cell lung cancer development and thus has carcinogenic properties.
AhR overexpression promotes NSCLC progression
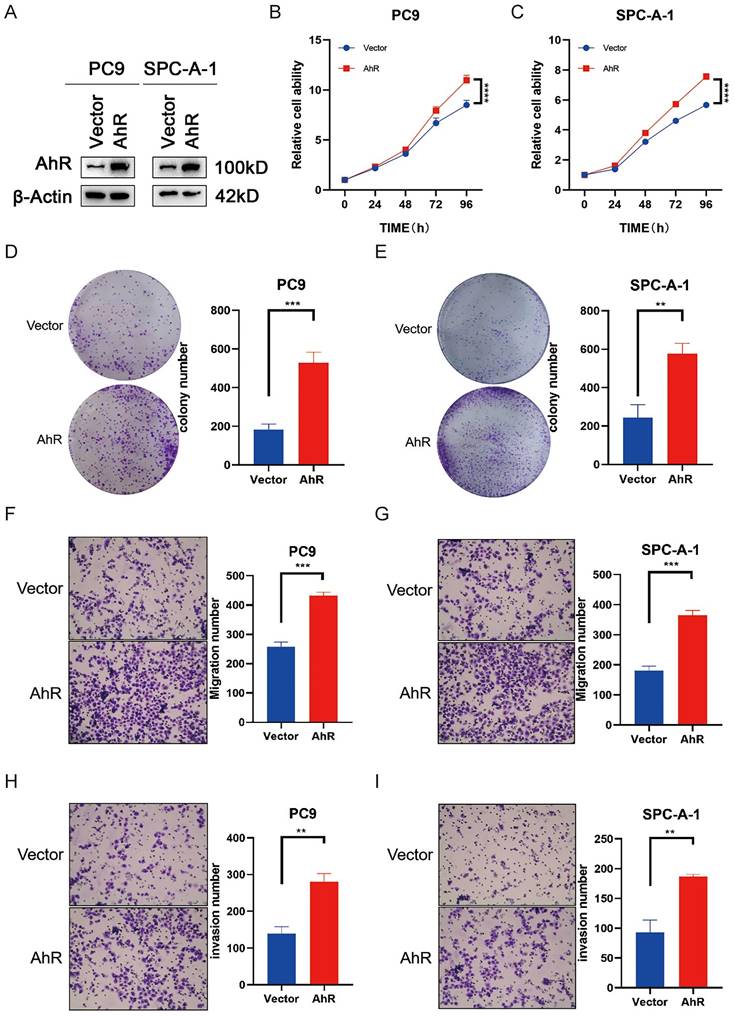
To clarify the physiological effects of AhR in NSCLC, we overexpressed AhR in PC9 and SPC-A-1 cells. Then, we assessed the overexpression efficiencies by western blot (Fig. 3A). AhR overexpression promoted PC9 and SPC-A-1 cell growth in vitro (Fig. 3B, C) and significantly improved their colony-forming abilities (Fig. 3D, E). In addition, the cell lines overexpressing AhR had stronger invasion and migration capabilities than the carrier cells (Fig. 3F-I). These results confirmed that AhR overexpression is related to cell growth, colony formation and cell migration and has carcinogenic influences.
AhR regulates ferroptosis through SLC7A11
When we knock down AhR, we found that there was a phenomenon related to ferroptosis in cells, for example, the total ROS level of A549 cells knocked down AhR would increas (Fig. 4A), suggesting that AhR may be related to ferroptosis.
Therefore, we performed a preliminarily ferroptosis-related gene expression analysis in lung ADC cells, finding Significant differential SLC7A11 expression after treating the cells with an AhR agonist and antagonist (Fig. 4B), suggesting that AhR may be related to SLC7A11.Furthermore, in order to explore the relationship between AhR and SLC7A11,we used the University of California Santa Cruz Genome Browser database and bioinformatics analyses showed that there might be AhR binding sites in the promoter region of SLC7A11 (Fig. 4C). We also identified a positive correlation between AhR and SLC7A11 (Fig. 4D). The Cancer Genome Atlas database analysis also showed higher SLC7A11 expression in ADC and SCC tissue than in adjacent tissues (Fig. 4E, F). Furthermore, high SLC7A11 expression was unfavorable for survival prognoses (Fig. 4G, I). Together, these results indicated that AhR might inhibit tumor cell ferroptosis by regulating SLC7A11 expression.
SLC7A11 positively correlates with AhR in NSCLC
Owing to previous analysis and experiments, we hypothesized a positive correlation between AhR and SLC7A11. To clarify this relationship, we detected the SLC7A11 protein level (by immunoblot) in lung cancer cell lines after overexpressing and knocking down AhR. The SLC7A11 protein level increased in the AhR overexpression cells (Fig. 5A) and decreased in the AhR knockdown cells (Fig. 5B). For further clarification, we transfected the AhR expression plasmid into the AhR knockdown A549 cell line, and then detected the AhR and SLC7A11 protein levels by western blot, which confirmed their positive relationship (Fig. 5C).
