Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(10):3113-3120. doi:10.7150/jca.75456 This issue Cite
Research Paper
Short-term safety and Long-term efficacy of multivisceral resection in pT4b gastric cancer patients without distant metastasis: a 20-year experience in China National Cancer Center
Department of Pancreatic and Gastric Surgical Oncology, National Cancer Center/National Clinical Research for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
#These authors contributed equally to this study.
Received 2022-5-24; Accepted 2022-7-12; Published 2022-8-15
Abstract
Background: Multivisceral resection is occasionally necessary for pT4b gastric cancer patients to achieve negative margin. The purpose of this study is to assess the short-term safety and long-term efficacy of this approach.
Methods: A single-center, retrospective analysis was conducted for pT4b gastric cancer patients after curative-intent multivisceral resection from the China National Cancer Center Gastric Cancer Database (NCCGCDB) from 1998 to 2018. The postoperative complications, recurrence patterns, long-term survival, and prognostic factors were analyzed.
Results: A total of 210 patients were included in the study. The most common combined resection organs were multiple organs (30.5%), pancreas (20.5%), colon (16.7%), and liver (9.0%). Seventeen patients (8.1%) developed postoperative complications and hospital death was observed in one patient (0.5%). The most common postoperative complications were anastomotic leak (4.3%) and intra-abdominal infection (5.7%). The 3-year and 5-year disease-free survival (DFS) rates for the patients investigated were 38.0% and 33.8%, respectively, and the 3-year and 5-year overall survival (OS) rates were 48.2% and 39.1%, respectively. Multivariate Cox regression analysis proved that negative nerve invasion was independent risk factors for DFS (HR: 2.202, 95%CI: 1.144-4.236, P=0.018) and OS (HR: 2.219, 95%CI: 1.164-4.231, P=0.015).
Conclusions: Multivisceral resection in pT4b gastric cancer patients without distant metastasis was effective and had an acceptable safety profile.
Keywords: gastric cancer, T4b, multivisceral resection (MVR), postoperative complications, recurrence, survival
Background
Gastric cancer (GC) is one of the most common fatal malignancies with high risk of metastasis and tumor recurrence [1]. Considering that locally advanced GC sometimes invade the surrounding organs (T4b), such as pancreas, colon, and liver [2], the multivisceral resection (MVR) surgery is necessary to achieve a negative margin [3, 4]. Generally, MVR surgery is considered to have higher cost with increased risk of postoperative complications and mortality [5]. The short-term safety and long-term efficacy have been widely debated over the years.
Notably, most of the previous studies included the patients with clinical T4b (cT4b) [2, 4, 6-10]. However, in some cT4b patients, the tumor itself did not directly invade the surrounding organs due to the inflammatory response [6]. Therefore, partial GC patients with cT4b included in the previous study (pathologically confirmed T4a, pT4a) might not require extended MVR. Moreover, the previous study demonstrated that the median overall survival of GC patients with pT4a were higher than pT4b who underwent MVR surgery (22.6 months vs. 17.7 months) [6].
To date, only a few studies evaluated the safety and efficacy of MVR surgery focusing on pathologic T4b (pT4b) patients [3, 6, 11]. In Korea Cancer Center Hospital, 243 GC patients with pT4b were retrospectively reviewed, and the results demonstrated that the postoperative mortality rate was 0.8% and the media overall survival (OS) was 26 months [11]. Another National Cancer Database (NCDB) study showed that the mortality rate within 30 days of MVR surgery was 7.5% and the media OS was 12.9 months in pT4b GC patients underwent MVR surgery [6]. However, neither of the two studies explored the recurrence pattern of pT4b GC patients after MVR surgery. Meanwhile, the rates of postoperative complications and mortality varies greatly.
Therefore, we designed this study to assess the short-term safety, recurrence pattern and long-term survival of MVR surgery in pT4b GC patients based on the China National Cancer Center Gastric Cancer Database (NCCGCDB).
Materials and Methods
Patients
We retrospectively collected the clinicopathologic characteristics of pT4b patients who underwent potential curative MVR surgery from the China National Cancer Center Gastric Cancer Database (NCCGCDB). The details of NCCGCDB have been previously described and recognized [12]. The inclusion criteria were as follows: (i). Age more than 18 years; (ii). Adenocarcinoma of stomach; (iii). Postoperative pathology confirmed T4b. The exclusion criteria included: (i). Patients with other tumor history; (ii). Patients who confirmed with distant metastasis; (iii). Patients with incomplete clinical data.
The definition of pT4b was that gastric cancer directly invaded the adjacent structures, including the spleen, colon, liver, diaphragm, pancreas, abdominal wall, adrenal gland, kidney, small intestine, and retroperitoneum, according to the guidelines of the National Comprehensive Cancer Network (NCCN, version 5.2021). The requirement for written informed consent by patients was waived due to the retrospective nature of the study. Eventually, a total of 210 patients were enrolled into the final analysis.
