3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(3):434-445. doi:10.7150/jca.81588 This issue Cite
Research Paper
Neoadjuvant Chemotherapy Compared with Surgery for Oesophageal Carcinoma: A Retrospective Study and Missing Evidence
1. Department of Thoracic Surgery, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, Henan 450008, P. R. China
2. Department of Statistics, LinkDoc Technology Co., Ltd., Beijing, China
Abstract
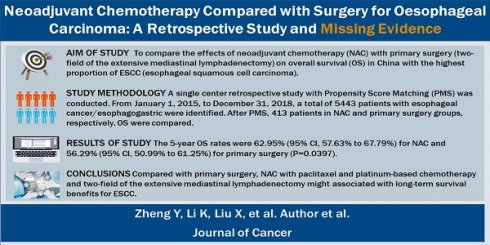
Background: Negative evidence for the use of neoadjuvant chemotherapy (NAC) to treat oesophageal squamous cell carcinoma (ESCC) has been reported in Western countries in the past century. However, in China, most ESCC patients underwent paclitaxel and platinum-based NAC without evidence from local RCTs. Empiricism or a lack of evidence does not necessarily mean that the evidence is negative. However, there was no way to compensate for the missing evidence. The only way to obtain evidence is by conducting a retrospective study using propensity score matching (PSM) to compare the effects of NAC and primary surgery on overall survival (OS) and disease-free survival (DFS) among ESCC patients in China, which is the country with the highest prevalence of ESCC patients.
Methods: From January 1, 2015, to December 31, 2018, a total of 5443 patients with oesophageal cancer/oesophagogastric junction carcinoma who underwent oesophagectomy were retrospectively identified at Henan Cancer Hospital. After PSM, 826 patients were selected for the retrospective study and divided into the NAC and primary surgery groups. The median follow-up period was 54.08 months. Toxicity and tumour responses to NAC, intraoperative and postoperative outcomes, recurrence, DFS and OS were analysed.
Results: The postoperative complication rates were not significantly different between the two groups. The 5-year DFS rates were 57.48% (95% CI, 52.05% to 62.53%) for the NAC group and 49.93% (95% CI, 44.56% to 55.05%) for the primary surgery group (P=0.0129). The 5-year OS rates were 62.95% (95% CI, 57.63% to 67.79%) for the NAC group and 56.29% (95% CI, 50.99% to 61.25%) for the primary surgery group (P=0.0397).
Conclusion: Compared with primary surgery, NAC with paclitaxel and platinum-based chemotherapy and two-field extensive mediastinal lymphadenectomy might be associated with long-term survival benefits among ESCC patients.
Keywords: oesophageal cancer, surgery, lymphadenectomy, neoadjuvant chemotherapy

 Global reach, higher impact
Global reach, higher impact