We also performed a ChIP analysis to detect AhR in the promoter region of SLC7A11. In PC9 cells, AhR was recruited into the SLC7A11 promoter. Furthermore, Ahr recruitment into the SLC7A11 promoter region was greater in PC9 cells overexpressing AhR than in the control cells (Fig. 5D).
AhR overexpression inhibits ferroptosis in NSCLC
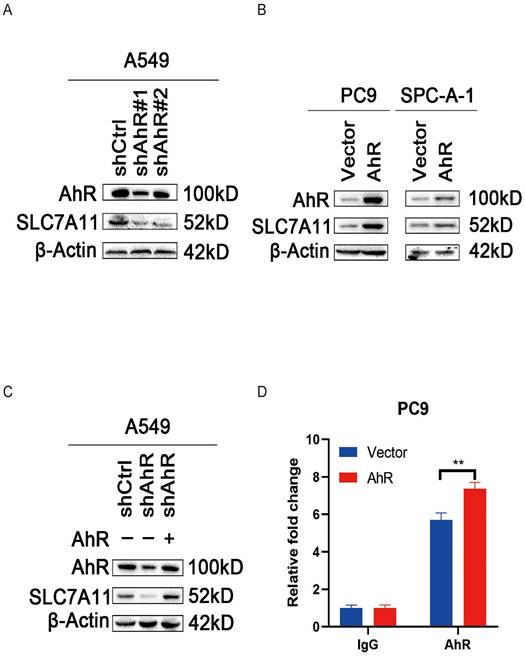
SLC7A11 is a key molecule regulating ferroptosis, and we found that AhR positively correlated with SLC7A11, prompting us to explore the role of AhR in ferroptosis. First, we treated PC9 and SPC-A-1 cell lines overexpressing AhR with Erastin, a SLC7A11 inhibitor, to evaluate cell growth inhibition, the drug concentration followed previously described instructions [28, 29]. Ahr overexpression decreased the growth inhibition of Erastin-treated PC9 and SPC-A-1 cell lines (Fig. 6A, B). Morphological observations of control cells (PC9 cells treated with DMSO) versus Erastin-treated PC9 and SPC-A-1 cells overexpressing AhR supported this result (Fig. 6C, D). These results suggested that AhR inhibits Erastin-induced cancer cell death.
Iron, glutathione (GSH), and lipid ROS concentrations in cells are surrogate markers of ferroptosis [21, 32]. We observed that PC9 and SPC-A-1 cells treated with Erastin had a higher concentration of iron than the control group, but AhR overexpression inhibited this increase (ferrous ions and total iron concentrations; Fig. 6E-H). Furthermore, Erastin treatment decreased the total GSH level, but AhR overexpression inhibited this reduction (Fig. 6I, J). Finally, Erastin treatment increased the lipid ROS level, but AhR overexpression inhibited this increase (Fig. 6K, L). These findings indicated that AhR overexpression inhibits Erastin-induced cancer cell ferroptosis.
AhR knockdown promotes ferroptosis in NSCLC
To further clarify the role of AhR in ferroptosis in NSCLC, we tested the effects of Erastin on growth inhibition of AhR-knockdown A549 cells treated with Erastin. Knocking down AhR enhanced the Erastin-induced inhibitory effect on A549 cell growth (Fig. 7A); the morphological observation aligned with this finding (Fig. 7B).
Aryl hydrocarbon receptor (AhR) overexpression promotes NSCLC progression. A) AhR protein levels in PC9 and SPC-A-1 cells overexpressing AhR (western blot). B, C) Cell viability in PC9 and SPC-A-1 cells with stably overexpressed AhR (n = 5; MTS assay). Data are presented as means ± standard deviations (SDs); ****p <0.0001. D, E) Colony formation abilities of PC9 and SPC-A-1 cells with stably overexpressed AhR (n = 3; colony formation assay in plates). Data are presented as means ± SDs; **p <0.01. Representative image of F, G) AhR-overexpressing PC9 and SPC-A-1 cell migration (n = 3) and H, I) AhR-overexpressing PC9 and SPC-A-1 cell invasion (n = 3).
Aryl hydrocarbon receptor (AhR) regulates ferroptosis via solute carrier family 7 member 11 (SLC7A11). A) The total ROS level was higher in AhR-knockdown A549 cells than in A549 cells without knockdown. B) AhR binding site predictions in the SLC7A11 promoter region using a University of California Santa Cruz Genome Browser database analysis and Algorithmics and Genetics Group (i.e., ALGGEN) website. C) Bioinformatics analysis identified a positive correlation between AhR and SLC7A11. D) SLC7A11 expression in A549 cells was significantly upregulated after treatment with an AhR agonist; expression did not change after treatment with an AhR agonist. E, F) The Cancer Genome Atlas database analysis showed that SLC7A11 expression in LUAD and LUSC is higher than that in adjacent tissues. G, I) High SLC7A11 expression is unfavorable for survival and prognosis.
