3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(10):3022-3030. doi:10.7150/jca.73365 This issue Cite
Review
The Role of the S100 Protein Family in Glioma
Department of Neurosurgery, the First Affiliated Hospital of Anhui Medical University, Hefei, 230022, China.
#These authors contributed equally to this work.
Received 2022-3-28; Accepted 2022-6-2; Published 2022-8-1
Abstract
The S100 protein family consists of 25 members and share a common structure defined in part by the Ca2+ binding EF-hand motif. Multiple members' dysregulated expression is associated with progression, diagnosis and prognosis in a broad range of diseases, especially in tumors. They could exert wide range of functions both in intracellular and extracellular, including cell proliferation, cell differentiation, cell motility, enzyme activities, immune responses, cytoskeleton dynamics, Ca2+ homeostasis and angiogenesis. Gliomas are the most prevalent primary tumors of the brain and spinal cord with multiple subtypes that are diagnosed and classified based on histopathology. Up to now the role of several S100 proteins in gliomas have been explored. S100A8, S100A9 and S100B were highly expression in serum and may present as a marker correlated with survival and prognosis of glioma patients. Individual member was confirmed as a new regulator of glioma stem cells (GSCs) and a mediator of mesenchymal transition in glioblastoma (GBM). Additionally, several members up- or downregulation have been reported to involve in the development of glioma by interacting with signaling pathways and target proteins. Here we detail S100 proteins that are associated with glioma, and discuss their potential effects on progression, diagnosis and prognosis.
Keywords: S100 protein, glioma, progression, diagnosis, prognosis
S100 protein family: Types, structure and functions
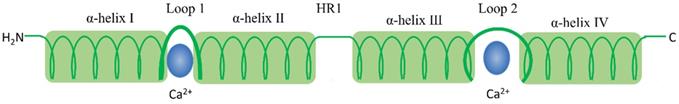
The S100 protein family are low molecular weight and tissue/cell type specific proteins which are exclusively expression in vertebrates [1]. At least 25 protein members have been identified since Moore first separated S100A1 and S100B from bovine brain in 1965 [2, 3]. Of these, encoding genes of 21 family members (S100A1-S100A18, trichohylin, filaggrin and repetin) distribute on chromosome locus 1q21, while other S100 proteins include S100P, S100Z, S100B and S100G locate at chromosome loci 4p16, 5q14, 21q22 and Xp22, respectively [4]. Due to the constituents can be dissolved in ammonium sulfate solution at neutral pH, the subcellular fractions were termed as “S100”. These family of proteins are Ca2+-dependent proteins (and, except for S100A10, a Ca2+-independent protein), characterized by the presence of a complex grouping of EF-hand motif [5]. EF-hand motif was first identified by Kretsinger and Nockolds in 1973, which comprises of two α-helices with an intervening 12-residue calcium-binding loop [6]. Further structural analysis of S100 protein show that each of them has two different EF-hand-shape structure domains. Based on the above exceptional structure characteristics, S100 proteins can be exist as homodimers, heterodimers and oligomers, and perform distinct functions [4]. To be specific, the two EF-hand motifs of each S100 protein contain two distinct different Ca2+-binding domains: C terminal (carboxy-terminal), composed of 12 amino acids, characterized high Ca2+-binding affinity; while N terminal (amino-terminal), formed by 14 residues, has a weaker calcium affinity. Additionally, a region between the two distinct EF-hand domains is called “hinge”, consists of 10-12 residues, which can increase the Ca2+-binding affinity [7]. With Ca2+ ion binding to the EF-hand motifs, these proteins conformation will be rearranged, leading to hydrophobic regions exposed, which are thought the site of target protein binding. It is that Ca2+ interacts with S100 protein targets to regulate a large number of cellular functions [8] (Fig. 1 and Fig. 2).
S100 protein structural organization. 1) S100 protein monomer is composed by EF-hand motifs, which contains two distinct different Ca2+-binding domains: 1) C terminal, characterized high Ca2+-binding affinity; 2) N terminal, has a weaker calcium affinity. Each monomer contains four α helical domains α-helix I, α-helix II, α-helix III, and α-helix IV. Helical loop 1 and loop 2 separate α-helix I and α-helix II, and α-helix III and α-helix IV, respectively. A flexible linker or hinge region (HR1) is also located between H-II and H-III.
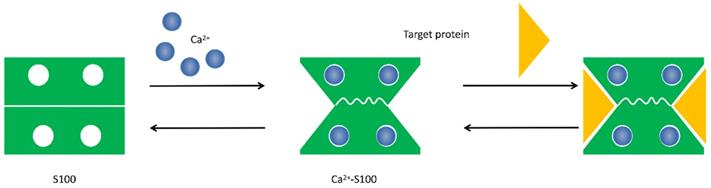
Conformational change of S100 protein. Ca2+ ion binds to the EF-hand motifs induce a conformational rearrangement, allowing the S100 protein to bind its cellular targets and regulate a large number of cellular functions.
S100 proteins' functions in cancer
The physical and structural characteristics of S100 proteins indicate that they are trigger or activator proteins. Ca2+ binding is the way of most S100 protein families regulate structure and function, which allows them to change conformation that exposes hydrophobic regions in the molecules and act as Ca2+ sensors that can translate alterations in intracellular Ca2+ levels into a cellular response [9, 10]. In addition to bind to Ca2+, individual S100 proteins also can bind to Zn2+, Cu2+ and Mn2+ to exert a series of intracellular and extracellular regulatory effects [11]. Interestingly, although the majority of S100 protein are calcium-dependent, several calcium-independent S100 proteins such as S100A10 have been reported [4].