Short-term and Long-term outcomes
The main short-term outcomes were operative difficulty, postoperative complications, and perioperative mortality. The operative difficulty was reflected from the aspects of operative time, blood transfusion, and postoperative hospital stay. The main long-term outcomes were disease-free survival time (DFS) and overall survival time (OS). DFS was defined as the time from surgery to the locoregional and systemic recurrence. OS was defined as the time from surgery to the death or last follow-up. Recurrence pattern was classified as locoregional recurrence, peritoneal metastasis, and distant metastasis, which has been described in detail in previous studies [13, 14].
Postoperative follow-up
The postoperative follow-up was performed through outpatient clinical visits, telephone contact, and death registries. Finally, 53 patients were lost to follow-up and the follow-up rate was 74.8%. The median duration of follow-up was 22 months (rang, 1-192 months).
Statistical analysis
The basic clinicopathologic features of the patients was presented using descriptive statistics. Categorical variables were presented with counts and proportions, while continuous variables were presented with medians and standard deviation (SD). Categorical variables were compared using the chi-square test and Fisher's exact test. Continuous variables were compared using the Mann-Whitney U test. Survival analysis was conducted using the Cox proportional hazards model. In the multivariate models, we included the factors with P≤0.2 in the univariate analysis and other important factors might affect the survival outcomes. All the survival analysis was performed with the SPSS software (SPSS Inc., Chicago, IL, USA, version 22.0). The survival curves were depicted according to the Kaplan-Meier method through GraphPad Prism software (GraphPad Software, La Jolla, CA, USA, version 8.0.2). Results with a two-tailed P<0.05 were considered as statistically significant.
Results
Clinicopathologic characteristics
The basic clinicopathologic characteristics of the 210 pT4b GC patients who underwent MVR surgery were displayed in the Table 1. The median age of all patients was 61 years (range, 24-82 years). Most patients (87.1%) were proved to have locoregional lymph nodes metastasis. In addition, most pT4b patients had large tumor size and poor differentiation. The most common combined resection organs were multiple organs (30.5%), pancreas (20.5%), colon (16.7%), and liver (9.0%). Although all the patients underwent potentially radical surgery, 12 patients (5.7%) were confirmed to have the positive surgical margins.
Clinicopathologic features of pT4b gastric cancer patients who underwent MVR surgery
| Characteristic | n=210 | 100% |
|---|---|---|
| Age (mean±SD) | 61±11 | |
| ≤65 | 138 | 65.7% |
| >65 | 72 | 34.3% |
| Gender | ||
| Male | 153 | 72.9% |
| Female | 57 | 27.1% |
| Tumor location | ||
| Proximal | 110 | 52.4% |
| Distal | 87 | 41.4% |
| Total | 13 | 6.2% |
| Neoadjuvant therapy | ||
| No | 189 | 90.0% |
| Yes | 21 | 10.0% |
| Gastric stump carcinoma | ||
| No | 145 | 69.0% |
| Yes | 24 | 11.4% |
| Unknown | 41 | 19.5% |
| Surgical approach | ||
| Open | 196 | 93.3% |
| Laproscope | 14 | 6.7% |
| Gastric surgery | ||
| Total gastrectomy | 30 | 14.3% |
| Sub gastrectomy | 180 | 85.7% |
| Tumor size (pathology) | ||
| <5cm | 62 | 29.5% |
| ≥5cm | 148 | 70.5% |
| Differentiation | ||
| Well and Moderate | 49 | 23.3% |
| Poor and Undifferentiated | 161 | 76.7% |
| Borrman classification | ||
| I | 14 | 6.7% |
| II | 45 | 21.4% |
| III | 98 | 46.7% |
| IV | 46 | 21.9% |
| Unknown | 7 | 3.3% |
| Lauren classification | ||
| Intestinal type | 28 | 13.3% |
| Diffuse type | 30 | 14.3% |
| Mixed type | 23 | 11.0% |
| Unknown | 129 | 61.4% |
| pN stage | ||
| N0 | 27 | 12.9% |
| N1 | 26 | 12.4% |
| N2 | 53 | 25.2% |
| N3 | 104 | 49.5% |
| pTNM stage | ||
| IIIA | 27 | 12.9% |
| IIIB | 79 | 37.6% |
| IIIC | 104 | 49.5% |
| Lymphatic vessels invasion | ||
| Positive | 104 | 49.5% |
| Negative | 106 | 50.5% |
| Blood vessels invasion | ||
| Positive | 105 | 50.0% |
| Negative | 105 | 50.0% |
| Nerve invasion | ||
| Positive | 65 | 31.0% |
| Negative | 145 | 69.0% |
| Margin involved | ||
| R0 | 198 | 94.3% |
| R1/R2 | 12 | 5.7% |
| Combined organs removed | ||
| Pancreas | 43 | 20.5% |
| Liver | 19 | 9.0% |
| Colon | 35 | 16.7% |
| Spleen | 5 | 2.4% |
| Other (abdominal wall, diaphragm, gallbladder, kidney) | 44 | 21.0% |
| Multiple organs | 64 | 30.5% |
| Blood transfusion | ||
| Yes | 99 | 47.1% |
| No | 111 | 52.9% |
| Postoperative complications | ||
| No | 193 | 91.9% |
| Yes | 17 | 8.1% |
| Adjuvant treatment | ||
| Yes | 81 | 38.6% |
| No | 15 | 7.1% |
| Unknown | 114 | 54.3% |
Short-term outcomes
Regarding the surgical difficulty, as shown in the Table 1, most patients (93.3%) received open operation, and only 14 patients (6.7%) received laparoscopic surgery. The mean operation time were 209.8 minutes in all patients, and the operation time was longer in patients underwent gastrectomy combined with spleen resection. More than 50% of the patients required intraoperative blood transfusion, especially for patients combined pancreas resection and combined liver resection. Moreover, patients who received combined pancreas resection and multiple organs resection were more likely to have a longer stay in hospital (Table 2).
