3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(4):505-518. doi:10.7150/jca.76591 This issue Cite
Research Paper
Network Pharmacology Combined with Experimental Validation Reveals the Anti-tumor Effect of Duchesnea indica against Hepatocellular Carcinoma
1. Department of Central Laboratory Medicine, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 200071, China
2. Shanghai Tenth People's Hospital, Tongji University, School of Medicine, Shanghai, 200072, China
3. Department of Hospital-Acquired Infection Control, Shanghai municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 200071, China
*These authors contributed equally to this article
Abstract
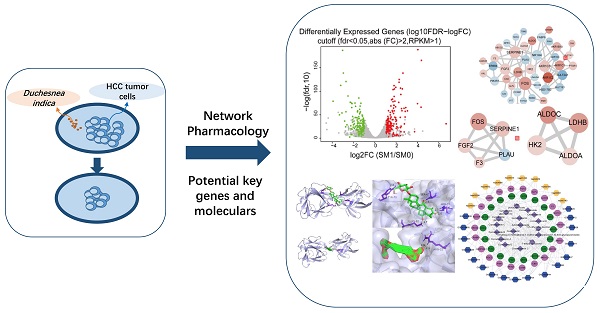
Context: Duchesnea indica is effective against hepatocellular carcinoma (HCC); however, its underlying mechanism of action remains unclear.
Objective: The present study aimed to investigate the potential mechanism of action and effects of D. indica components against HCC.
Materials and methods: First, the effects of D. indica against HCC were investigated in vitro and in vivo. For in vitro experiments, HCC cell lines were treated with D. indica solutions at different concentrations (0, 1, 2 mg/mL) and then assessed for cell apoptosis, proliferation, migration, invasion, and angiogenic ability. For in vivo experiments, 24 mice were randomly divided into the following four groups: model group and D. indica low-, medium-, and high-dose groups. Tumor growth and CD34 and Ki67 expression levels were assessed to determine the effects of D. indica on cell proliferation and angiogenic ability. Furthermore, transcriptome sequencing and differential expression analyses were used to identify D. indica-induced differentially expressed genes (DEGs) in HCC cells. Additionally, mass spectrometry was conducted to identify the chemical components of D. indica. Four databases were used to predict the target proteins of these chemical components in HCC. HCC-associated genes were identified from two databases. By intersecting the identified DEGs; target proteins; and HCC-associated genes, key D. indica-regulated HCC-related genes were identified. Subsequently, protein-protein interaction network, network pharmacology, and molecular docking were used to identify the active compounds in D. indica and their likely gene targets.
Results: In vitro experiments demonstrated that D. indica induced tumor cell apoptosis and inhibited cell proliferation, migration, invasion, and angiogenic potential. In vivo experiments demonstrated that D. indica inhibited tumor growth in a dose-dependent manner. Bioinformatic analyses identified 49 key D. indica-regulated HCC-related genes, of which FOS, SERPINE1, AKR1C3, and FGF2 were the most significant. Mass spectrometry identified the following five molecules in D. indica with potential anti-HCC activity: 4′, 5, 7-trihydroxyflavone; ethyl protocatechuate; 3, 5-dihydroxy-benzoic acid; curculigosaponin A; and curculigine G. Molecular docking validated the interaction between D. indica active compounds and their target proteins in HCC.
Conclusions: The present study confirmed the therapeutic effects of D. indica against HCC and identified the key genes and active components that may contribute to its mechanism of action, thereby providing a basis for further research on targeted therapeutics for HCC.
Keywords: Duchesnea indica, hepatocellular carcinoma, metabolism, tumorigenesis, angiogenesis

 Global reach, higher impact
Global reach, higher impact