Based on the aforementioned processes and features, the functions of S100 proteins are diverse in cancer. Multiple members of the S100 family dysregulation are involved in tumor growth, apoptosis, differentiation, metastasis, angiogenesis and immune evasion in vivo [12, 13]. Furthermore, extracellular S100 proteins interact with a variety of cell-surface receptors that initiate a cascade of signal transduction to realize these functions, including 1) receptor for advanced glycosylation end products (RAGE; also known as AGER), 2) G protein-coupled receptors, 3) Toll-like receptor 4 (TLR4), 4) scavenger receptors, 5) fibroblast growth factor receptor 1 (FGFR1), 6) CD166 antigen, 7) interleukin-10 receptor (IL-10R), 8) extracellular matrix metalloproteinase inducer (EMMPRIN; also known as basigin), 9) the bioactive sphingolipid ceramide 1-phosphate [12, 14-17]. For example, S100A10, calcium-independent S100 protein, which is be identified as a novel biomarker in pancreatic ductal adenocarcinoma. The expression level of S100A10 mRNA and protein are significantly increased in human pancreatic tumors compared to normal ducts and nonductal stroma, and the knockdown of its expression could reduce surface plasminogen activation, invasiveness, and in vivo growth of pancreatic cancer cell lines [18]. Recent studies reveal that S100A8/A9 mediated signaling through RAGE and TLR4 in activating specific downstream genes to promote tumor growth and metastasis [19, 20].
S100 Proteins and Gliomas
Gliomas are the most common type of malignant brain tumors which are heterogeneous group of tumors developing from glial cells in the central nervous system. According to their histopathological characteristics, they are divided into high-grade and low-grade glioma. Especially high-grade glioma has an unfavorable prognosis with the median survival of only 12-15 months [21-23]. Involving evidence indicates that S100 protein family are closely related to glioma in tumor progression, metastasis, invasion, and so on [24-27]. Here, we reviewed the literature related to S100 proteins and their functions in gliomas (Fig. 3).
S100A4
S100A4, also known as metastasin (Mts1), fibroblast-specific protein (FSP1), 18A2, pEL98, p9Ka, 42A, CAPL, and calvasculin, is localized in the nucleus, cytoplasm, and extracellular space. Its gene consists of four exons, of which the first two are noncoding [28, 29]. Different to other S100 proteins, S100A4 has a quite long and very basic C-terminal loop following helix 4, which makes it particularly unique [29, 30]. At molecular level, stimulating with Zn2+ induce its conformation changed that allows it to bind target proteins to enhance series of processes. Recent studies have demonstrated that S100A4 is directly involved in tumor metastasis and such as breast, non-small-cell lung, and glioma [30-33]. In addition, it also has a certain correlation with cell survival, motility, invasion and epithelial-mesenchymal transition (EMT) [28, 29, 34].
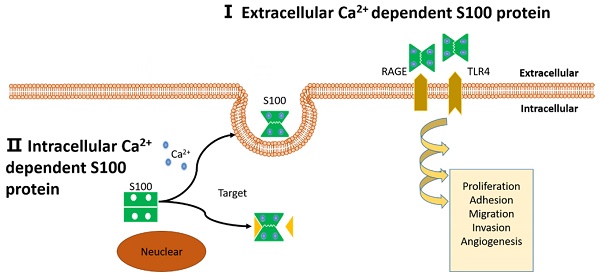
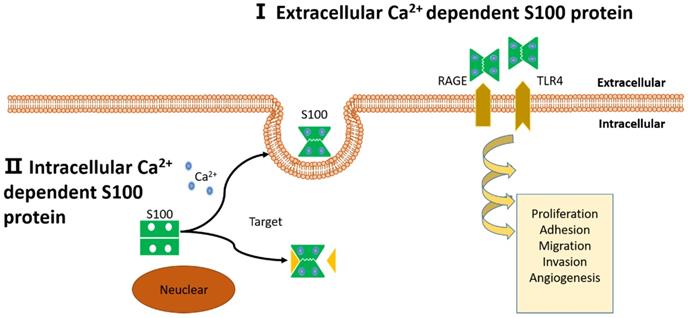
Schematic representation of proposed intracellular and extracellular effects of S100 proteins. (I): Extracellular Ca2+ dependent S100 proteins: S100 proteins interact with membrane surface receptors and activate a cascade of signaling responses to regulate pro-tumorigenic processes. (II): Intracellular Ca2+ independent S100 proteins: S100 proteins interact with target protein and activate a cascade of signaling responses or be secreted out of the cell.
S100A4 protein also plays an important role in glioma. An early study about the role of intracellular S100A4 for migration in rat astrocytes, which yielded some unexpected findings, S100A4 actually reduces the migratory capacity of white matter astrocytes [24]. A further study indicated that low-grade glioma, which do not express S100A4, would be more incline to migrate along meninges and blood vessels, while S100A4 positive malignant glioma prefer to spread in areas of white matter. And this result showed that S100A4 may be an important factor in the pathogenesis of highly malignant brain tumor [35]. However, Roberto Hernan et al. demonstrated that ERBB2 (HER-2/neu) may promote the metastasis of medulloblastoma via the up-regulation of S100A4 and several other prometastatic genes [33]. In addition, a study by Kin-Hoe Chow et al. found that S100A4 expression is closely connected with tumorsphere formation and tumor initiating abilities in vivo. Selectively removing S100A4-expressing cells was able to sufficiently block tumor growth both in vitro and in vivo; and meanwhile, S100A4 is also identified as a critical regulator of glioma stem cells self-renewal both in mouse and patient-derived glioma tumorspheres in this research. Additionally, this study discovered that S100A4 acts as an upstream regulator of the master EMT in glioblastomas, due to it can regulate SNAIL2, ZEB and other mesenchymal transition regulators. Their research highlighted that S100A4 is not only a new biomarker and a regulator of glioma stem cells but also a mediator of mesenchymal transition and stemness in glioblastomas [36]. S100A4 plays a significant role in some pathways as a mediator. A study by Ji Liang et al. showed that neutrophil-promoting malignant glioma progression was inhibited by S100A4 deregulation [37]. Another study by Diana Aguilar-Morante et al. found that C/EBPβ (Enhancer Binding Protein β) could increase S100A4 levels by activating S100A4 promoter expression directly in murine GL261 and human T98G glioblastoma cells, and their data indicated that S100A4 play a crucial role of cell invasion and could mediate the observed effects of C/EBPβ on invasiveness of glioblastoma cells [25] (Table 1).