In terms of surgical safety, 17 patients (8.1%) developed postoperative complications and hospital death was observed in 1 patient (0.5%) due to intraabdominal bleeding. The most common postoperative complications were anastomotic leak (4.3%) and intra-abdominal infection (5.7%). Combined resection of pancreas and multiple organs have higher risk of postoperative complications and mortality.
Long-term survival outcomes
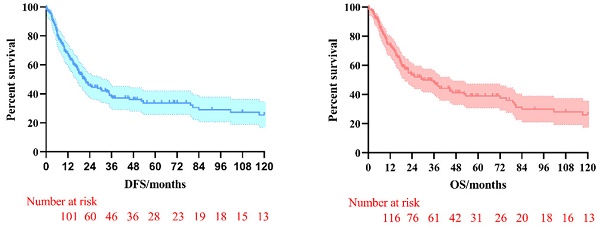
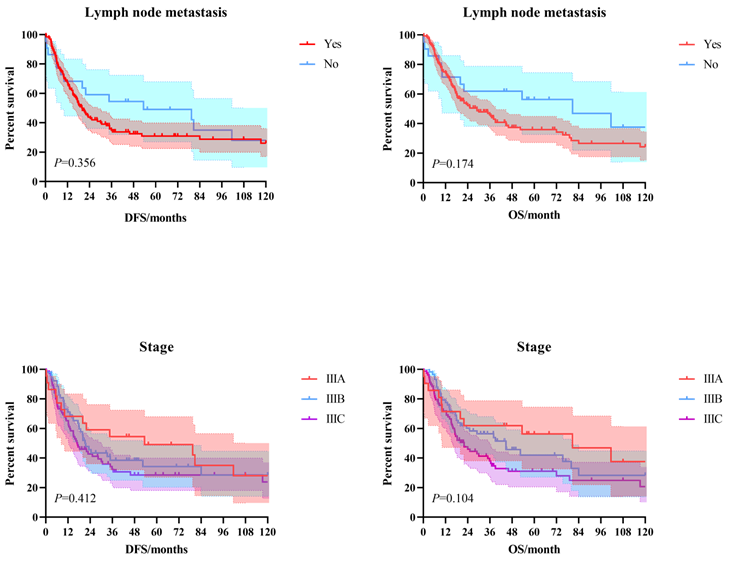
The 3-year and 5-year disease-free survival (DFS) rates for the patients investigated were 38.0% and 33.8%, respectively, and the 3-year and 5-year overall survival (OS) rates were 48.2% and 39.1%, respectively. The survival curves of DFS and OS were shown in Figure 1. Subgroup survival curve analysis according to the lymph node metastasis status and TNM stage found no statistical differences (Figure 2). Furthermore, we compared the survival curves of DFS and OS according to the combined resection organs and found that the patients received combined resection of pancreas and multiple organs were tend to have worse survival (Figure 3).
The survival curves of DFS and OS in pT4b gastric cancer patients who underwent MVR surgery.
The survival curves of DFS and OS according to the lymph node metastasis status and TNM stage.
The survival curves of DFS and OS according to the combined resection organs.
