Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(12):3368-3377. doi:10.7150/jca.71592 This issue Cite
Research Paper
Hexokinase 2 Is a Pivot for Lovastatin-induced Glycolysis-to-Autophagy Reprogramming in Triple-Negative Breast Cancer Cells
1. Key Laboratory of Translational Cancer Stem Cell Research, Department of Pathophysiology, Hunan Normal University School of Medicine, Changsha, Hunan 410013, China.
2. Department of Biochemistry and Molecular Biology, Jishou University, Jishou, Hunan, China.
3. Department of Nursing, Hunan Normal University School of Medicine, Changsha, Hunan 410013, China.
* These authors contributed equally to this work.
Received 2022-1-30; Accepted 2022-7-3; Published 2022-9-21
Abstract
Triple-negative breast cancer (TNBC) is the most aggressive subtype of breast cancer with limited therapeutic options available. We have recently demonstrated that lovastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor, suppresses TNBC cell proliferation and stemness properties in vitro and in vivo. However, the mechanisms through which lovastatin inhibits TNBC cells are not fully understood. Here, we used 1H NMR-based metabolomic profiling to investigate lovastatin-induced metabolic changes in TNBC cell line MDA-MB-231. Among the 46 metabolites identified, lactate demonstrated the highest variable importance in projection (VIP) score. Glycolysis stress test revealed that lovastatin significantly decreased the extracellular acidification rate (ECAR) in MDA-MB-231 cells. Furthermore, lovastatin treatment down-regulated the levels of glycolysis-related proteins including GLUT1, PFK1, and PKM2 in MDA-MB-231 but not non-TNBC MDA-MB-453 cells. In addition, lovastatin induced autophagy as evidenced by increased LC3 puncta formation and LC3-II/I ratio, increased AMPK phosphorylation, and decreased Akt phosphorylation. We also revealed the interaction between the glycolytic enzyme hexokinase 2 (HK2) and the mitochondrial membrane protein voltage-dependent anion channel 1 (VDAC1), an important regulator of autophagy. Further bioinformatics analysis revealed that VDAC1 was expressed at a higher level in breast cancer than normal tissues and higher level of VDAC1 predicted poorer survival outcomes in breast cancer patients. The present study suggests that lovastatin might exert anti-tumor activity by reprogramming glycolysis toward autophagy in TNBC cells through HK2-VDAC1 interaction.
Keywords: Lovastatin, Triple-negative breast cancer, Metabolomics, Glycolysis, Autophagy
Introduction
In spite of the progress in screening and early diagnosis, breast cancer remains the most common female malignancy globally [1]. Characterized by the lack of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), TNBC accounts for 10-20% of all breast cancer cases and has a median overall survival time of less than 18 months in the metastatic setting [2]. Although targeted therapeutic strategies have been extensively explored in TNBC, the efficacy is still limited for various reasons including the occurrence of treatment-related adverse events, etc [3]. Therefore, it is a very urgent task to explore novel therapeutic drugs for TNBC.
Even in the presence of abundant oxygen, a majority of tumor cells produce substantial amounts of energy through enhanced glycolytic metabolism rather than oxidative phosphorylation. This phenomenon, known as the Warburg effect [4], has been well recognized in a variety of solid cancer types. The enzymes and transporters involved in the aerobic glycolytic pathway, in association with the intracellular signaling pathways, play critical roles in metabolic reprogramming of cancer cells. Over the last decade, there has been immense interest in targeting key glycolysis-related enzymes such as hexokinases (HKs), phosphofructokinases (PFKs), pyruvate kinases (PKs), and glucose transporters (GLUTs), as a possible avenue for cancer therapy. Since TNBC has well-known dysregulation in glucose metabolism [5], agents targeting the molecules involved in the hyperactivity of glycolysis may provide a potential therapeutic option for this type of hard-to-treat cancer.
Statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are widely used as lipid-lowering drugs due to their advantages of low adverse events and pleiotropic effects other than HMG-CoA reductase inhibition [6]. Lovastatin is the first US FDA-approved naturally occurring lipophilic statin, whose anti-tumor effect has gained enormous attention. In clinical trials, the risk of developing breast cancer has been shown to be reduced after long-term use of lovastatin [7]. Mechanistically, lovastatin can inhibit proliferation, migration, and invasion and induce apoptosis in vitro and in vivo in a variety of cancer types including breast cancer [7, 8]. Recently, we have demonstrated that lovastatin could inhibit the stemness properties, reverse the HER2-negative phenotype, and inhibit metastasis of TNBC cancer stem cells in preclinical models [9-11]. However, how lovastatin exerts its inhibitory effects on TNBC remains largely elusive.