S100A6
S100A6, also known as calcyclin, is located in the cytoplasm and nucleus in wide of cell types include adult normal tissues and several tumor cell types. Besides binding of Ca2+ with two EF-hand motifs, it also binds Zn2+ by not yet identified structures [38, 39]. A large number of studies has proved that S100A6 is over-expressed in several diseases, especially in malignancies, such as non-small-cell lung cancer [40], gastric cancer [41] and pancreatic carcinoma [42]. Recent studies have found that it is involved in the regulation of various cellular processes, including cell proliferation [41], cell apoptosis [43], migration [44], and so on. Moreover, several studies reported that S100A6 exert its extracellular or intracellular roles through interacting with binding or target proteins and activating the downstream signaling pathways [38, 45]. For instance, study by Duan L et al. showed that a colorectal carcinoma cell line with relatively high S100A6 expression, leading to the inhibition of cell proliferation, migration and MAPK (mitogen-activated protein kinase) activity, further study suggest that S100A6 promotes the growth and migration by activating ERK1/2 (extracellular regulated protein kinase) and p38/MAPKs in colorectal carcinoma, and modulating of these pathways may be employed for colorectal carcinoma prevention and therapy [46]. And another study indicated that S100A6 may promote nasopharyngeal carcinoma development via the activation of p38/MAPK signaling pathways and may be a new prognostic marker [47].
Dysregulated expression of S100A6 associates with glioma progression have been reported. A previous study has been shown to clearly distinguish between low-grade (WHO grade I and II) and high-grade (WHO grade III and IV) astrocytic tumors after altering the level of S100A6 protein expression [48]. However, another study by Camby I et al. indicated that S100A6 is highly expressed in human astrocytic tumors, but this level of its expression cannot show a significantly functional change of the degree of tumor malignancy. Hence, it cannot be used as a discriminatory marker between the different grades [49]. Furthermore, in ependymoma, a study by V Rand et al. have clearly proved that S100A6 is differentially expressed in ependymoma arising in different regions of the brain, and is significantly associated with supratentorial tumors [50]. J C Lindsey et al. screened S100 genes for evidence of epigenetic regulation in medulloblastoma through a pharmacological expression reactivation approach, which found that S100A6 upregulated expression in multiple medulloblastoma cell lines after treatment with DNA methyltransferase inhibitor, 5′-aza-2′-deoxycytidine. Additionally, this study demonstrated S100A6 hypermethylation was significantly associated with the aggressive large cell/anaplastic morphophenotype [51]. As mentioned earlier, overexpression of S100A6 is associated with cell motility in malignant tumor. The study by J Kucharczak et al. suggest that, gastrin could mediate cell motility in glioblastoma cells through activating gastrin-induced overexpression of the S100A6 gene product (tenascin-C) [52]. Unfortunately, the mechanisms and signal pathways of S100A6 associated to tumor progression has, to present, not been studied in glioma.
S100A8/9
S100A8 and S100A9, also called myeloid-related proteins (myeloid related protein 8 (MRP-8) and myeloid related protein 14 (MRP-14)) [53, 54]. As the name suggest these proteins are specific highly expressed in myeloid cells, and a study indicated both the two proteins account for approximately 45% of cytosolic protein in neutrophils and approximately 5% in monocytes [54]. Although S100A8 and S100A9 are able to form monomers, heterodimers, homodimers and trimers, it is reported that the S100A8/A9 heteromer (also known as calprotectin) is the clearly preferred complex due to its high stability [54, 55], And besides, they are frequently co-expressed and their expression is regulated together [54].
Up- or downregulation and effects of S100 proteins in different subtype of gliomas
| Types of S100 proteins | Subtypes of gliomas | Up- or downregulation | Functions | References |
|---|---|---|---|---|
| S100A4 | Low grade gliomas | - | incline to migrate along meninges and blood vessels | [35] |
| Malignant gliomas | ↑ | prefer to spread in areas of white matter | ||
| Medulloblastoma | ↑ | promote metastasis | [33] | |
| Glioblastoma | ↑ | a regulator of glioma stem cells and mediator of mesenchymal transition and stemness | [36] | |
| S100A6 | Astrocytic tumours | ↑ | doesn't show a significantly change in function of the level of tumour malignancy | [49] |
| Ependymoma | ↑ | can be used to distinguish clinically and biologically relevant subgroups | [50] | |
| S100A8 | Glioblastoma | ↑ | presente a correlation with survival of GBM patients | [66] |
| Glioma | ↑ | promote cell proliferation, invasion, and migration | [69] | |
| S100A9 | GSCs | ↑ | regulates GSCs proliferation | [73] |
| S100A8 and S100A9 | Glioblastoma | ↑ | dependent on integrin signaling to promote migration and invasion at medium concentration | [66] |
| S100B | Glioma | ↑ | may be a valuable serum biomarker to predict the prognosis in glioma patients | [84,85,86,87] |
| Astrocytoma | ↑ | contribute to astrocytomas progression by inhibiting p53 functions | [92] | |
| S100P | Glioblastoma | ↑ | promote cell proliferation, migration, invasion and anchorage independent growth | [94] |
| S100A13 | Astroglioma | ↑ | correlate with tumour grading and microvessel density | [101] |
| S100A11 | Glioblastoma | ↑ | play crucial role in proliferation, EMT, migration, invasion and neurosphere formation | [105,106] |
↑,Upregulation; ↓,Downregulation; -, Do not expression.
S100A8/S100A9 was original discovered as an immunogenic protein which was secreted by neutrophils with potent anti-microbial properties and then it is identified as a critical pro-inflammatory cytokine in acute and chronic inflammation [56, 57]. Recently, researches have demonstrated that S100A8 and S100A9 dimers could interact with multiple receptors like RAGE (receptor for advanced glycation end products), TLR (toll-like receptor) and Wnt/β-catenin to promote tumor developments and invasiveness [58-60]. In addition, S100A8 and S100A9 were reported highly expressed in tumors and primary expressed within tumors by immune cells. And their expression can induce the recruitment of myeloid cells and myeloid-derived suppressor cells resulted in tumor growth, metastasis, and formation of premetastatic niche [19, 61-64].