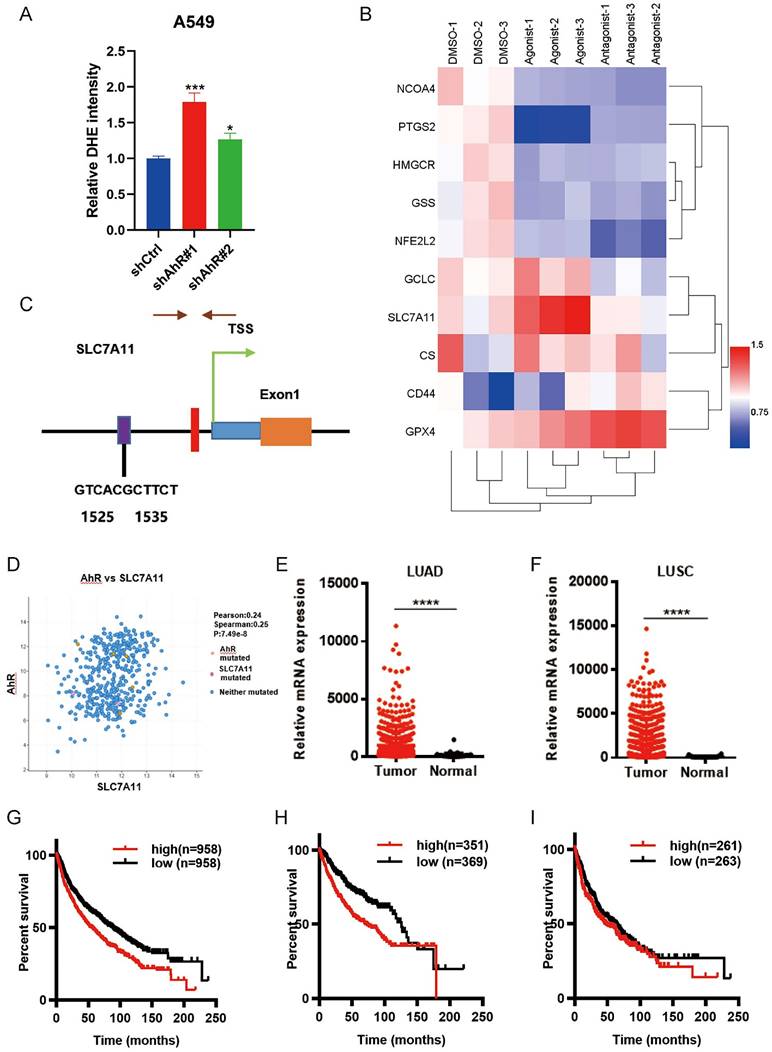
Solute carrier family 7 member 11 (SLC7A11) positively correlates with aryl hydrocarbon receptor (AhR) in non-small cell lung cancer (NSCLC). A) AhR and SLC7A11 protein levels in AhR-knockdown A549 cells (western blot). B) AhR and SLC7A11 protein levels in AhR-overexpressing PC9 and SPC-A-1 cells (western blot). C) AhR and SLC7A11 in AhR-knockdown A549 cells transfected into an AhR-overexpression plasmid (western blot). D) AhR in the promoter region of SLC7A11 using two primer sites (chromatin immunoprecipitation assay).
We also measured the total iron and ferrous concentrations in A549 cells after knocking down AhR, which increased compared to cells without AhR knockdown (Fig. 7C, D). Furthermore, the total GSH level was obviously lower in AhR knockdown cells than in those without knockdown (Fig. 7E). Finally, the lipid ROS level of A549 cells after AhR knockdown significantly increased after Erastin treatment compared to the DMSO group (Fig. 7F). Therefore, AhR knockdown promotes ferroptosis of Erastin-induced cancer cells.