The connection between glycolysis and autophagy has been an area of intense investigation in recent years. It has been demonstrated that glycolysis regulates autophagy through multiple mechanisms, which plays a critical role in the acquisition of malignant phenotypes of cancer cells [12]. A group of mitochondrial pore-forming proteins, known as voltage-dependent anion channels (VDACs), are critical for the maintenance of mitochondrial integrity. Located at the interface between the cytosol and the mitochondria, VDACs are the docking sites for several proteins important for autophagy regulation. VDAC1, the most abundant and best characterized VDAC isoform, has become a new pharmacologically targetable molecule for cancer therapy [13]. Autophagy induction by statins has been documented in several types of cancer cells including melanoma, prostate, and breast cancer [14-16]. Recently, we have demonstrated that lovastatin promotes the formation of autophagosomes in TNBC cancer stem cells (unpublished observation). However, it is not known whether lovastatin induces autophagy through the regulation of metabolic reprogramming. In this study, we found that lovastatin exerts anti-tumor activity by reprogramming glycolysis toward autophagy in TNBC cells. Our findings provide rationale for designing a novel therapeutic strategy based on the connection between glycolysis and autophagy in TNBC.
Materials and Methods
Antibodies
Antibodies against HK2 and VDAC1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Proteintech (Chicago, IL, USA), respectively. Antibodies against LC3, mTOR, mTORSer2448, AMPK, AMPKThr172 and, AktSer473 were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against Tubulin and GAPDH were purchased from Bioworld Technology (Philadelphia, PA, USA) and Zhongshan Golden Bridge (Beijing, China), respectively. Anti-rabbit IgG (H+L) affinity purified secondary antibody was obtained from Vector Laboratories (Burlingame, CA, USA).
Cell culture and reagents
MDA-MB-231, MDA-MB-468, and MDA-MB-453 human breast cancer cells were purchased from the Cell Resource Center of Shanghai Institutes for Biological Sciences, maintained in Dulbecco's high glucose (25 mM glucose) modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were cultured at 37 °C in a humidified incubator with 5% CO2. Lovastatin was purchased from Sigma (M2147, St Louis, MO), dissolved in DMSO as a stock solution of 30 mM, which was aliquoted and stored frozen at -20 °C.
Cell treatment
MDA-MB-231, MDA-MB-468, and MDA-MB-453 human breast cancer cells were seeded in culture dishes or plates (about 1.5 × 104 cells/cm2) and allowed to grow overnight before treatment. On the next day, lovastatin was added to the cells at various concentrations and the cells were cultured under normoxia (21% O2) or hypoxia (1% O2) for the desired period of time as previously described [17].
1H NMR-based metabolomic profiling
1H nuclear magnetic resonance (NMR) analysis based on metabolite identification was conducted as previously described [18]. Following the normalization processing, independent-sample t test was further used to investigate alterations in endogenous metabolites using SPSS (version 13) software. The peaks with adjusted P values < 0.05 were deemed statistically significant. Principal component analysis (PCA) and partial-least-squares discriminant analysis (PLS-DA) were performed using the R statistical package. Data was visualized by employing the ggplot2. The PCA scores plot was used to reveal the metabolomic profiles of lovastatin-treated cells. The influence of variables was predicted by their variable importance in projection (VIP) scores. A VIP score above 1.00 was considered statistically significant.
Measurement of glucose uptake and lactate production
The cells were seeded in six-well plates (2 × 105, 8 × 105, and 4 × 105 cells per well for MDA-MB-231, MDA-MB-468, and MDA-MB-453, respectively) in triplicate wells and incubated overnight. On the next day, various concentrations of lovastatin (0, 1, and 3 μM) were added to the medium and the culture was continued for up to 48 h. The medium was collected, and glucose and lactate levels were then measured using the Automatic Biochemical Analyzer (AU680, Beckman, USA) at the Clinical Biochemical Laboratory of Xiangya Hospital, Central South University. The protein concentration of the cells in each well were measured and used for normalization.
Glycolysis stress test
The cells were seeded on XF24 multi-well plates (2 × 104, 8 × 104, and 5 × 104 cells per well for MDA-MB-231, MDA-MB-468, and MDA-MB-453, respectively) in triplicate and were pre-incubated overnight at 37°C with 5% CO2, followed by incubation with various concentrations of lovastatin (0, 1, and 3 μM) for 48 h. The glycolysis stress test was performed in the Seahorse XF24 Extracellular Flux Analyzer (Seahorse Bioscience, USA) according to the manufacturer's protocol. The relative levels of glycolysis, glycolytic capacity and glycolytic reserve were calculated based on extracellular acidification rate (ECAR) data. The data were normalized by the protein concentration in each well.
Western blotting
After lovastatin (0, 1 and 3 μM) treatment, MDA-MB-231 and MDA-MB-453 cells were harvested and lysed in cell lysis buffer with PMSF and phosphatase inhibitors added. The protein concentration was determined and equal amounts of protein from each lysate were run on the SDS-PAGE gel. After electrophoretic separation, the proteins were transferred to a PVDF membrane, followed by primary and secondary antibody incubation and ECL development according to our standard procedure [11]. The protein bands were quantified using the ImageJ software.
Immunofluorescence-confocal microscopy
The cells were grown on coverglasses and treated with lovastatin or vehicle for 48 h as described [11]. Then the cells were fixed with 4% paraformaldehyde and subjected to indirect immunofluorescence. The cells were imaged using a Leica TCS SP8 Confocal Microscope (Wetzlar, Germany). Bidirectional scanning and 2× line averaging was used on a 2,048 × 2,048 resolution with zoom adjusted according to the field of interest.