Recently, with rapid progress of proteomics, the identification of biomarkers are of great interest for early tumor growth, recurrence, and therapeutic response in oncology [65]. A study by Anjali Arora et al. showed that S100A8 and S100A9 were highly expression both in the tissue and proteins in the serum, but only S100A8 presented a correlation with survival of GBM patients. Furthermore, this study found that S100A8 and S100A9 dependent on integrin signaling to promote migration and invasion at medium concentration [66]. Similarly, the study of Paul R. Gielen et al. also found glioma patients have increased S100A8/9 serum levels, at the same time, this study confirmed that S100A8/9 protein expression was statistical significantly increased in myeloid-derived suppressor cells of patients with glioma [67]. However, Gautam P et al. revealed that S100A9 levels was significantly elevated in individual plasma specimens from GBM patients [68]. In addition, S100A8 and S100A9 are associated with progression and prognosis in glioma. The study by Jinsheng Xiong reported that S100A8 is significantly highly expression in glioma samples. Downregulated its expression inhibited cell proliferation, invasion, as well as migration of glioma. And circMAN2B2 could promote this biology processes by regulating the miR-1205/S100A8 axis [69]. Furthermore, a previous study identified S100A8 and S100A9 involved neuron-to-astrocyte signaling process that may be important for astrocytoma formation, more specifically, the increased expression of S100A8 and S100A9 in neurons is an early and critical step in tumorigenic Kras-induced gliosis, which offers some important insights into their potential role in pre-cancer [70]. There is growing evidence that GSCs are responsible for glioma formation and ongoing growth [71, 72]. Studies of Song Chen et al. showed that S100A9 is highly expressed in GSCs with grade dependence and S100A9 regulates GSCs proliferation both in vitro and in vivo [73]. Altogether, S100A8 and S100A9 play a significant role in proliferation, invasion, migration as well as glioma stem cell stemness via multiple target proteins and signal pathways. Detecting biomarkers of S100A8 and S100A9 in serum and tissue may be a diagnostic and prognostic maker.
S100B
S100B, a Ca2+ binding peptide with a molecular weight of 20 kDA, existing as a homodimer composed of two beta subunits (EF-hand motifits). It is produced primarily by the astrocytes or spilled from damaged astrocytic cells and enter the bloodstream or extracellular space [5, 74]. In vitro and in vivo experiments has been demonstrated it is involved in regulating cellular activities such as metabolism, motility and proliferation [75]. S100B protein performs important functions in central nervous system (CNS) and is concentration-dependent. At low concentrations, it appears to exert neuroprotective effects against oxidative stress, but at high concentrations it produces neurodegenerative or apoptosis-inducing effects by increasing the expression of pro-inflammatory cytokines [26, 76, 77]. S100B also over-expressed in tumors and modulated tumorigenesis via multiple signal pathways. For instance, S100B is elevated in primary malignant melanoma and interacts directly with p53, which are likely promoting tumor progression by excessive downregulating TP53 levels and activity [78]. Additionally, S100B has been considered to contribute to tumorigenesis by regulate cell proliferation and differentiation by activating the mitogenic kinases Ndr [79] and Akt (protein kinase B) [80].
S100B is actively secreted by astrocytes or spilled from damaged astrocytic cells and could release into blood when the blood-brain barrier (BBB) is destroyed. Therefore, elevated serum level of S100B is a suggested marker in numerous nervous system diseases [81-83]. Recently, several studies have indicated that serum levels of the S100B protein may be a valuable serum biomarker to predict the prognosis in glioma patients. The study by MAAIKE J. VOS et al. suggest that serum S100B might be a prognostic variable in cerebral glioma patients [84]. However, F. K. Holla et al reported that while serum S100B seemed to have no prognostic value in newly diagnosed glioma patients, it may be valuable in terms of survival prognosis in patients with recurrent glioma [85]. These findings are basically consistent with other studies [86, 87]. Tumor-associated macrophages(TAMs) is an important component of inflammatory cells in tumor microenvironment and various chemokine are involved in TAM trafficking, and have been implicated in various aspects of cancer such as cell growth, angiogenesis and immunosuppression [88]. A study demonstrated that over-expression of S100B in glioma promoted tumor growth by CCL2(C-C motif ligand 2) upregulation and TAM chemoattraction in murine models [89]. Further study has identified Duloxetine, an S100B inhibitor, is able to shift TAM polarization into pro-inflammatory subtypes, which suggested that it may have anti-tumor properties [90]. Furthermore, the S100B protein has been proposed to significantly contribute to glioma development by interacting with protein signaling pathways directly or indirectly. Flora Brozzi, et al. analyzed the effects of inhibition S100B expression in astrocytoma cell line GL15 and the Müller cell line MIO-M1 by knockdown S100B gene expression with small interference RNA technique, these results suggest that S100B might involve in the regulation of cell morphology, differentiation and migration through src-dependent activation of PI3K [27]. Leying Zhang, et al. evaluated the effect of S100B-RAGE function on macrophages/microglia function in a murine glioma model, which found that glioma-mediated activation of STAT3 (signal transduction and activators of transcription 3) in macrophages/microglia might partly occur by the RAGE pathway and low levels of S100B induced STAT3 and inhibited microglia activation. Their findings suggest that the RAGE pathway may exert an important function in STAT3 induced glioma-associated macrophages/microglia, which may be mediated by S100B [91]. In addition, a study reported that the S100B protein could reduce tumor suppressor p53 DNA binding and transcriptional activity, and meanwhile indicated that since S100B levels are significantly elevated in glioma and astrocytoma, it may contribute to glioma and astrocytoma progression by inhibiting p53 functions [92].
S100P
S100P is a 95-amino-acid protein which was first purified and characterized from the placenta by Becker et al. in 1992. The “P” in its name indicates that it was purified first from placenta [93]. There is increasing evidence suggesting that the deregulated expression of S100P associated with the tumor growth, progression and metastasis of various types of human cancer [94], such as breast [95], nasopharyngeal carcinoma [96] and pancreatic cancer [97]. Evidence has been shown that S100P protein could mediate these processes by binding of Ca2+ ions, receptor for advanced glycation end products, cytoskeletal protein ezrin, calcyclin-binding protein/Siah-1-interacting protein and cathepsin D. Additionally, S100P could be applied as diagnostic marker, therapy target and prognostic/predictive indicator in a variety of different tumor types [94, 98].
