3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(12):3326-3332. doi:10.7150/jca.76719 This issue Cite
Research Paper
Therapy-related Acute Lymphoblastic Leukaemia has a Unique Genetic Profile Compared to De Novo Acute Lymphoblastic Leukaemia
1. Division of Hematology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul 03722, Republic of Korea.
2. Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul 03722, Republic of Korea.
*These authors contributed equally to this work.
Abstract
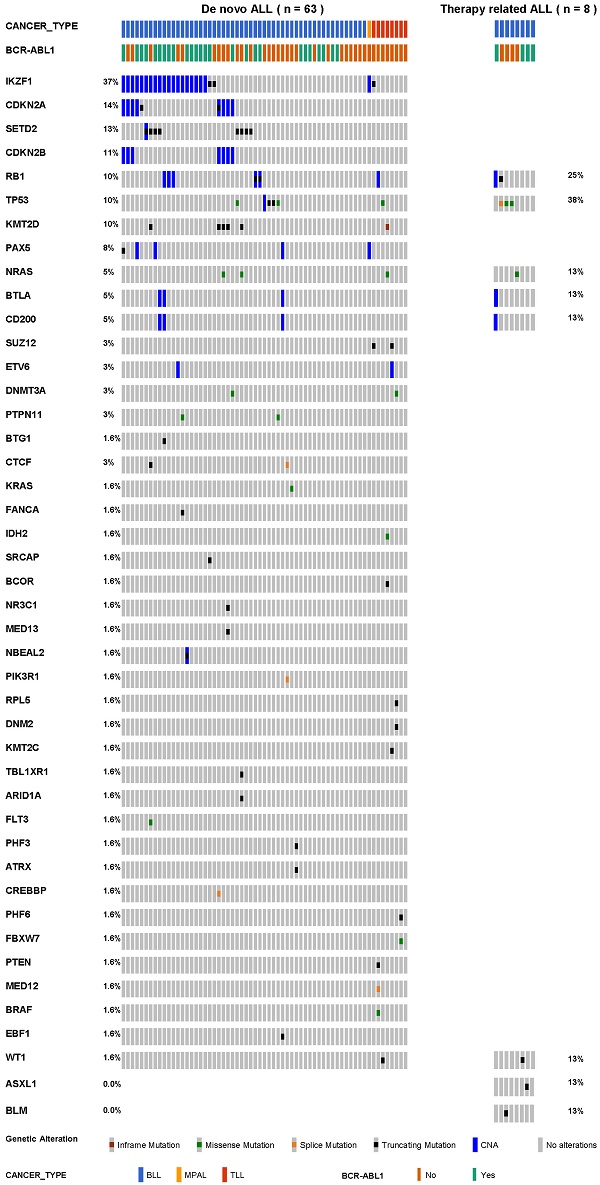
Background: Unlike therapy-related myeloid neoplasms, therapy-related acute lymphoblastic leukaemia (tr-ALL) is poorly defined due to its rarity. However, increasing reports have demonstrated that tr-ALL is a distinct entity with adverse genetic features and clinical outcomes.
Methods: We compared the clinicopathological characteristics and outcomes of patients diagnosed with tr-ALL (n = 9) or de novo ALL (dn-ALL; n = 162) at a single institution from January 2012 to March 2021. The mutational landscapes of eight tr-ALL and 63 dn-ALL patients were compared from a comprehensive next-generation sequencing panel.
Results: All tr-ALL patients had the B-cell phenotype. The most frequently mutated genes were IKZF1 (37%), CDKN2A (14%), SETD2 (13%), and CDKN2B (11%) in dn-ALL, whereas TP53 (38%) and RB1 (25%) mutations were most common in tr-ALL. tr-ALL patients did not show a statistically significant difference in overall survival (p = 0.70) or progression-free survival (p = 0.94) compared to dn-ALL patients.
Conclusions: In this study, we determined the clinical and genetic profiles of Korean patients with tr-ALL. We found alterations in genes constituting the TP53/RB1 pathway are more frequent in tr-ALL. Due to the rarity of the disease, multi-institutional studies involving a larger number of patients are required in future study.
Keywords: next-generation sequencing, therapy-related acute lymphoblastic leukaemia, germline predisposition, de novo acute lymphoblastic leukaemia, mutation

 Global reach, higher impact
Global reach, higher impact