Co-immunoprecipitation (co-IP) assay
After treatment with lovastatin, the cells were lysed in 1× cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA) with 1 mM phenylmethanesulfonyl fluoride (Sigma, St Louis, MO, USA) and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA, USA). Co-immunoprecipitation between HK2 and VDAC1 was carried out using the Dynabeads Protein G Immunoprecipitation Kit (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer's protocol.
Hexokinase activity assay
The cells were seeded into a 6-well plate and grown for 24 h, and then incubated with different concentrations of lovastatin for 24 h. The cells were counted and cell pellets were obtained by centrifugation and then lysed with 1× lysis buffer (Cell Signaling Technology, Danvers, MA, USA) containing 1 mM PMSF (Sigma, St Louis, MO, USA) and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA, USA). The protein concentration in cell lysate was determined by BCA assay, and the HK activity in cell lysate was analyzed with Micro HK Assay Kit (Solarbio, Beijing, China) following the instructions of the manufacturer.
Transmission electron microscopy
Autophagosomes and autolysosomes were observed by routine transmission electron microscopy (TEM). Briefly, the cells were treated with vehicle or lovastatin (1 μM) for 24 h, fixed, post-fixed, and subjected to embedding, sectioning, and TEM observation as described in our recent publication [11].
Bioinformatics analysis
The breast cancer gene expression and patient annotation datasets of The Cancer Genome Atlas (TCGA) were retrieved from the Genomic Data Commons (GDC) (https://portal.gdc.cancer.gov/) of the National Cancer Institute. Gene set enrichment analysis (GSEA) (https://www.gsea-msigdb.org/gsea/msigdb/index.jsp) was performed using the GSEA 4.1.0 program [19]. The differentially expressed genes were obtained by dividing breast cancer patients into high and low expression groups according to the median of HK2 gene expression level. The correlation of HK2 expression with the autophagy-related markers in breast cancer was analyzed using the online database Gene Expression Profiling Interactive Analysis 2 (GEPIA2) (http://gepia2.cancer-pku.cn/#index) [20]. Protein-protein interaction (PPI) network generated using the STRING database (https://string-db.org/) [21] was applied to analyze the functional groups of autophagy-related proteins in breast cancer. UALCAN (http://ualcan.path.uab.edu/) [22] was applied to analyze the transcriptional levels of HK2 and VDAC1 in primary breast cancer tissues from TCGA database. The prognostic value of HK2 and VDAC1 in TNBC patients were analyzed using Kaplan-Meier plotter (http://kmplot.com/analysis/).
Statistical analysis
All the quantitative data were presented as mean ± SEM. Statistical analyses (ANOVA, unpaired Student's t test) were carried out using SigmaPlot (version 12.5). P < 0.05 was considered as statistically significant.
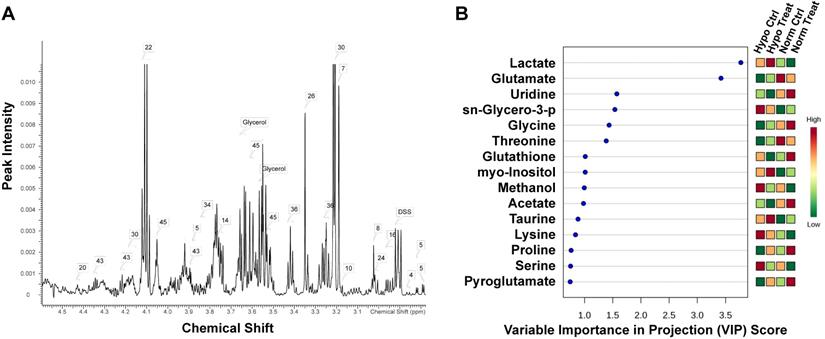
Lovastatin alters the metabolomic profile in TNBC MDA-MB-231 cells. (A) 1H NMR-based metabolomics detected in 15-cm culture dishes for 72 h treated with lovastatin in MDA-MB-231 cells. The attribution range was 3.0ppm_5.0ppm. The assigned metabolites were identified with a serial number. (B) The VIP scores depicting the 15 top-ranked metabolites that contributed to the separation of MDA-MB-231 breast cancer cells treated with lovastatin or DMSO. A VIP score above 1.00 was considered statistically significant. The colored boxes on the right indicate the relative concentrations of the corresponding metabolite in each group under study. The scale bar depicts the relative metabolite levels (red, high abundance; green, low abundance). Hypo: hypoxia; Norm: normoxia; Ctrl: control
Results
Lovastatin alters the metabolomic profile in TNBC cells
In order to gain insight into the effect of lovastatin on metabolic reprogramming in breast cancer cells, we performed global metabolomic profiling using MDA-MB-231 as a representative TNBC cell line by an NMR-based metabolomics approach. The typical 500 MHz 1H NMR spectra with peak assignments for the extracts from MDA-MB-231 cells were shown in Figure 1A. The PCA score plot for 1H NMR data of MDA-MB-231 cells showed a clear separation between normoxia and hypoxia groups, with the lovastatin-treated group and the control group overlapping with each other (Figure S1). To get a better separation, partial least-squares discriminant analysis (PLS-DA) was performed to explore the differences in metabolomics among the treatment and control groups (Figure S2). Among the metabolites identified, marked decrease in the levels of lactate, glutamate, glycine, threonine, methanol, lysine, and serine was observed in the lovastatin-treated MDA-MB-231 cells compared with the control cells, with lactate being on the top list of VIP scores (Figure 1B).