Concerning to glioma, a study about immunocytochemical comparison of S100P, glial fibrillary acidic protein and vimentin in human glial tumors found that S100P was positive in most astroglial tumors and half of the oligodendrogliomas [99]. Another study by Jennifer Nicole Sims et al. demonstrated that silence the expression of S100P dramatically inhibited cell proliferation, migration, invasion and anchorage independent growth in glioblastoma cells. At the same time, this study observed reduced cell migration, spheroid formation and expansion treated with di-ethylhexylphthalate (the most well-known phthalate) following S100P knockdown. Which suggested that S100P may be a potential therapeutic target and can be used as a biomarker for drug response [100]. However, there have been few studies on S100P in glioma and the functional role of S100P and mechanisms in glioma has not been clearly elucidated until now.
Other S100 proteins (S100A13, S100A16 and S100A11)
To our knowledge, these three proteins have not been extensively studied in glioma. One study investigated the relationship between the expression of S100A13 in human astroglioma in relation to tumor grade and vascularization, which results indicated that S100A13 is overexpressed in human high grade astrocytic gliomas and correlates with tumor grading and microvessel density [101]. S100A16 is an astrocyte specific protein upregulate in tumors of different origins [102], Study by Szeliga M et al. found that transfection with liver-type glutaminase (LGA) cDNA increased the expression of LGA mRNA and protein and the ability of the cells to degrade glutamine, which result in reduction of survival, migration and proliferation of T98G glioma cells. And microarray analysis found decreased expression of S100A16 deserves attention in the context of LGA-induced phenotypic alterations [103]. S100A11, also named S100C or calgizzarin, was found that played a significant role in GBM [104]. Study by Tu et al. demonstrated that S100A11 plays key role in proliferation, EMT, migration, invasion and neurosphere formation in GBM cells and associated with poor survival of GBM patients [105]. Another study by Yin-Hsun Feng et al found that Allopregnanolone could suppress GBM cell survival by decreasing DPYSL3 (dihydropyrimidinase-like-3)/S100A11 expression and inducing DNA damage [106].
Conclusion and perspective
S100 proteins are frequently expression in gliomas and their dysregulated expression are closely associated with tumor progression, diagnosis and prognosis. In this review, we summarized current findings and progresses about S100 proteins in gliomas that have contributed to our understanding of the relationship between S100 proteins and gliomas. There is a considerable amount of literature on the contributions of S100 proteins to tumor progression, diagnosis and prognosis in glioma and its subtypes. However, the molecular mechanisms of S100 protein including S100A6, S100P, S100A13 and S100A16 is still unclear at present. As the research moves along, an increasing number of researches may reveal the underlying mechanisms S100 proteins in the progression of glioma and S100 protein will also act as an important prognosis marker and therapeutic target gradually.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kligman D, Hilt DC. The S100 protein family. Trends in biochemical sciences. 1988;13:437-43
2. Marenholz I, Lovering RC, Heizmann CW. An update of the S100 nomenclature. Biochimica et biophysica acta. 2006;1763:1282-3
3. Moore BW. A soluble protein characteristic of the nervous system. Biochemical and biophysical research communications. 1965;19:739-44
4. Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. The Biochemical journal. 2006;396:201-14
5. Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. The international journal of biochemistry & cell biology. 2001;33:637-68
6. Kretsinger RH, Nockolds CE. Carp muscle calcium-binding protein. II. Structure determination and general description. The Journal of biological chemistry. 1973;248:3313-26
7. Donato R. S-100 proteins. Cell calcium. 1986;7:123-45
8. Zimmer DB, Cornwall EH, Landar A, Song W. The S100 protein family: history, function, and expression. Brain research bulletin. 1995;37:417-29
9. Yap KL, Ames JB, Swindells MB, Ikura M. Diversity of conformational states and changes within the EF-hand protein superfamily. Proteins. 1999;37:499-507
10. Schäfer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends in biochemical sciences. 1996;21:134-40
11. Hermann A, Donato R, Weiger TM, Chazin WJ. S100 calcium binding proteins and ion channels. Frontiers in pharmacology. 2012;3:67
12. Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ. et al. Functions of S100 proteins. Current molecular medicine. 2013;13:24-57
13. Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nature reviews Cancer. 2015;15:96-109
14. von Bauer R, Oikonomou D, Sulaj A, Mohammed S, Hotz-Wagenblatt A, Gröne HJ. et al. CD166/ALCAM mediates proinflammatory effects of S100B in delayed type hypersensitivity. Journal of immunology (Baltimore, Md: 1950). 2013;191:369-77
15. Dmytriyeva O, Pankratova S, Owczarek S, Sonn K, Soroka V, Ridley CM. et al. The metastasis-promoting S100A4 protein confers neuroprotection in brain injury. Nature communications. 2012;3:1197
16. Hibino T, Sakaguchi M, Miyamoto S, Yamamoto M, Motoyama A, Hosoi J. et al. S100A9 is a novel ligand of EMMPRIN that promotes melanoma metastasis. Cancer research. 2013;73:172-83
17. Hankins JL, Ward KE, Linton SS, Barth BM, Stahelin RV, Fox TE. et al. Ceramide 1-phosphate mediates endothelial cell invasion via the annexin a2-p11 heterotetrameric protein complex. The Journal of biological chemistry. 2013;288:19726-38