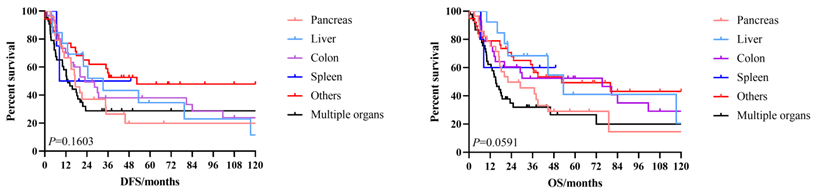
Operative difficulty and short-term safety of pT4b gastric cancer patients who underwent MVR surgery
| Variables | Total (n=210) | Spleen (n=5) | Colon (n=35) | Pancreas (n=43) | Liver (n=19) | Other* (n=44) | Multiple organs (n=64) | P-value |
|---|---|---|---|---|---|---|---|---|
| Mean operative time /min | 209.8 | 250.8 | 199.6 | 209 | 209.6 | 184.8 | 222.3 | 0.057 |
| Blood transfusion | <0.001 | |||||||
| No | 99 | 3 | 20 | 14 | 9 | 10 | 42 | |
| Yes | 111 | 2 | 15 | 27 | 20 | 34 | 22 | |
| Blood transfusion /ml | 890.8 | 1500 | 878 | 871.9 | 968.4 | 740 | 927.5 | 0.201 |
| Mean postoperative hospital stay /d | 16.5 | 18.4 | 16.8 | 19 | 17.1 | 11.9 | 18.5 | <0.001 |
| Postoperative complications | 0.007 | |||||||
| No | 193 | 3 | 33 | 39 | 19 | 44 | 55 | |
| Yes | 17 | 2 | 2 | 4 | 0 | 0 | 9 | |
| Anastomotic leak | 9 | 1 | 2 | 2 | 0 | 0 | 4 | |
| Intra-abdominal infections | 12 | 2 | 2 | 2 | 0 | 0 | 6 | |
| Intra-abdominal hemorrhage | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Gastrointestinal hemorrhage | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Postoperative intestinal obstruction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Gastroparesis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pulmonary complications | 4 | 0 | 0 | 2 | 0 | 0 | 2 | |
| Pancreatic fistula | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
*Including: abdominal wall, diaphragm, gallbladder, kidney.
When conducting the multivariate Cox regression survival analysis, we found that negative nerve invasion was independent risk factors for DFS (HR: 2.202, 95%CI: 1.144-4.236, P=0.018) (Table 3) and OS (HR: 2.219, 95%CI: 1.164-4.231, P=0.015) (Table 4).
Recurrence pattern
A total of 114 patients (54.3%) developed recurrences. Additionally, 28 patients were followed up for over five years and no evidence of recurrence were found. Among the 34 patients with documented recurrence, 18 patients developed only locoregional recurrence, 2 patients developed distant metastasis, and 8 patients developed only peritoneal metastasis. The specific recurrence sites were demonstrated in Table 5.
Discussions
Radical surgery (R0) is the only potentially curable treatment for GC patients. For pT4b GC patients, MVR surgery is necessary to achieve R0 resection. However, this operation remains debatable due to surgical difficulty and high incidence of postoperative complications and mortality. Therefore, we integrated 20-year experience to explore this clinically important question. Our results demonstrated that MVR surgery had relatively acceptable short-term safety and long-term efficacy. The current study provides further evidence to support MVR surgery in the future clinical practice to some extent.
The rates of postoperative complications and mortality were 8.1% and 0.5% in the present study, which were relatively lower than the previous studies. Previous studies indicated that the postoperative complications rate ranged from 15% to 53.4%, and mortality rate ranged from 1.0% to 8.6% [2, 4, 6, 7, 9-11, 15, 16]. Such discrepancy might have several reasons. Firstly, regional difference in GC prevalence might contribute to the different risk of surgical complications and death. In areas of high GC incidence, such as Korea, the postoperative complications and mortality rates of MVR surgery were 15% and 2% [11]. However, in Brazil, the postoperative complications and mortality rates of MVR surgery could achieve 53.4% and 8.6% [7]. Secondly, the difference of combined resection organs might lead to the different results. Several investigators have suggested that combined pancreas resection in MVR surgery of T4b GC patients could arise the risk of postoperative complications and mortality [11]. Although Tran et al. found that MVR surgery with pancreas resection was not associated with an increased frequency of postoperative complications and mortality [10]. The gastrectomy combined with pancreaticoduodenectomy was considered to have high risk of postoperative death and complications [3, 10]. Thirdly, the number of cases in the present study and previous studies were relatively small, which could potentially bias the results.
Several studies have showed a substantial survival advantage of MVR surgery in T4b GC patients, comparing with gastrectomy alone or palliative surgery [6, 17-20]. However, some other studies have demonstrated that GC patients with MVR surgery achieved worse survival than those with gastrectomy alone [7, 15]. Overall, the 5-year OS rate of MVR surgery for GC patients was 13.8%~36.8% [2, 4, 11, 15, 17, 20]. In the present study, the 5-year OS rate of pT4b GC patients who received MVR surgery was 39.1%, which was a little higher than the previous results. This may be related to the fact that the previous results have illustrated that prognosis in the patients combined with pancreas resection, especially in patients who underwent pancreaticoduodenectomy, was poor than patients combined other organs resection during MVR surgery [3, 10, 11]. In a Korea retrospective study, the 5-year OS rates of pT4b patients combined pancreas resection group and combined other organs resection were 23.3% and 42.1% (P=0.002), while in the pancreaticoduodenectomy group, the 5-year OS rates was 0% [11]. Similarly, a significant survival difference among pT4b patients without and with pancreas resection was reported in Taiwan (32% vs. 13%, P=0.004). However, in the current study, approximately one in five patients underwent combined pancreas resection alone and only four patients underwent gastrectomy combined pancreaticoduodenectomy surgery.
