3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(13):3415-3426. doi:10.7150/jca.63514 This issue Cite
Research Paper
Silibinin inhibits the migration, invasion and epithelial-mesenchymal transition of prostate cancer by activating the autophagic degradation of YAP
Department of Urology, the First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, Shaanxi 710061, P.R. China.
Abstract
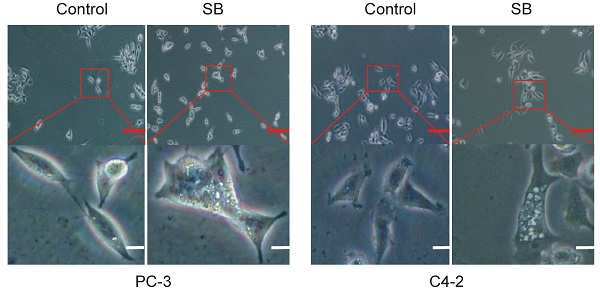
Silibinin (SB), a flavonoid extracted from milk thistle seeds, has been found to exert antitumor effects in numerous tumor types. Our previous study reported that SB had anti-metastatic effects in prostate cancer (PCa). However, the exact underlying molecular mechanisms remain to be determined. The present study aimed to investigate the effects of SB on the migration, invasion and epithelial-mesenchymal transition (EMT) of castration-resistant PCa (CRPC) cells using wound healing, Transwell assays, and western blotting. The results revealed that SB treatment significantly inhibited the migration and invasion of CRPC cell lines. Moreover, SB was confirmed to activate autophagy, as determined using LC3 conversion, LC3 turnover and LC3 puncta assays. Further mechanistic studies indicated that the expression levels of Yes-associated protein (YAP) were downregulated in an autophagy-dependent manner after SB treatment. In addition, the SB-induced autophagic degradation of YAP was associated with the anti-metastatic effects of SB in CRPC. In conclusion, the findings of the present study suggested that SB might inhibit the migration, invasion and EMT of PCa cells by regulating the autophagic degradation of YAP, thus representing a potential novel treatment strategy for metastatic CRPC.
Keywords: silibinin, migration, invasion, epithelial-mesenchymal transition, autophagy, Yes-associated protein

 Global reach, higher impact
Global reach, higher impact