18. Bydoun M, Sterea A, Liptay H, Uzans A, Huang WY, Rodrigues GJ. et al. S100A10, a novel biomarker in pancreatic ductal adenocarcinoma. Molecular oncology. 2018;12:1895-916
19. Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Molecular cancer research: MCR. 2011;9:133-48
20. Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. Journal of immunology (Baltimore, Md: 1950). 2008;181:4666-75
21. Cahill D, Turcan S. Origin of Gliomas. Seminars in neurology. 2018;38:5-10
22. Perry A, Wesseling P. Histologic classification of gliomas. Handbook of clinical neurology. 2016;134:71-95
23. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P. et al. An integrated genomic analysis of human glioblastoma multiforme. Science (New York, NY). 2008;321:1807-12
24. Takenaga K, Kozlova EN. Role of intracellular S100A4 for migration of rat astrocytes. Glia. 2006;53:313-21
25. Aguilar-Morante D, Morales-Garcia JA, Santos A, Perez-Castillo A. CCAAT/enhancer binding protein β induces motility and invasion of glioblastoma cells through transcriptional regulation of the calcium binding protein S100A4. Oncotarget. 2015;6:4369-84
26. Hu J, Van Eldik LJ. S100 beta induces apoptotic cell death in cultured astrocytes via a nitric oxide-dependent pathway. Biochimica et biophysica acta. 1996;1313:239-45
27. Brozzi F, Arcuri C, Giambanco I, Donato R. S100B Protein Regulates Astrocyte Shape and Migration via Interaction with Src Kinase: IMPLICATIONS FOR ASTROCYTE DEVELOPMENT, ACTIVATION, AND TUMOR GROWTH. The Journal of biological chemistry. 2009;284:8797-811
28. Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. The Journal of biological chemistry. 2006;281:677-80
29. Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. The American journal of pathology. 2010;176:528-35
30. Ismail TM, Fernig DG, Rudland PS, Terry CJ, Wang G, Barraclough R. The basic C-terminal amino acids of calcium-binding protein S100A4 promote metastasis. Carcinogenesis. 2008;29:2259-66
31. Lee WY, Su WC, Lin PW, Guo HR, Chang TW, Chen HH. Expression of S100A4 and Met: potential predictors for metastasis and survival in early-stage breast cancer. Oncology. 2004;66:429-38
32. Kimura K, Endo Y, Yonemura Y, Heizmann CW, Schafer BW, Watanabe Y. et al. Clinical significance of S100A4 and E-cadherin-related adhesion molecules in non-small cell lung cancer. International journal of oncology. 2000;16:1125-31
33. Hernan R, Fasheh R, Calabrese C, Frank AJ, Maclean KH, Allard D. et al. ERBB2 up-regulates S100A4 and several other prometastatic genes in medulloblastoma. Cancer research. 2003;63:140-8
34. Lo JF, Yu CC, Chiou SH, Huang CY, Jan CI, Lin SC. et al. The epithelial-mesenchymal transition mediator S100A4 maintains cancer-initiating cells in head and neck cancers. Cancer research. 2011;71:1912-23
35. Takenaga K, Nygren J, Zelenina M, Ohira M, Iuchi T, Lukanidin E. et al. Modified expression of Mts1/S100A4 protein in C6 glioma cells or surrounding astrocytes affects migration of tumor cells in vitro and in vivo. Neurobiology of disease. 2007;25:455-63
36. Chow KH, Park HJ, George J, Yamamoto K, Gallup AD, Graber JH. et al. S100A4 Is a Biomarker and Regulator of Glioma Stem Cells That Is Critical for Mesenchymal Transition in Glioblastoma. Cancer research. 2017;77:5360-73
37. Liang J, Piao Y, Holmes L, Fuller GN, Henry V, Tiao N. et al. Neutrophils promote the malignant glioma phenotype through S100A4. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20:187-98
38. Leśniak W, Słomnicki Ł P, Filipek A. S100A6 - new facts and features. Biochemical and biophysical research communications. 2009;390:1087-92
39. Donato R, Sorci G, Giambanco I. S100A6 protein: functional roles. Cellular and molecular life sciences: CMLS. 2017;74:2749-60
40. De Petris L, Orre LM, Kanter L, Pernemalm M, Koyi H, Lewensohn R. et al. Tumor expression of S100A6 correlates with survival of patients with stage I non-small-cell lung cancer. Lung cancer (Amsterdam, Netherlands). 2009;63:410-7
41. Yang YQ, Zhang LJ, Dong H, Jiang CL, Zhu ZG, Wu JX. et al. Upregulated expression of S100A6 in human gastric cancer. Journal of digestive diseases. 2007;8:186-93
42. Vimalachandran D, Greenhalf W, Thompson C, Lüttges J, Prime W, Campbell F. et al. High nuclear S100A6 (Calcyclin) is significantly associated with poor survival in pancreatic cancer patients. Cancer research. 2005;65:3218-25
43. Joo JH, Yoon SY, Kim JH, Paik SG, Min SR, Lim JS. et al. S100A6 (calcyclin) enhances the sensitivity to apoptosis via the upregulation of caspase-3 activity in Hep3B cells. Journal of cellular biochemistry. 2008;103:1183-97
44. Li Z, Tang M, Ling B, Liu S, Zheng Y, Nie C. et al. Increased expression of S100A6 promotes cell proliferation and migration in human hepatocellular carcinoma. Journal of molecular medicine (Berlin, Germany). 2014;92:291-303
45. Filipek A, Leśniak W. Current view on cellular function of S100A6 and its ligands, CacyBP/SIP and Sgt1. Postepy biochemii. 2018;64:242-52
46. Duan L, Wu R, Zou Z, Wang H, Ye L, Li H. et al. S100A6 stimulates proliferation and migration of colorectal carcinoma cells through activation of the MAPK pathways. International journal of oncology. 2014;44:781-90
47. Li A, Shi D, Xu B, Wang J, Tang YL, Xiao W. et al. S100A6 promotes cell proliferation in human nasopharyngeal carcinoma via the p38/MAPK signaling pathway. Molecular carcinogenesis. 2017;56:972-84
48. Camby I, Nagy N, Lopes MB, Schäfer BW, Maurage CA, Ruchoux MM. et al. Supratentorial pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas and glioblastomas are characterized by a differential expression of S100 proteins. Brain pathology (Zurich, Switzerland). 1999;9:1-19
49. Camby I, Lefranc F, Titeca G, Neuci S, Fastrez M, Dedecken L. et al. Differential expression of S100 calcium-binding proteins characterizes distinct clinical entities in both WHO grade II and III astrocytic tumours. Neuropathology and applied neurobiology. 2000;26:76-90