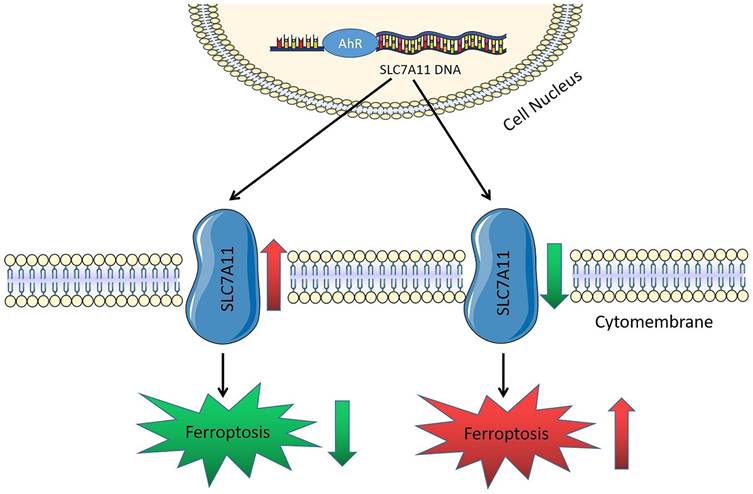
Overall, AhR may regulate iron, GSH, and lipid ROS levels in lung cancer cells by promoting SLC7A11 transcription, thereby inhibiting lung cancer cell ferroptosis and promoting non-small cell lung cancer development (Fig. 8).
Discussions
This study provides new evidence that AhR plays an important role in non-small cell lung cancer development. For the first time, we demonstrate that AhR, a ferroptosis inhibitor, influences carcinogenesis by promoting SLC7A11, an antioxidant system-related gene. Ferroptosis is a type of programmed cell death and a promising new target for treating tumors. Studies have reported strong relationships between ferroptosis and tumor progression [32-36], for example, ferroptosis-mediated radiation therapy for tumors [32] and tumor killing by immune cells [37]. Additionally, reports indicate that ferroptosis-related genes have vital roles in pancreatic cancer [38-40], suggesting that ferroptosis may be a novel way to inhibit cancer development.
Aryl hydrocarbon receptor (AhR) overexpression inhibits NSCLC cell ferroptosis. A, B) PC9 (A) and SPC-A-1 (B) cell responses to Erastin (5 μM) ± Ferrostatin (1 μM) with or without AhR overexpression. C, D) A representative image showing PC9 (A) and SPC-A-1 (B) cell responses to Erastin (5 μM) ± Ferrostatin (1 μM) with or without AhR overexpression. E, F) Total iron (E) and ferrous iron (F) levels in PC9 cells with or without AhR overexpression. G, H) Total iron (G) and ferrous iron (H) levels in SPC-A-1 cells with or without AhR overexpression. I, J) The total relative glutathione (GSH) levels in PC9 (I) and SPC-A-1 (J) cells with or without AhR overexpression. K, L) The lipid reactive oxygen species (ROS) level was measured by C11-BODIPY staining coupled to flow cytometry in Erastin-treated PC9 (K) and SPC-A-1 (L) cells after AhR overexpression. *p <0.05, **p <0.01, ***p <0.001.
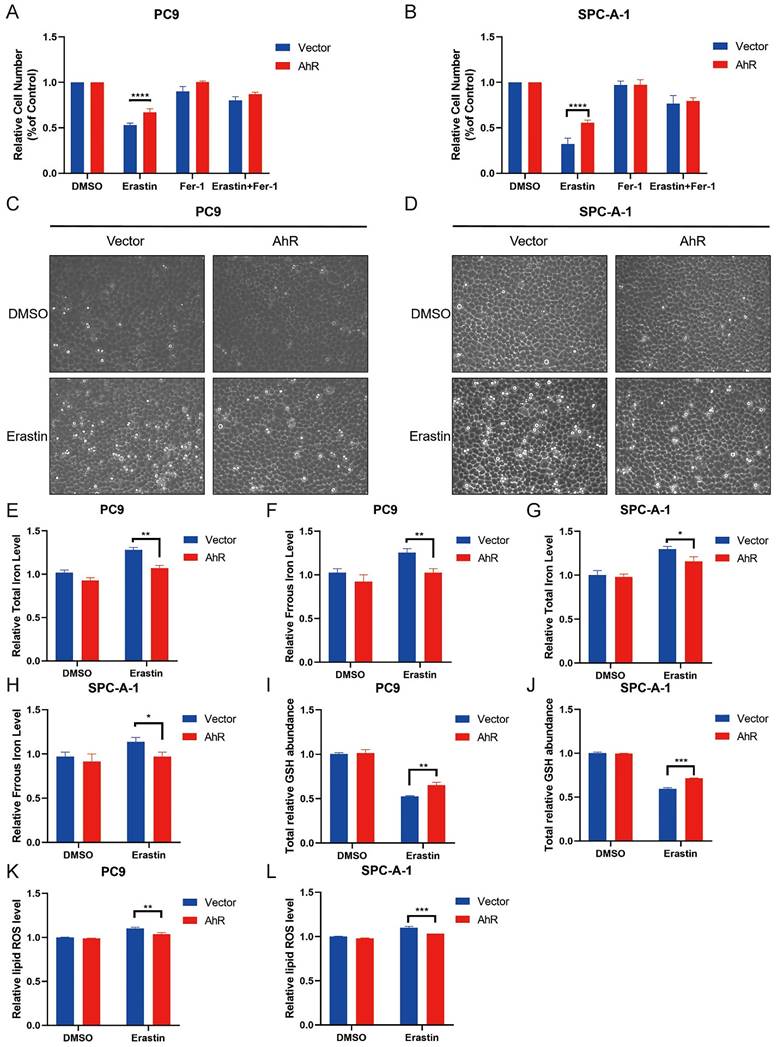
Aryl hydrocarbon receptor (AhR) knockdown promotes NSCLC cell ferroptosis. A) A549 cell responses to Erastin (5 μM) ± Ferrostatin (1 μM) with or without AhR knockdown. B) A representative image showing A549 cell responses to Erastin (5μM) ± Ferrostatin (1 μM) with or without AhR knockdown. C) The total iron levels in A549 cells with or without AhR knockdown. D) The ferrous iron levels in A549 cells with or without AhR knockdown. E) The total relative glutathione (GSH) levels in A549 cells with or without AhR knockdown. F) The lipid reactive oxygen species (ROS) level was measured by C11-BODIPY staining coupled to flow cytometry in Erastin-treated A549 cells after AhR knockdown. *p <0.05, **p <0.01, ***p <0.001.