Lovastatin suppresses glycolysis but contrarily increases HK2 protein level in TNBC cells
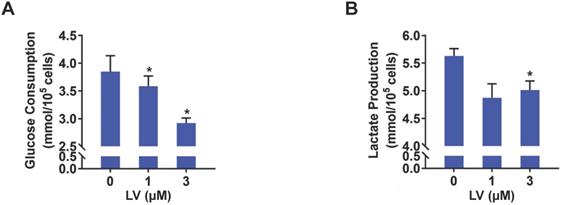
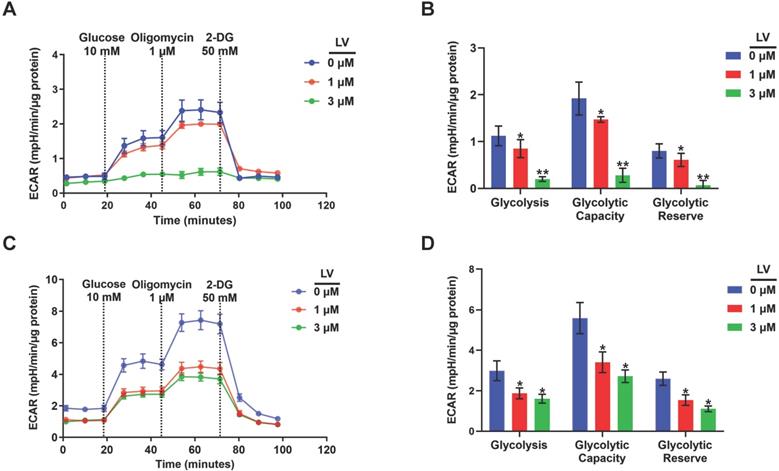
Based on the findings from metabolomic profiling showing a significant decrease in lactate level induced by lovastatin, we further examined the levels of glucose consumption and lactate production in control and lovastatin-treated cells. Consistent with the results of metabolomics analysis, less amount of glucose consumed from the medium (Figure 2A) and less amount of lactate secreted into the medium (Figure 2B) were observed in lovastatin-treated MDA-MB-231 cells compared with the control cells. However, lovastatin did not significantly influence glucose consumption and lactate production of HER2-positive MDA-MB-453 cells (Figure S3). Seahorse glycolysis stress test showed that lovastatin significantly decreased the ECAR associated with glycolysis in MDA-MB-231 (Figure 3A, B) and MDA-MB-468 (Figure 3C, D) cells.
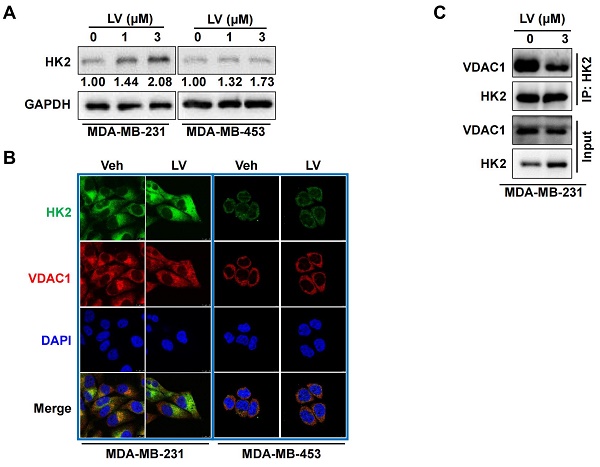
The effect of lovastatin on the glycolytic enzymes was further examined by Western blotting. The protein levels of GLUT1, PFK1, and PKM2 were all decreased in lovastatin-treated MDA-MB-231 but not MDA-MB-453 cells compared with the control cells (data not shown). Interestingly, we found that the protein level of HK2 was not decreased but rather increased by lovastatin treatment in MDA-MB-231 cells (Figure 4A). We asked whether this increase in the protein level would enhance the enzymatic activity of HK. We found that although HK2 expression was increased, the total kinase activity was contrarily decreased (Figure S4). We thus speculate that the increased HK2 might play a non-glycolytic function.
Lovastatin decreases glucose consumption and lactate production in breast cancer cells. (A, B) The levels of glucose consumption (A) and lactate production (B) examined in culture medium collected from control or lovastatin-treated (48 h) MDA-MB-231 cells. * P < 0.05, ** P < 0.01, compared with the control.