50. Rand V, Prebble E, Ridley L, Howard M, Wei W, Brundler MA. et al. Investigation of chromosome 1q reveals differential expression of members of the S100 family in clinical subgroups of intracranial paediatric ependymoma. British journal of cancer. 2008;99:1136-43
51. Lindsey JC, Lusher ME, Anderton JA, Gilbertson RJ, Ellison DW, Clifford SC. Epigenetic deregulation of multiple S100 gene family members by differential hypomethylation and hypermethylation events in medulloblastoma. British journal of cancer. 2007;97:267-74
52. Kucharczak J, Pannequin J, Camby I, Decaestecker C, Kiss R, Martinez J. Gastrin induces over-expression of genes involved in human U373 glioblastoma cell migration. Oncogene. 2001;20:7021-8
53. Abtin A, Eckhart L, Gläser R, Gmeiner R, Mildner M, Tschachler E. The antimicrobial heterodimer S100A8/S100A9 (calprotectin) is upregulated by bacterial flagellin in human epidermal keratinocytes. The Journal of investigative dermatology. 2010;130:2423-30
54. Strupat K, Rogniaux H, Van Dorsselaer A, Roth J, Vogl T. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis. Journal of the American Society for Mass Spectrometry. 2000;11:780-8
55. Pröpper C, Huang X, Roth J, Sorg C, Nacken W. Analysis of the MRP8-MRP14 protein-protein interaction by the two-hybrid system suggests a prominent role of the C-terminal domain of S100 proteins in dimer formation. The Journal of biological chemistry. 1999;274:183-8
56. Gebhardt C, Németh J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochemical pharmacology. 2006;72:1622-31
57. Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in Inflammation. Frontiers in immunology. 2018;9:1298
58. Hermani A, De Servi B, Medunjanin S, Tessier PA, Mayer D. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Experimental cell research. 2006;312:184-97
59. Källberg E, Vogl T, Liberg D, Olsson A, Björk P, Wikström P. et al. S100A9 interaction with TLR4 promotes tumor growth. PloS one. 2012;7:e34207
60. Duan L, Wu R, Ye L, Wang H, Yang X, Zhang Y. et al. S100A8 and S100A9 are associated with colorectal carcinoma progression and contribute to colorectal carcinoma cell survival and migration via Wnt/β-catenin pathway. PloS one. 2013;8:e62092
61. Cross SS, Hamdy FC, Deloulme JC, Rehman I. Expression of S100 proteins in normal human tissues and common cancers using tissue microarrays: S100A6, S100A8, S100A9 and S100A11 are all overexpressed in common cancers. Histopathology. 2005;46:256-69
62. Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature cell biology. 2006;8:1369-75
63. Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K. et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nature cell biology. 2008;10:1349-55
64. Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM. et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. The Journal of experimental medicine. 2008;205:2235-49
65. Kočevar N, Hudler P, Komel R. The progress of proteomic approaches in searching for cancer biomarkers. New biotechnology. 2013;30:319-26
66. Arora A, Patil V, Kundu P, Kondaiah P, Hegde AS, Arivazhagan A. et al. Serum biomarkers identification by iTRAQ and verification by MRM: S100A8/S100A9 levels predict tumor-stroma involvement and prognosis in Glioblastoma. Scientific reports. 2019;9:2749
67. Gielen PR, Schulte BM, Kers-Rebel ED, Verrijp K, Bossman SA, Ter Laan M. et al. Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients with glioblastoma highly express S100A8/9 and arginase and suppress T cell function. Neuro-oncology. 2016;18:1253-64
68. Gautam P, Nair SC, Gupta MK, Sharma R, Polisetty RV, Uppin MS. et al. Proteins with altered levels in plasma from glioblastoma patients as revealed by iTRAQ-based quantitative proteomic analysis. PloS one. 2012;7:e46153
69. Xiong J, Wang T, Tang H, Lv Z, Liang P. Circular RNA circMAN2B2 facilitates glioma progression by regulating the miR-1205/S100A8 axis. Journal of cellular physiology. 2019;234:22996-3004
70. Ryu MJ, Liu Y, Zhong X, Du J, Peterson N, Kong G. et al. Oncogenic Kras expression in postmitotic neurons leads to S100A8-S100A9 protein overexpression and gliosis. The Journal of biological chemistry. 2012;287:22948-58
71. Rich JN, Eyler CE. Cancer stem cells in brain tumor biology. Cold Spring Harbor symposia on quantitative biology. 2008;73:411-20
72. Sharifzad F, Ghavami S, Verdi J, Mardpour S, Mollapour Sisakht M, Azizi Z. et al. Glioblastoma cancer stem cell biology: Potential theranostic targets. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2019;42:35-45
73. Chen S, Zhao H, Deng J, Liao P, Xu Z, Cheng Y. Comparative proteomics of glioma stem cells and differentiated tumor cells identifies S100A9 as a potential therapeutic target. Journal of cellular biochemistry. 2013;114:2795-808
74. Michetti F, D'Ambrosi N, Toesca A, Puglisi MA, Serrano A, Marchese E. et al. The S100B story: from biomarker to active factor in neural injury. Journal of neurochemistry. 2019;148:168-87
75. Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochemical and biophysical research communications. 2004;322:1111-22
76. Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microscopy research and technique. 2003;60:614-32
77. Mrak RE, Griffinbc WS. The role of activated astrocytes and of the neurotrophic cytokine S100B in the pathogenesis of Alzheimer's disease. Neurobiology of aging. 2001;22:915-22
78. Lin J, Yang Q, Yan Z, Markowitz J, Wilder PT, Carrier F. et al. Inhibiting S100B restores p53 levels in primary malignant melanoma cancer cells. The Journal of biological chemistry. 2004;279:34071-7
79. Millward TA, Heizmann CW, Schäfer BW, Hemmings BA. Calcium regulation of Ndr protein kinase mediated by S100 calcium-binding proteins. The EMBO journal. 1998;17:5913-22