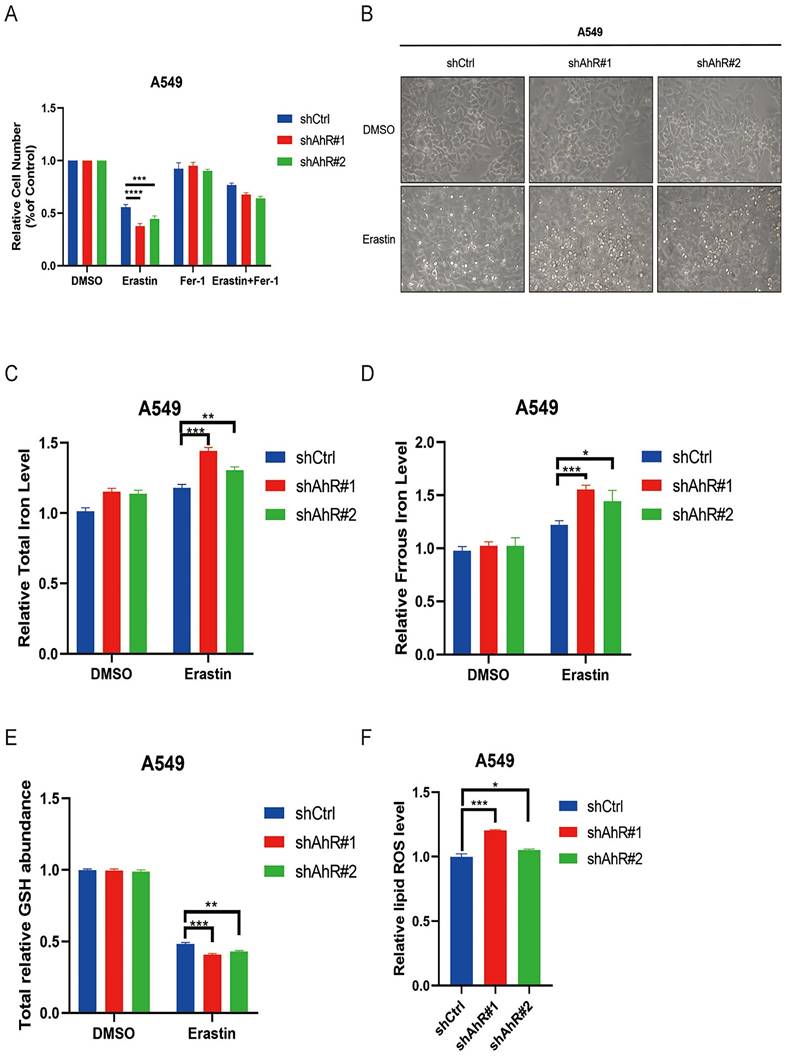
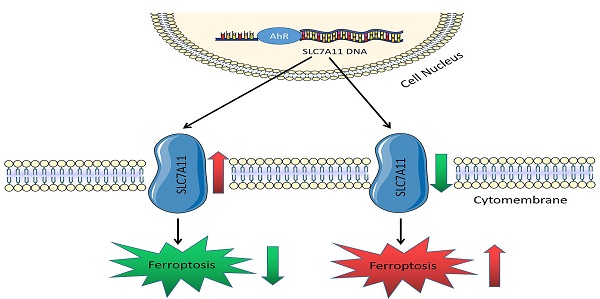
Schematic working model of solute carrier family 7 member 11 (SLC7A11) regulation by aryl hydrocarbon receptor (AhR). AhR is a transcription factor. The SLC7A11 promoter region increases its expression by recruiting AhR, inhibiting ferroptosis, and promoting cancer development.