Lovastatin decreases glucose metabolism in breast cancer cells. (A) ECAR measured in MDA-MB-231 cells treated with vehicle, or lovastatin at 1 or 3 μM. (B) Quantifications of the ECAR results of MDA-MB-231 cells, showed glycolysis, glycolytic capacity, and glycolytic reserve, respectively. (C) ECAR measured MDA-MB-468 cells treated with vehicle, or lovastatin at 1 or 3 μM. (D) Quantifications of the ECAR results of MDA-MB-468 cells, showed glycolysis, glycolytic capacity, and glycolytic reserve, respectively. * P < 0.05, ** P < 0.01, compared with the control.
Lovastatin decreases colocalization of HK2 and VDAC1 in TNBC cells
Because the interaction between HK2 and VDAC1 is a key event in determining cell fate [23], we examined whether lovastatin also affected this interaction in TNBC cells. To this end, we examined the localization of HK2 and VDAC1 in lovastatin-treated and control MDA-MB-231 cells by immunofluorescence-confocal microscopy. Unexpectedly, although the protein level of HK2 was increased, colocalization of HK2 and VDAC1 was decreased after treatment with lovastatin compared with the control group (Figure 4B). We further used co-IP to confirm their interaction at the protein level. Consistent with the findings from immunofluorescence, co-IP assay revealed that the interaction between HK2 and VDAC1 was also decreased (Figure 4C). These results suggested that although lovastatin increased the protein level of HK2, its interaction with VDAC1 is contrarily decreased.
Lovastatin promotes autophagy in TNBC cells
To investigate whether lovastatin promotes autophagy in TNBC cells, we first performed transmission electron microscopy to reveal the presence of autophagosome/autolysosome in TNBC cells. Lovastatin indeed triggered autophagy in MDA-MB-231 cells as demonstrated by increased formation of autophagosomes and autolysosomes (Figure 5A). Increased LC3 puncta formation was observed by immunofluorescence-confocal microscopy in lovastatin-treated MDA-MB-231 cells but not MDA-MB-453 cells (Figure 5B). Consistent with the results of immunofluorescence, lovastatin increased the LC3-II/I ratio in MDA-MB-231 cells as revealed by Western blotting (Figure 5C). In addition, we demonstrated the decreased levels of mTORSer2448 and AktSer473 and the increased level of AMPKThr172 in lovastatin-treated MDA-MB-231 but not MDA-MB-453 cells (Figure 5D).
Lovastatin decreases the interaction of VDAC1 and HK2 in TNBC cells. (A) The protein level of HK2 analyzed by Western blotting in MDA-MB-231 cells treated with lovastatin or vehicle for 48 h. (B) Representative confocal images of immunofluorescence staining for HK2 (green) and VDAC1 (red) in MDA-MB-231 and MDA-MB-453 cells after treatment with lovastatin (3 μM, 48 h) or vehicle. Blue, DAPI staining of the nucleus. Original magnification: 630×. (C) Co-immunoprecipitation analysis of the interaction of between HK2 and VDAC1 in MDA-MB-231 cells using an anti-HK2 antibody followed by Western blotting with anti-HK2 or anti-VDAC1 antibody. V or Veh, vehicle; L or LV, lovastatin. * P < 0.05, ** P < 0.01, compared with the control.
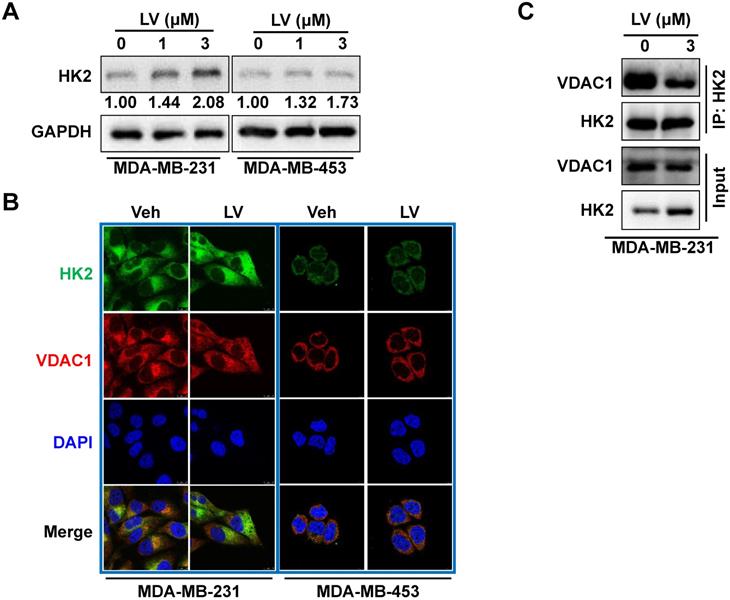
Lovastatin promotes autophagy in TNBC cells. (A) Representative transmission electron microscopic images showing the autophagosomes/autolysosomes (indicated by arrows) in MDA-MB-231 cells after treatment with vehicle or lovastatin (1 μM, 24 h). Scale bar = 2 μm. Right, quantification of the number of autophagosomes/autolysosomes per cell. (B) Representative confocal images of immunofluorescence staining for LC3 in MDA-MB-231 and MDA-MB-453 cells after treatment for 48 h with lovastatin (3 μM) or vehicle. Blue, DAPI staining of the nucleus. Original magnification: 630×. (C) Western blotting of whole cell lysates of MDA-MB-231 cells and MDA-MB-453 cells for LC3. (D) Phosphorylated vs. total mTOR and AMPK after treatment for 48 h with lovastatin at different concentrations. Numbers under the blots are quantifications of band intensities expressed as LC3-II/I ratio or phosphorylated vs. total protein ratio. V or Veh, vehicle; L or LV, lovastatin. * P < 0.05, ** P < 0.01, compared with the control.