80. Arcuri C, Bianchi R, Brozzi F, Donato R. S100B increases proliferation in PC12 neuronal cells and reduces their responsiveness to nerve growth factor via Akt activation. The Journal of biological chemistry. 2005;280:4402-14
81. Kapural M, Krizanac-Bengez L, Barnett G, Perl J, Masaryk T, Apollo D. et al. Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain research. 2002;940:102-4
82. Steiner J, Bogerts B, Schroeter ML, Bernstein HG. S100B protein in neurodegenerative disorders. Clinical chemistry and laboratory medicine. 2011;49:409-24
83. Kleindienst A, Ross Bullock M. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. Journal of neurotrauma. 2006;23:1185-200
84. Vos MJ, Postma TJ, Martens F, Uitdehaag BM, Blankenstein MA, Vandertop WP. et al. Serum levels of S-100B protein and neuron-specific enolase in glioma patients: a pilot study. Anticancer research. 2004;24:2511-4
85. Holla FK, Postma TJ, Blankenstein MA, van Mierlo TJM, Vos MJ, Sizoo EM. et al. Prognostic value of the S100B protein in newly diagnosed and recurrent glioma patients: a serial analysis. Journal of neuro-oncology. 2016;129:525-32
86. Ilhan-Mutlu A, Wagner L, Widhalm G, Wöhrer A, Bartsch S, Czech T. et al. Exploratory investigation of eight circulating plasma markers in brain tumor patients. Neurosurgical review. 2013;36:45-55 discussion -6
87. Rajendra A, Spinella PC, Drott HR, Dominguez TE, Sutton L, Helfaer M. S-100beta protein-serum levels in children with brain neoplasms and its potential as a tumor marker. Journal of neuro-oncology. 2004;67:345-9
88. Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Current opinion in immunology. 2010;22:231-7
89. Wang H, Zhang L, Zhang IY, Chen X, Da Fonseca A, Wu S. et al. S100B promotes glioma growth through chemoattraction of myeloid-derived macrophages. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:3764-75
90. Gao H, Zhang IY, Zhang L, Song Y, Liu S, Ren H. et al. S100B suppression alters polarization of infiltrating myeloid-derived cells in gliomas and inhibits tumor growth. Cancer letters. 2018;439:91-100
91. Zhang L, Liu W, Alizadeh D, Zhao D, Farrukh O, Lin J. et al. S100B attenuates microglia activation in gliomas: possible role of STAT3 pathway. Glia. 2011;59:486-98
92. Lin J, Blake M, Tang C, Zimmer D, Rustandi RR, Weber DJ. et al. Inhibition of p53 transcriptional activity by the S100B calcium-binding protein. The Journal of biological chemistry. 2001;276:35037-41
93. Becker T, Gerke V, Kube E, Weber K. S100P, a novel Ca(2+)-binding protein from human placenta. cDNA cloning, recombinant protein expression and Ca2+ binding properties. European journal of biochemistry. 1992;207:541-7
94. Arumugam T, Logsdon CD. S100P: a novel therapeutic target for cancer. Amino acids. 2011;41:893-9
95. Kikuchi K, McNamara KM, Miki Y, Iwabuchi E, Kanai A, Miyashita M. et al. S100P and Ezrin promote trans-endothelial migration of triple negative breast cancer cells. Cellular oncology (Dordrecht). 2019;42:67-80
96. Liu Y, Wang C, Shan X, Wu J, Liu H, Liu H. et al. S100P is associated with proliferation and migration in nasopharyngeal carcinoma. Oncology letters. 2017;14:525-32
97. Nakayama H, Ohuchida K, Yonenaga A, Sagara A, Ando Y, Kibe S. et al. S100P regulates the collective invasion of pancreatic cancer cells into the lymphatic endothelial monolayer. International journal of oncology. 2019;55:211-22
98. Jiang H, Hu H, Tong X, Jiang Q, Zhu H, Zhang S. Calcium-binding protein S100P and cancer: mechanisms and clinical relevance. Journal of cancer research and clinical oncology. 2012;138:1-9
99. Nakopoulou L, Kerezoudi E, Thomaides T, Litsios B. An immunocytochemical comparison of glial fibrillary acidic protein, S-100p and vimentin in human glial tumors. Journal of neuro-oncology. 1990;8:33-40
100. Sims JN, Graham B, Pacurari M, Leggett SS, Tchounwou PB, Ndebele K. Di-ethylhexylphthalate (DEHP) modulates cell invasion, migration and anchorage independent growth through targeting S100P in LN-229 glioblastoma cells. International journal of environmental research and public health. 2014;11:5006-19
101. Landriscina M, Schinzari G, Di Leonardo G, Quirino M, Cassano A, D'Argento E. et al. S100A13, a new marker of angiogenesis in human astrocytic gliomas. Journal of neuro-oncology. 2006;80:251-9
102. Marenholz I, Heizmann CW. S100A16, a ubiquitously expressed EF-hand protein which is up-regulated in tumors. Biochemical and biophysical research communications. 2004;313:237-44
103. Szeliga M, Obara-Michlewska M, Matyja E, Łazarczyk M, Lobo C, Hilgier W. et al. Transfection with liver-type glutaminase cDNA alters gene expression and reduces survival, migration and proliferation of T98G glioma cells. Glia. 2009;57:1014-23
104. He H, Li J, Weng S, Li M, Yu Y. S100A11: diverse function and pathology corresponding to different target proteins. Cell Biochem Biophys. 2009;55:117-26
105. Tu Y, Xie P, Du X, Fan L, Bao Z, Sun G. et al. S100A11 functions as novel oncogene in glioblastoma via S100A11/ANXA2/NF-κB positive feedback loop. J Cell Mol Med. 2019;23:6907-18
106. Matsunuma R, Chan DW, Kim BJ, Singh P, Han A, Saltzman AB. et al. DPYSL3 modulates mitosis, migration, and epithelial-to-mesenchymal transition in claudin-low breast cancer. Proc Natl Acad Sci U S A. 2018;115:E11978-e87
Author contact
![]() Corresponding authors: Xingliang Dai, the First Affiliated Hospital of Anhui Medical University, Hefei, 230022, China. E-mail: daixingliangedu.cn; Hongwei Cheng, the First Affiliated Hospital of Anhui Medical University, Hefei, 230022, China. E-mail: hongwei.chengedu.cn.
Corresponding authors: Xingliang Dai, the First Affiliated Hospital of Anhui Medical University, Hefei, 230022, China. E-mail: daixingliangedu.cn; Hongwei Cheng, the First Affiliated Hospital of Anhui Medical University, Hefei, 230022, China. E-mail: hongwei.chengedu.cn.

 Global reach, higher impact
Global reach, higher impact