Transcription factor AhR is a protein with several functions [41-43], including cell growth, development, proliferation, and tumor differentiation [7, 44]. Its functions and mechanisms often vary based on tissue and cell type and their molecular microenvironment [45-49]. AhR is highly expressed in NSCLC, but there is no change in RNA level, the previous research results of our group have confirmed that this is based on the effect of de-ubiquitination [18]. Therefore, AhR is important for tumorigenesis, but how it affects tumor progression remains unclear. Elucidating these mechanisms will allow the identification of new targets for treating AhR-positive cancer. Similarly, AhR expression varies among NSCLCs, and the mechanism underlying the abnormal AhR expression remains unclear. Additionally, the results are inconsistent, with several reports indicating AhR downregulation in lung cancer [50], but others reported AhR overexpressed [51, 52], consistent with our findings. Furthermore, some reports indicating AhR overexpression promoted NSCLC development.
Ferroptosis is characterized by an overwhelming, iron-dependent accumulation of lethal lipid ROS [53-55]. We demonstrated that AhR overexpression decreased the lipid ROS and iron concentrations and increased the total GSH concentration in human lung ADC cell lines, supporting AhR inhibition in ferroptosis. Ferroptosis is also characterized by an increase in lipid peroxide, which may be caused by compound-mediated inhibition of GSH peroxidase 4 (GPX4) or indirectly targeting GPX4 through GSH consumption. Additionally, system xc- is an important antioxidant system in cells, in which SLC7A11 is responsible for the main transport activity with high specificity for cystine and glutamate [22, 55]. System xc- intracellular glutamate is used to exchange extracellular cystine [24, 25], and then cystine is synthesized into GSH, catalyzed by glutamate-cysteine ligase and GSH synthetase. GSH is a reducing cofactor of the membrane lipid repair enzyme GPX4. Thus, increasing the activity of SLC7A11 increases the absorption of cystine [56, 57], which affects GSH synthesis, increasing GPX4 activity [58, 59]. Consequently, the antioxidant capacity of the cells is enhanced, inhibiting ferroptosis. Erastin induces ferroptosis, inducing ferroptosis by inhibiting the cysteine/glutamate reverse transporter system [60]. Erastin also inhibits SLC7A11, providing a reasonable way to study the role of AhR in ferroptosis in NSCLC. In this study, Erastin was mainly used to inhibit SLC7A11 and induce ferroptosis.In addition, the limitation of this experiment is that a complete pathway has not been found.
In conclusion, we demonstrated that AhR inhibits iron-dependent cell death (i.e., ferroptosis) in human non-small cell lung cancer cells by binding to the promoter region of SLC7A11, a transcription factor. Thus, it promotes SLC7A11 transcription, influencing tumor cell ferroptosis. Specifically, AhR upregulated SLC7A11 expression. Moreover, high SLC7A11 levels increased cystine absorption, promoted GSH synthesis, increased GPX4 activity, enhanced the antioxidant capacity of lung cancer cells, inhibited lung cancer cell ferroptosis, and promoted cancer development. These findings highlight the indispensable role of AhR in tumor development via ferroptosis regulation, which may be a new treatment strategy for NSCLC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [81972638, W.L.] and the Central South University Research Programme of Advanced Interdisciplinary Studies [2023QYJC030].