Univariate and multivariate Cox regression analysis for disease-free survival of pT4b gastric cancer patients who underwent MVR surgery
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR [95%CI] | P-value | HR [95%CI] | P-value | |
| Age | ||||
| ≤65 | Reference | |||
| >65 | 0.809[0.519-1.262] | 0.350 | ||
| Gender | ||||
| Male | Reference | |||
| Female | 1.266[0.803-1.996] | 0.310 | ||
| Tumor location | ||||
| Proximal | Reference | |||
| Distal | 1.006[0.669-1.512] | 0.978 | ||
| Total | 0.627[0.250-1.574] | 0.320 | ||
| Neoadjuvant therapy | ||||
| No | Reference | |||
| Yes | 0.983[0.494-1.958] | 0.962 | ||
| Gastric stump carcinoma | ||||
| No | Reference | |||
| Yes | 0.706[0.323-1.541] | 0.382 | ||
| Unknown | 1.067[0.672-1.694] | 0.784 | ||
| Surgical approach | ||||
| Open | Reference | |||
| Laproscope | 1.305[0.630-2.702] | 0.473 | ||
| Gastric surgery | ||||
| Total gastrectomy | Reference | |||
| Sub gastrectomy | 0.976[0.543-1.756] | 0.936 | ||
| Tumor size (pathology) | ||||
| <5 cm | Reference | Reference | ||
| ≥5 cm | 1.586[1.022-2.461] | 0.040 | 1.372[0.862-2.184] | 0.183 |
| Differentiation | ||||
| Well and Moderate | Reference | |||
| Poor and Undifferentiated | 0.928[0.571-1.507] | 0.762 | ||
| Borrman classification | ||||
| I | Reference | |||
| II | 1.401[0.488-4.024] | 0.531 | ||
| III | 1.066[0.381-2.980] | 0.903 | ||
| IV | 1.621[0.561-4.686] | 0.372 | ||
| Unknown | 1.436[0.261-7.883] | 0.677 | ||
| Lauren classification | ||||
| Intestinal type | Reference | Reference | ||
| Diffuse type | 0.860[0.434-1.704] | 0.665 | 0.738[0.336-1.622] | 0.450 |
| Mixed type | 0.504[0.199-1.280] | 0.150 | 0.408[0.143-1.163] | 0.093 |
| Unknown | 1.043[0.604-1.802] | 0.879 | 0.615[0.295-1.284] | 0.196 |
| pN stage | ||||
| N0 | Reference | Reference | ||
| N1 | 2.271[1.067-4.835] | 0.033 | 1.471[0.624-3.468] | 0.378 |
| N2 | 0.862[0.431-1.723] | 0.675 | 0.748[0.343-1.629] | 0.464 |
| N3 | 1.442[0.795-2.617] | 0.228 | 1.311[0.665-2.586] | 0.434 |
| pTNM stage | ||||
| IIIA | Reference | |||
| IIIB | 1.157[0.617-2.170] | 0.649 | ||
| IIIC | 1.426[0.786-2.586] | 0.243 | ||
| Lymphatic vessels invasion | ||||
| Positive | Reference | Reference | ||
| Negative | 0.743[0.498-1.108] | 0.145 | 2.353[0.278-19.942] | 0.433 |
| Blood vessels invasion | ||||
| Positive | Reference | Reference | ||
| Negative | 0.728[0.488-1.086] | 0.120 | 0.295[0.034-2.572] | 0.269 |
| Nerve invasion | ||||
| Positive | Reference | Reference | ||
| Negative | 1.666[1.071-2.590] | 0.024 | 2.202[1.144-4.236] | 0.018 |
| Margin involved | ||||
| R0 | Reference | |||
| R1/R2 | 1.492[0.721-3.088] | 0.281 | ||
| Combined organs removed | ||||
| Pancreas | Reference | Reference | ||
| Liver | 0.791[0.364-1.719] | 0.554 | 0.842[0.370-1.916] | 0.681 |
| Colon | 0.764[0.406-1.441] | 0.406 | 0.700[0.361-1.358] | 0.292 |
| Spleen | 0.752[0.174-3.245] | 0.703 | 1.233[0.242-6.278] | 0.801 |
| Other (abdominal wall, diaphragm, gallbladder, kidney) | 0.494[0.254-0.962] | 0.038 | 0.710[0.334-1.509] | 0.373 |
| Multiple organs | 1.104[0.615-1.981] | 0.741 | 0.985[0.529-1.834] | 0.963 |
| Blood transfusion | ||||
| Yes | Reference | |||
| No | 0.974[0.655-1.448] | 0.897 | ||
| Postoperative complications | ||||
| No | Reference | Reference | ||
| Yes | 1.803[0.937-3.471] | 0.078 | 1.461[0.709-3.010] | 0.304 |
| Adjuvant treatment | ||||
| Yes | Reference | Reference | ||
| No | 0.806[0.344-1.888] | 0.619 | 0.536[0.209-1.377] | 0.195 |
| Unknown | 1.015[0.673-1.529] | 0.944 | 1.026[0.649-1.623] | 0.911 |
Univariate and multivariate Cox regression analysis for overall survival of pT4b gastric cancer patients who underwent MVR surgery
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR [95%CI] | P-value | HR [95%CI] | P-value | |
| Age | ||||
| ≤65 | Reference | |||
| >65 | 0.923[0.597-1.426] | 0.717 | ||
| Gender | ||||
| Male | Reference | |||
| Female | 1.260[0.798-1.991] | 0.321 | ||
| Tumor location | ||||
| Proximal | Reference | |||
| Distal | 0.965[0.643-1.449] | 0.864 | ||
| Total | 0.626[0.249-1.569] | 0.317 | ||
| Neoadjuvant therapy | ||||