HK2 is associated with autophagy in breast cancer patients
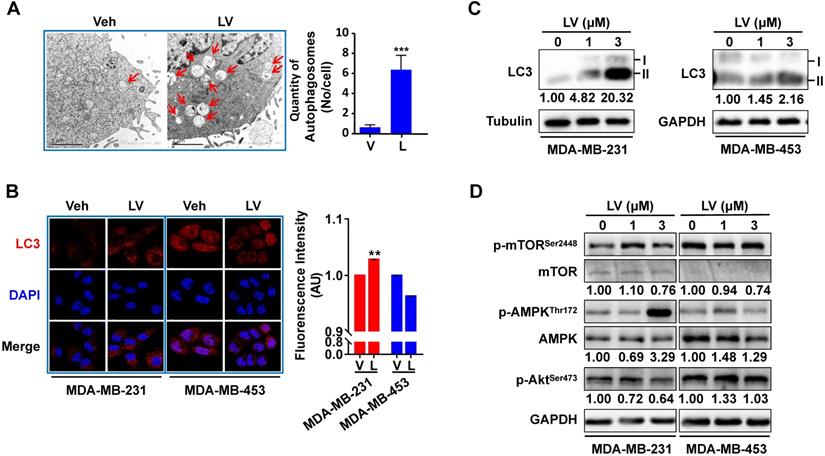
To further explore the relationship between HK2 and autophagy, we performed bioinformatics analysis comparing between the high and low HK2 expression groups in breast cancer patients. GSEA revealed that in the KEGG database, not only the glycolysis-related pathways but also the mTOR signaling pathway, which is known to be associated with autophagy, were enriched in breast cancer patients with HK2high expression (Figure 6A and Table S1). Then, we analyzed the correlation between autophagy-related marker sets in breast cancer patients using the GEPIA2 and STRING databases. We found significant correlation between HK2 and the autophagy-related gene set, including mechanistic target of rapamycin kinase (mTOR), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), pyruvate dehydrogenase kinase 1 (PDK1), AKT serine/threonine kinase 1 (AKT1), TBC1 domain family member 7 (TBC1D7), protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1), Ras homolog MTORC1 binding (RHEB), mTOR associated protein LST8 homolog (mLST8), ribosomal protein S6 kinase B1 (RPS6KB1) in breast cancer patients through the GEPIA2 database (Figure 6B). Furthermore, the STRING analysis revealed functional and structural relationships between HK2 and autophagy-related proteins (Figure 6C). These results suggested that HK2 is indeed associated with autophagy in breast cancer patients.
Expression levels of HK2 and VDAC1 are correlated with TNBC patient survival
Next, we explored the relationship between the expression of HK2 and VDAC1 and the prognosis of breast cancer patients. The protein levels of HK2 and VDAC1 and the effects on the prognosis of breast cancer patients were analyzed based on online databases. UALCAN analysis revealed that the protein level of HK2 was lower in breast cancer tissues compared with non-cancer tissues (Figure 7A). In addition, Kaplan-Meier analysis revealed that higher expression of HK2 protein was correlated with better overall survival in TNBC patients (Figure 7B). In contrast, higher VDAC1 protein level was found in breast cancer tissues compared with non-cancer tissues (Figure 7C) and was correlated with poorer overall survival in TNBC patients (Figure 7D). These results suggested that HK2 and VDAC1 are differentially expressed between breast cancer and non-cancer tissues and their expression levels are associated with TNBC patient survival.
Biological pathways related to HK2 expression in breast cancer. (A) Functional enrichment of HK2high expression breast cancer patients analyzed by GSEA for KEGG pathways. The mTOR signaling pathway, which is related to autophagy, is highlighted by the red box. (B) The correlation between HK2 and autophagy-related genes analyzed by the GEPIA2 database. (C) The protein-protein interaction network of HK2 and autophagy-related proteins constructed by the STRING database.