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians. 2016;66:115-32
2. Zeng H, Zheng R, Guo Y, Zhang S, Zou X, Wang N. et al. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer. 2015;136:1921-30
3. Singleman C, Holtzman NG. PCB and TCDD derived embryonic cardiac defects result from a novel AhR pathway. Aquatic toxicology (Amsterdam, Netherlands). 2021;233:105794
4. Masaki T, Okazawa M, Asano R, Inagaki T, Ishibashi T, Yamagishi A. et al. Aryl hydrocarbon receptor is essential for the pathogenesis of pulmonary arterial hypertension. Proceedings of the National Academy of Sciences of the United States of America. 2021 118
5. Fernández-Gallego N, Sánchez-Madrid F, Cibrian D. Role of AHR Ligands in Skin Homeostasis and Cutaneous Inflammation. Cells. 2021 10
6. Walczak K, Langner E, Makuch-Kocka A, Szelest M, Szalast K, Marciniak S. et al. Effect of Tryptophan-Derived AhR Ligands, Kynurenine, Kynurenic Acid and FICZ, on Proliferation, Cell Cycle Regulation and Cell Death of Melanoma Cells-In Vitro Studies. Int J Mol Sci. 2020 21
7. Shinde R, McGaha TL. The Aryl Hydrocarbon Receptor: Connecting Immunity to the Microenvironment. Trends in immunology. 2018;39:1005-20
8. Cheng J, Li W, Kang B, Zhou Y, Song J, Dan S. et al. Tryptophan derivatives regulate the transcription of Oct4 in stem-like cancer cells. Nat Commun. 2015;6:7209
9. Mao C, Wang M, Qian B, Ouyang L, Shi Y, Liu N. et al. Aryl hydrocarbon receptor activated by benzo (a) pyrene promotes SMARCA6 expression in NSCLC. American journal of cancer research. 2018;8:1214-27
10. Che X, Dai W. Aryl Hydrocarbon Receptor: Its Regulation and Roles in Transformation and Tumorigenesis. Current drug targets. 2019;20:625-34
11. Wang GZ, Zhang L, Zhao XC, Gao SH, Qu LW, Yu H. et al. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat Commun. 2019;10:1125
12. Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9:3294
13. Narasimhan S, Stanford Zulick E, Novikov O, Parks AJ, Schlezinger JJ, Wang Z. et al. Towards Resolving the Pro- and Anti-Tumor Effects of the Aryl Hydrocarbon Receptor. Int J Mol Sci. 2018 19
14. Scoville SD, Nalin AP, Chen L, Chen L, Zhang MH, McConnell K. et al. Human AML activates the aryl hydrocarbon receptor pathway to impair NK cell development and function. Blood. 2018;132:1792-804
15. Cheong JE, Sun L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy - Challenges and Opportunities. Trends in pharmacological sciences. 2018;39:307-25
16. Liu S, Piatigorsky J. Regulation of mouse small heat shock protein αb-crystallin gene by aryl hydrocarbon receptor. PloS one. 2011;6:e17904
17. Yan B, Liu S, Shi Y, Liu N, Chen L, Wang X. et al. Activation of AhR with nuclear IKKα regulates cancer stem-like properties in the occurrence of radioresistance. Cell Death Dis. 2018;9:490
18. Ouyang L, Yan B, Liu Y, Mao C, Wang M, Liu N. et al. The deubiquitylase UCHL3 maintains cancer stem-like properties by stabilizing the aryl hydrocarbon receptor. Signal Transduct Target Ther. 2020;5:78
19. Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox biology. 2019;23:101107
20. Stoyanovsky DA, Tyurina YY, Shrivastava I, Bahar I, Tyurin VA, Protchenko O. et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free radical biology & medicine. 2019;133:153-61
21. Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ. et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273-85
22. Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell research. 2016;26:1021-32
23. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-72
24. Koppula P, Zhang Y, Shi J, Li W, Gan B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. The Journal of biological chemistry. 2017;292:14240-9
25. Chen D, Fan Z, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 2017;36:5593-608
26. Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57-62
27. Liu T, Jiang L, Tavana O, Gu W. The Deubiquitylase OTUB1 Mediates Ferroptosis via Stabilization of SLC7A11. Cancer Res. 2019;79:1913-24
28. Jiang Y, Mao C, Yang R, Yan B, Shi Y, Liu X. et al. EGLN1/c-Myc Induced Lymphoid-Specific Helicase Inhibits Ferroptosis through Lipid Metabolic Gene Expression Changes. Theranostics. 2017;7:3293-305
29. Mao C, Wang X, Liu Y, Wang M, Yan B, Jiang Y. et al. A G3BP1-Interacting lncRNA Promotes Ferroptosis and Apoptosis in Cancer via Nuclear Sequestration of p53. Cancer Res. 2018;78:3484-96
30. Shi Y, Tao Y, Jiang Y, Xu Y, Yan B, Chen X. et al. Nuclear epidermal growth factor receptor interacts with transcriptional intermediary factor 2 to activate cyclin D1 gene expression triggered by the oncoprotein latent membrane protein 1. Carcinogenesis. 2012;33:1468-78
31. Jiang Y, Yan B, Lai W, Shi Y, Xiao D, Jia J. et al. Repression of Hox genes by LMP1 in nasopharyngeal carcinoma and modulation of glycolytic pathway genes by HoxC8. Oncogene. 2015;34:6079-91
32. Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH. et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell research. 2020;30:146-62