| No | Reference | |||
| Yes | 0.839[0.446-1.580] | 0.587 | ||
| Gastric stump carcinoma | ||||
| No | Reference | |||
| Yes | 0.985[0.539-1.800] | 0.961 | ||
| Unknown | 1.033[0.646-1.651] | 0.893 | ||
| Surgical approach | ||||
| Open | Reference | |||
| Laproscope | 1.046[0.505-2.167] | 0.903 | ||
| Gastric surgery | ||||
| Total gastrectomy | Reference | |||
| Sub gastrectomy | 0.813[0.459-1.443] | 0.480 | ||
| Tumor size (pathology) | ||||
| <5cm | Reference | Reference | ||
| ≥5cm | 1.613[1.043-2.495] | 0.032 | 1.410[0.882-2.254] | 0.151 |
| Differentiation | ||||
| Well and Moderate | Reference | Reference | ||
| Poor and Undifferentiated | 0.742[0.476-1.156] | 0.188 | 0.619[0.361-1.062] | 0.082 |
| Borrman classification | ||||
| I | Reference | Reference | ||
| II | 1.310[0.547-3.139] | 0.545 | 1.222[0.459-3.252] | 0.688 |
| III | 1.103[0.488-2.498] | 0.813 | 1.224[0.470-3.187] | 0.679 |
| IV | 1.852[0.776-4.421] | 0.165 | 1.623[0.584-4.514] | 0.353 |
| Unknown | 2.076[0.646-6.672] | 0.220 | 2.695[0.718-10.107] | 0.142 |
| Lauren classification | ||||
| Intestinal type | Reference | Reference | ||
| Diffuse type | 1.098[0.516-2.337] | 0.808 | 1.041[0.433-2.502] | 0.928 |
| Mixed type | 0.942[0.402-2.205] | 0.890 | 0.766[0.290-2.024] | 0.591 |
| Unknown | 1.642[0.896-3.010] | 0.109 | 0.871[0.386-1.969] | 0.741 |
| pN stage | ||||
| N0 | Reference | Reference | ||
| N1 | 1.697[0.722-3.988] | 0.225 | 1.218[0.483-3.071] | 0.677 |
| N2 | 1.108[0.548-2.242] | 0.776 | 0.760[0.342-1.691] | 0.502 |
| N3 | 1.750[0.930-3.293] | 0.083 | 1.646[0.803-3.371] | 0.173 |
| pTNM stage | ||||
| IIIA | Reference | |||
| IIIB | 1.234[0.632-2.409] | 0.539 | ||
| IIIC | 1.743[0.927-3.279] | 0.085 | ||
| Lymphatic vessels invasion | ||||
| Positive | Reference | |||
| Negative | 0.777[0.520-1.162] | 0.219 | ||
| Blood vessels invasion | ||||
| Positive | Reference | |||
| Negative | 0.809[0.541-1.209] | 0.301 | ||
| Nerve invasion | ||||
| Positive | Reference | Reference | ||
| Negative | 2.131[1.330-3.413] | 0.002 | 2.219[1.164-4.231] | 0.015 |
| Margin involved | ||||
| R0 | Reference | |||
| R1/R2 | 1.244[0.574-2.696] | 0.580 | ||
| Combined organs removed | ||||
| Pancreas | Reference | Reference | ||
| Liver | 0.573[0.242-1.356] | 0.205 | 0.814[0.313-2.113] | 0.672 |
| Colon | 0.683[0.357-1.308] | 0.251 | 0.711[0.354-1.428] | 0.337 |
| Spleen | 0.641[0.150-2.742] | 0.548 | 0.998[0.196-5.075] | 0.998 |
| Other (abdominal wall, diaphragm, gallbladder, kidney) | 0.574[0.307-1.076] | 0.083 | 0.923[0.448-1.899] | 0.828 |
| Multiple organs | 1.265[0.722-2.215] | 0.412 | 1.270[0.691-2.336] | 0.442 |
| Blood transfusion | ||||
| Yes | Reference | |||
| No | 0.939[0.629-1.400] | 0.756 | ||
| Postoperative complications | ||||
| No | Reference | Reference | ||
| Yes | 1.606[0.834-3.093] | 0.156 | 1.704[0.761-3.816] | 0.195 |
| Adjuvant treatment | ||||
| Yes | Reference | Reference | ||
| No | 1.140[0.523-2.482] | 0.742 | 0.830[0.342-2.014] | 0.681 |
| Unknown | 1.488[0.981-2.257] | 0.061 | 1.792[1.113-2.885] | 0.016 |
The specific recurrence sites of pT4b gastric cancer patients who underwent MVR surgery
| Recurrence and Metastasis sites | N=114 | 54.3% |
|---|---|---|
| Specific sites that were well recorded | 34 | 16.2% |
| Liver metastasis | 4 | 1.9% |
| Peritoneal metastasis | 9 | 4.3% |
| Locoregional areas recurrence | 11 | 5.2% |
| Supraclavicular lymph nodes metastasis | 1 | 0.5% |
| Remnant stomach recurrence | 12 | 5.7% |
| Ovarian metastasis | 2 | 1.0% |
| Lung metastasis | 2 | 1.0% |
| Brain metastasis | 1 | 0.5% |
| Specific sites that were not recorded | 80 | 38.1% |
It was worth noting that in our study, we found that the pN stage was not the independent prognostic factor in GC patients who received MVR surgery. This is in contrast with previous findings [2, 4, 6, 10, 11, 15, 21]. A possible reason for such contrary results may be that the current pN stage has insufficient homogeneity and discriminatory ability in predicting the survival of GC patients comparing with the positive lymph nodes ratio [22].