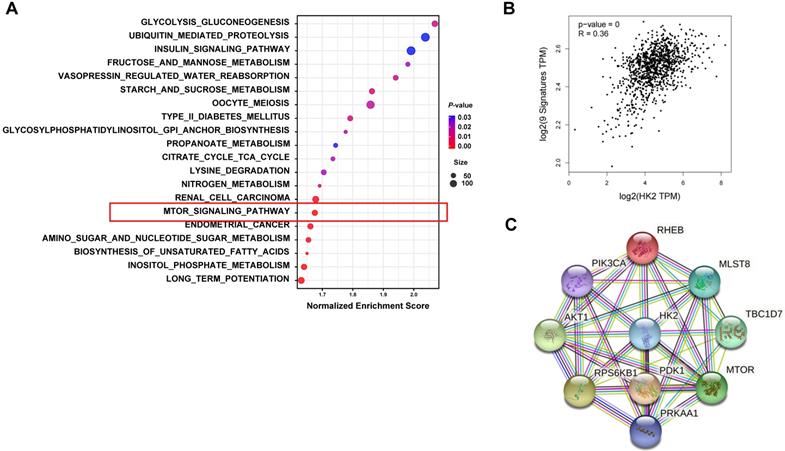
The expression of HK2 and VDAC1 and their prognosis in breast cancer. (A) UALCAN analysis for the protein level of HK2 in breast cancer patients. (B) Kaplan-Meier analysis revealing the relationship between the protein level of HK2 and overall survival in TNBC patients. (C) UALCAN analysis for the protein level of VDAC1 in breast cancer patients. (D) Kaplan-Meier analysis revealing the relationship between the protein level of VDAC1 and overall survival in TNBC patients.
Discussion
It is well appreciated that lovastatin induces metabolic reprogramming in cancer cells, but current metabolic studies of lovastatin exposure are mainly concentrated on the alterations of lipid metabolism. In this study, 1H NMR-based metabolomics approach was adopted to explore the altered metabolites and the affected metabolic pathways after the administration of lovastatin in TNBC MDA-MB-231 cells. Out of the 46 extracellular metabolites identified, lactate, an end product of glycolysis, was obviously decreased. This indicates that metabolic reprogramming might be one of the consequences of lovastatin treatment in TNBC cells. Since the “Warburg effect” is closely associated with cancer initiation and progression, nowadays, targeting the glycolytic pathway has become one of the active research areas in cancer therapy. A promising strategy for anti-tumor therapy could be the inhibition of glycolysis-related enzymes in cancers, thus generating a state of energy deprivation that may sensitize cancer cells to other anti-cancer therapies. Statins have been reported to exert anti-tumor effects by disrupting glucose uptake and metabolism [24, 25]. In our study, we found that lovastatin effectively suppressed the level of glucose uptake and lactate production and also down-regulated the expression of glycolysis-related proteins such as GLUT1, PFK1, and PKM2 in TNBC MDA-MB-231 cells.
It has been reported that the interaction between HK2 and VDAC1 predicts the roles they may have in the regulation of glycolysis and mitochondrial respiration. VDAC1, as the most abundant protein on mitochondrial outer membrane, controls the passages of metabolites, nucleotides, and ions between the cytosol and the mitochondrion [26]. Our study showed that lovastatin decreased the protein level of VDAC1 and its colocalization of HK2. Many factors are important in regulating mitochondrial localization of HK2 and its interaction with VDAC1. For example, it has been demonstrated that phosphorylation of HK2 at Threonine 473 by Akt promotes mitochondrial binding of VDAC1 [27, 28]. In addition, Sun et al. has established that HK overexpression stimulates VDAC1 phosphorylation through a PKCɛ-dependent pathway [29]. Moreover, GSK3β, of which the enzymatic activity is regulated by Akt, phosphorylates the binding site of HK2 on VDAC1, resulting in dissociation of HK2 from the mitochondrion. In this study, we found that lovastatin inhibited AktSer473 phosphorylation, which, we speculate, may be involved in enhanced phosphorylation of VDAC1 and reduced mitochondrial localization of HK2.
While the role of HK2 in autophagy has been documented in the literature, our study here represents the first observation to link glycolysis and autophagy in the case of TNBC regarding the anti-cancer effect of lovastatin. The combination of these two biological pathways might lead to new therapeutic strategies for TNBC. Tan et al. discovered that HK2 facilitates autophagy in response to glucose deprivation to protect cardiomyocytes, suggesting that HK2 functions as a molecular switch from glycolysis to autophagy [30]. However, the molecular determinants responsible for the crosstalk between glycolysis and autophagy in cancer remain largely unsolved. It is possible that HK2 connects the two biological processes through the Akt/mTORC1 signaling pathway [31]. Additional studies will be required to elucidate the exact role of HK2 in reprogramming glycolysis toward autophagy and to determine the mechanism underpinning this transition in TNBC cells. Our findings are helpful to understand the anti-cancer mechanism of lovastatin in TNBC cells and provide a theoretical basis for its repurposing as a therapeutic drug for TNBC, a subset of clinically challenging breast cancer.
Supplementary Material
Supplementary figures and table.
Acknowledgements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Funding
This work was supported by the National Natural Science Foundation of China (82173374, 81872167, 8170103011, 81702635), Key Grant of Research and Development in Hunan Province (2020DK2002), and Natural Science Foundation of Hunan (2019JJ40193).