33. Liu J, Zhang C, Wang J, Hu W, Feng Z. The Regulation of Ferroptosis by Tumor Suppressor p53 and its Pathway. Int J Mol Sci. 2020 21
34. Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z. et al. HCAR1/MCT1 Regulates Tumor Ferroptosis through the Lactate-Mediated AMPK-SCD1 Activity and Its Therapeutic Implications. Cell Rep. 2020;33:108487
35. Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science (New York, NY). 2020;368:85-9
36. Dierge E, Debock E, Guilbaud C, Corbet C, Mignolet E, Mignard L. et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell metabolism. 2021;33:1701-15.e5
37. Liao P, Wang W, Wang W, Kryczek I, Li X, Bian Y. et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer cell. 2022;40:365-78.e6
38. Ye Z, Zhuo Q, Hu Q, Xu X, Mengqi L, Zhang Z. et al. FBW7-NRA41-SCD1 axis synchronously regulates apoptosis and ferroptosis in pancreatic cancer cells. Redox biology. 2021;38:101807
39. Kremer DM, Nelson BS, Lin L, Yarosz EL, Halbrook CJ, Kerk SA. et al. GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat Commun. 2021;12:4860
40. Sui X, Zhang R, Liu S, Duan T, Zhai L, Zhang M. et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Frontiers in pharmacology. 2018;9:1371
41. Li YY, Wang XJ, Su YL, Wang Q, Huang SW, Pan ZF. et al. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta pharmacologica Sinica. 2022;43:1495-507
42. Wang L, Cheng B, Ju Q, Sun BK. AhR Regulates Peptidoglycan-Induced Inflammatory Gene Expression in Human Keratinocytes. Journal of innate immunity. 2022;14:124-34
43. da Silva JF, Bolsoni JA, da Costa RM, Alves JV, Bressan AFM, Silva LEV. et al. Aryl hydrocarbon receptor (AhR) activation contributes to high-fat diet-induced vascular dysfunction. British journal of pharmacology. 2022;179:2938-52
44. Nebert DW. Aryl hydrocarbon receptor (AHR): "pioneer member" of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of "sensors" of foreign and endogenous signals. Progress in lipid research. 2017;67:38-57
45. Stockinger B, Shah K, Wincent E. AHR in the intestinal microenvironment: safeguarding barrier function. Nature reviews Gastroenterology & hepatology. 2021;18:559-70
46. Qian C, Yang C, Lu M, Bao J, Shen H, Deng B. et al. Activating AhR alleviates cognitive deficits of Alzheimer's disease model mice by upregulating endogenous Aβ catabolic enzyme Neprilysin. Theranostics. 2021;11:8797-812
47. Toydemir G, Loonen LMP, Venkatasubramanian PB, Mes JJ, Wells JM, De Wit N. Coffee induces AHR- and Nrf2-mediated transcription in intestinal epithelial cells. Food chemistry. 2021;341:128261
48. Sa R, Guo M, Liu D, Guan F. AhR Antagonist Promotes Differentiation of Papillary Thyroid Cancer via Regulating circSH2B3/miR-4640-5P/IGF2BP2 Axis. Frontiers in pharmacology. 2021;12:795386
49. Dong H, Hao L, Zhang W, Zhong W, Guo W, Yue R. et al. Activation of AhR-NQO1 Signaling Pathway Protects Against Alcohol-Induced Liver Injury by Improving Redox Balance. Cellular and molecular gastroenterology and hepatology. 2021;12:793-811
50. Chen Y, Widschwendter M, Teschendorff AE. Systems-epigenomics inference of transcription factor activity implicates aryl-hydrocarbon-receptor inactivation as a key event in lung cancer development. Genome biology. 2017;18:236
51. Chang JT, Chang H, Chen PH, Lin SL, Lin P. Requirement of aryl hydrocarbon receptor overexpression for CYP1B1 up-regulation and cell growth in human lung adenocarcinomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:38-45
52. Ye M, Zhang Y, Gao H, Xu Y, Jing P, Wu J. et al. Activation of the Aryl Hydrocarbon Receptor Leads to Resistance to EGFR TKIs in Non-Small Cell Lung Cancer by Activating Src-mediated Bypass Signaling. Clinical cancer research: an official journal of the American Association for Cancer Research. 2018;24:1227-39
53. Chopra VS, Cande J, Hong JW, Levine M. Stalled Hox promoters as chromosomal boundaries. Genes Dev. 2009;23:1505-9
54. Chopra VS, Hong JW, Levine M. Regulation of Hox gene activity by transcriptional elongation in Drosophila. Current biology: CB. 2009;19:688-93
55. Chen X, Yu C, Kang R, Kroemer G, Tang D. Cellular degradation systems in ferroptosis. Cell death and differentiation. 2021;28:1135-48
56. Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free radical biology & medicine. 2020;152:175-85
57. Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054-81
58. Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free radical biology & medicine. 2019;133:144-52
59. Ding Y, Chen X, Liu C, Ge W, Wang Q, Hao X. et al. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. Journal of hematology & oncology. 2021;14:19
60. Skalska L, White RE, Franz M, Ruhmann M, Allday MJ. Epigenetic repression of p16(INK4A) by latent Epstein-Barr virus requires the interaction of EBNA3A and EBNA3C with CtBP. PLoS pathogens. 2010;6:e1000951
Author contact
![]() Corresponding authors: liuwenliangedu.cn; taoyongedu.cn
Corresponding authors: liuwenliangedu.cn; taoyongedu.cn

 Global reach, higher impact
Global reach, higher impact