Previous studies have shown that the majority recurrence occurred within two years after surgery in GC patients [23, 24]. Moreover, Zhu BY et al. found that 43.8% GC patients with T4 stage experienced recurrence after curative surgery, and peritoneal metastasis was the major recurrence pattern accounting for 62.2% [8]. Furthermore, for pT4b patients, the proportion of peritoneal metastasis even reached to 88.3% [8]. However, in the present study, 54.3% pT4b GC patients after MVR surgery developed recurrence, and the major recurrence pattern were locoregional recurrence. Possibly, this difference in results could be related to the fact that the first sites of recurrence were not recorded in many patients in our study.
Indeed, we do acknowledge that there were some limitations in this study. Firstly, some key data and clinical information were missing for some patients due to the retrospective nature of our study. Secondly, follow-up time was relatively short in some patients, and some patients were lost to follow-up. Thirdly, many patients were followed by other oncological centers, therefore, the adjuvant treatment strategies and first sites of recurrence were not well recorded. Despite this, we believe that our study has unique features and certain strengths. Firstly, our study mainly targeted pT4b GC patients, which avoided the interference of some pT4a GC patients. Secondly, to the best of our knowledge, the current study was the largest sample size study in China that have assessed the short-term safety and long-term efficacy of potential curative MVR surgery in pT4b GC patients.
Conclusions
In our study, we found that the rates of postoperative complications and mortality were 8.1% and 0.5%, and the 3-year and 5-year overall survival rates were 48.2% and 39.1% in pT4b gastric cancer patients without distant metastasis after MVR surgery. Therefore, MVR surgery in pT4b gastric cancer patients without distant metastasis was effective and had an acceptable safety profile.
Acknowledgements
Ethics approval and consent to participate
This was a retrospective, observational cohort study, therefore informed consent was waived by the National Cancer Center in China.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Author contributions
- (1) Guarantor of integrity of the entire study: Yingtai Chen, Dongbing Zhao;
- (2) Study concepts and design: Xiaojie Zhang, Yingtai Chen, Dongbing Zhao;
- (3) Provision of study materials or patients: Xiaojie Zhang, Lulu Zhao, Wanqing Wang;
- (4) Collection and assembly of data: Xiaojie Zhang, Lulu Zhao, Penghui Niu, Wanqing Wang;
- (5) Statistical analysis: Xiaojie Zhang, Lulu Zhao, Wanqing Wang, Yingtai Chen;
- (6) Manuscript preparation: All authors;
- (7) Manuscript editing: All authors.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021;71:209-49
2. Molina JC, Al-Hinai A, Gosseling-Tardif A, Bouchard P, Spicer J, Mulder D. et al. Multivisceral Resection for Locally Advanced Gastric and Gastroesophageal Junction Cancers-11-Year Experience at a High-Volume North American Center. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2019;23:43-50
3. Chang SC, Tang CM, Le PH, Kuo CJ, Chen TH, Wang SY. et al. Impact of Pancreatic Resection on Survival in Locally Advanced Resectable Gastric Cancer. Cancers. 2021;13(6):1289 1-16
4. Pacelli F, Cusumano G, Rosa F, Marrelli D, Dicosmo M, Cipollari C. et al. Multivisceral resection for locally advanced gastric cancer: an Italian multicenter observational study. JAMA surgery. 2013;148:353-60
5. Dias AR, Pereira MA, Ramos M, Oliveira RJ, Ribeiro U Jr, Zilberstein B. et al. Prediction scores for complication and recurrence after multivisceral resection in gastric cancer. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2020;46:1097-102
6. Aversa JG, Diggs LP, Hagerty BL, Dominguez DA, Ituarte PHG, Hernandez JM. et al. Multivisceral Resection for Locally Advanced Gastric Cancer. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2021;25:609-22
7. Dias AR, Pereira MA, Oliveira RJ, Ramos M, Szor DJ, Ribeiro U. et al. Multivisceral resection vs standard gastrectomy for gastric adenocarcinoma. Journal of surgical oncology. 2020;121:840-7