Author contributions
MW and YL performed the experiments; SC, MW, LX and JZ collected and analyzed the data; LX, QZ and CZ drafted the manuscript; GH, SD and SF reviewed the manuscript; GL and XD conceived and designed the research. All authors have read and approved the final submitted manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136:E359-86
2. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721-8
3. Garrido-Castro AC, Lin NU, Polyak K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019;9:176-98
4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
5. Shen L, O'Shea JM, Kaadige MR, Cunha S, Wilde BR, Cohen AL. et al. Metabolic reprogramming in triple-negative breast cancer through Myc suppression of TXNIP. Proc Natl Acad Sci U S A. 2015;112:5425-30
6. Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712-9
7. Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10-9
8. Yang T, Liu J, Luo F, Lin Q, Rosol TJ, Deng X. Anticancer properties of Monascus metabolites. Anti-cancer drugs. 2014;25:735-44
9. Song L, Tao X, Lin L, Chen C, Yao H, He G. et al. Cerasomal Lovastatin Nanohybrids for Efficient Inhibition of Triple-Negative Breast Cancer Stem Cells To Improve Therapeutic Efficacy. ACS Appl Mater Interfaces. 2018;10:7022-30
10. Yi H, Wu M, Zhang Q, Lu L, Yao H, Chen S. et al. Reversal of HER2 Negativity: An Unexpected Role for Lovastatin in Triple-Negative Breast Cancer Stem Cells. J Cancer. 2020;11:3713-6
11. Zheng C, Yan S, Lu L, Yao H, He G, Chen S. et al. Lovastatin Inhibits EMT and Metastasis of Triple-Negative Breast Cancer Stem Cells Through Dysregulation of Cytoskeleton-Associated Proteins. Front Oncol. 2021;11:656687
12. Chu Y, Chang Y, Lu W, Sheng X, Wang S, Xu H. et al. Regulation of Autophagy by Glycolysis in Cancer. Cancer management and research. 2020;12:13259-71
13. Magri A, Reina S, De Pinto V. VDAC1 as Pharmacological Target in Cancer and Neurodegeneration: Focus on Its Role in Apoptosis. Front Chem. 2018;6:108
14. Parikh A, Childress C, Deitrick K, Lin Q, Rukstalis D, Yang W. Statin-induced autophagy by inhibition of geranylgeranyl biosynthesis in prostate cancer PC3 cells. The Prostate. 2010;70:971-81
15. Al-Qatati A, Aliwaini S. Combined pitavastatin and dacarbazine treatment activates apoptosis and autophagy resulting in synergistic cytotoxicity in melanoma cells. Oncology letters. 2017;14:7993-9
16. Hu MB, Zhang JW, Gao JB, Qi YW, Gao Y, Xu L. et al. Atorvastatin induces autophagy in MDA-MB-231 breast cancer cells. Ultrastructural pathology. 2018;42:409-15
17. Yang T, Yao H, He G, Song L, Liu N, Wang Y. et al. Effects of Lovastatin on MDA-MB-231 Breast Cancer Cells: An Antibody Microarray Analysis. J Cancer. 2016;7:192-9
18. Hasanpour M, Saberi S, Iranshahi M. Metabolic Profiling and Untargeted 1H-NMR-Based Metabolomics Study of Different Iranian Pomegranate (Punica granatum) Ecotypes. Planta Med. 2020;86:212-9
19. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545-50
20. Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556-W60
21. Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J. et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607-D13
22. Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B. et al. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia. 2017;19:649-58
23. Zhang F, Angelova A, Garamus VM, Angelov B, Tu S, Kong L. et al. Mitochondrial Voltage-Dependent Anion Channel 1-Hexokinase-II Complex-Targeted Strategy for Melanoma Inhibition Using Designed Multiblock Peptide Amphiphiles. ACS Appl Mater Interfaces. 2021;13:35281-93
24. Malenda A, Skrobanska A, Issat T, Winiarska M, Bil J, Oleszczak B. et al. Statins impair glucose uptake in tumor cells. Neoplasia. 2012;14:311-23
25. Kuzyk CL, Anderson CC, Roede JR. Simvastatin Induces Delayed Apoptosis Through Disruption of Glycolysis and Mitochondrial Impairment in Neuroblastoma Cells. Clinical and translational science. 2020;13:563-72
26. Mazure NM. VDAC in cancer. Biochimica et biophysica acta Bioenergetics. 2017;1858:665-73
27. Roberts DJ, Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell death and differentiation. 2015;22:364
28. Roberts DJ, Tan-Sah VP, Smith JM, Miyamoto S. Akt phosphorylates HK-II at Thr-473 and increases mitochondrial HK-II association to protect cardiomyocytes. The Journal of biological chemistry. 2013;288:23798-806
29. Sun L, Shukair S, Naik TJ, Moazed F, Ardehali H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Molecular and cellular biology. 2008;28:1007-17
30. Tan VP, Miyamoto S. HK2/hexokinase-II integrates glycolysis and autophagy to confer cellular protection. Autophagy. 2015;11:963-4
31. Roberts DJ, Tan-Sah VP, Ding EY, Smith JM, Miyamoto S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Molecular cell. 2014;53:521-33
Author contact
![]() Corresponding authors: Guifei Li, liguifeimededu.cn, Xiyun Deng, dengxiyunmededu.cn
Corresponding authors: Guifei Li, liguifeimededu.cn, Xiyun Deng, dengxiyunmededu.cn

 Global reach, higher impact
Global reach, higher impact