8. Zhu BY, Yuan SQ, Nie RC, Li SM, Yang LR, Duan JL. et al. Prognostic Factors and Recurrence Patterns in T4 Gastric Cancer Patients after Curative Resection. Journal of Cancer. 2019;10:1181-8
9. Mita K, Ito H, Katsube T, Tsuboi A, Yamazaki N, Asakawa H. et al. Prognostic Factors Affecting Survival After Multivisceral Resection in Patients with Clinical T4b Gastric Cancer. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2017;21:1993-9
10. Tran TB, Worhunsky DJ, Norton JA, Squires MH 3rd, Jin LX, Spolverato G. et al. Multivisceral Resection for Gastric Cancer: Results from the US Gastric Cancer Collaborative. Annals of surgical oncology. 2015;22(Suppl 3):S840-7
11. Min JS, Jin SH, Park S, Kim SB, Bang HY, Lee JI. Prognosis of curatively resected pT4b gastric cancer with respect to invaded organ type. Annals of surgical oncology. 2012;19:494-501
12. Zhao L, Huang H, Zhao D, Wang C, Tian Y, Yuan X. et al. Clinicopathological Characteristics and Prognosis of Proximal and Distal Gastric Cancer during 1997-2017 in China National Cancer Center. Journal of oncology. 2019;2019:9784039
13. Ma LX, Espin-Garcia O, Lim CH, Jiang DM, Sim HW, Natori A. et al. Impact of adjuvant therapy in patients with a microscopically positive margin after resection for gastric and esophageal cancers. Journal of gastrointestinal oncology. 2020;11:356-65
14. Woo JW, Ryu KW, Park JY, Eom BW, Kim MJ, Yoon HM. et al. Prognostic impact of microscopic tumor involved resection margin in advanced gastric cancer patients after gastric resection. World journal of surgery. 2014;38:439-46
15. Yang Y, Hu J, Ma Y, Chen G, Liu Y. Multivisceral resection for locally advanced gastric cancer: A retrospective study. American journal of surgery. 2021;221:1011-7
16. Hasselgren K, Sandström P, Gasslander T, Björnsson B. Multivisceral Resection in Patients with Advanced Abdominal Tumors. Scandinavian journal of surgery: SJS: official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2016;105:147-52
17. Lai KK, Fang WL, Wu CW, Huang KH, Chen JH, Lo SS. et al. Surgical impact on gastric cancer with locoregional invasion. World journal of surgery. 2011;35:2479-84
18. Kim JH, Jang YJ, Park SS, Park SH, Kim SJ, Mok YJ. et al. Surgical outcomes and prognostic factors for T4 gastric cancers. Asian journal of surgery. 2009;32:198-204
19. Carboni F, Lepiane P, Santoro R, Lorusso R, Mancini P, Sperduti I. et al. Extended multiorgan resection for T4 gastric carcinoma: 25-year experience. Journal of surgical oncology. 2005;90:95-100
20. Martin RC 2nd, Jaques DP, Brennan MF, Karpeh M. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Annals of surgery. 2002;236:159-65
21. Lee CM, Lee S, Lee D, Park S. How Does Combined Resection Affect the Clinical Outcomes After Laparoscopic Surgery for Serosa-Positive Gastric Cancer?: A Retrospective Cohort Study to Investigate the Short-Term Outcomes of Laparoscopic Combined Resection in Patients With T4b Gastric Cancer. Frontiers in oncology. 2019;9:1564
22. Zhang M, Ding C, Xu L, Ou B, Feng S, Wang G. et al. Comparison of a Tumor-Ratio-Metastasis Staging System and the 8th AJCC TNM Staging System for Gastric Cancer. Frontiers in oncology. 2021;11:595421
23. Kang WM, Meng QB, Yu JC, Ma ZQ, Li ZT. Factors associated with early recurrence after curative surgery for gastric cancer. World journal of gastroenterology. 2015;21:5934-40
24. Chiang CY, Huang KH, Fang WL, Wu CW, Chen JH, Lo SS. et al. Factors associated with recurrence within 2 years after curative surgery for gastric adenocarcinoma. World journal of surgery. 2011;35:2472-8
Author contact
![]() Corresponding authors: Dongbing Zhao, MD, Professor, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No.17 Panjiayuan Nanli, Beijing, 100021, China. E-mail: dbzhaoac.cn; Ying-Tai Chen, MD, Professor, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No.17 Panjiayuan Nanli, Beijing 100021, China. E-mail: yingtaichencom.
Corresponding authors: Dongbing Zhao, MD, Professor, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No.17 Panjiayuan Nanli, Beijing, 100021, China. E-mail: dbzhaoac.cn; Ying-Tai Chen, MD, Professor, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No.17 Panjiayuan Nanli, Beijing 100021, China. E-mail: yingtaichencom.

 Global reach, higher impact
Global reach, higher impact