3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(10):3013-3021. doi:10.7150/jca.73112 This issue Cite
Research Paper
Down-regulation of MSMO1 promotes the development and progression of pancreatic cancer
1. Department of Gastrointestinal Surgery, The First Hospital of China Medical University, China.
2. Department of General Surgery, The People's Hospital of Liaoning Province, Shenyang, China.
Received 2022-3-22; Accepted 2022-6-14; Published 2022-8-1
Abstract
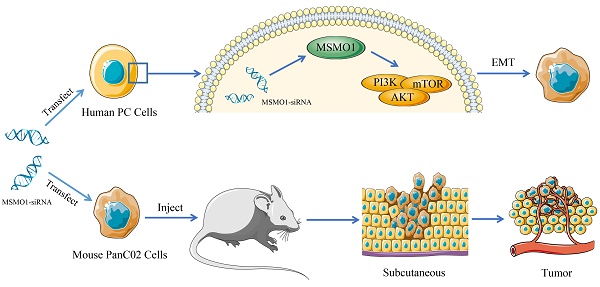
Background: Methylsterol monooxygenase 1 (MSMO1), as a completely unique tumor biomarker, plays a vital role in the malignant progression of various cancer. Until now, the potential function and pathway of MSMO1 in the development of pancreatic cancer (PC) has not been explored yet, to our knowledge.
Methods: We systematically explored the detail function of MSMO1 in Epithelial-mesenchymal transition (EMT) and cell proliferation of PC in vitro and in vivo.
Results: MSMO1 expression was much lower in PC tissues than that in paired normal pancreas. MSMO1 positive expression was negatively associated with T stage, lymph node metastasis and vascular permeation of PC patients. Meanwhile, positive MSMO1 expression indicated a significantly better prognosis and an independent favorable prognostic factor. MSMO1 silencing promoted cell invasion and migration via activating EMT and PI3K-AKT-mTOR pathway [p-PI3K (Tyr458), p-AKT (Ser473) and p-mTOR (Ser2448)] in Capan-2, Panc-1 and SW1990 cells. In vivo, subcutaneous tumor size was enhanced by MSMO1 silencing following with the consistent change of EMT and PI3K/AKT signaling shown in vitro. The motivation of EMT and PI3K-AKT-mTOR pathway was also demonstrated in MSMO1 silencing mouse PANC02 cells.
Conclusion: Down-regulation of MSMO1 in PC was associated with advanced progression and poor prognosis of PC patients. MSMO1 acts as a tumor suppressor via inhibiting the aggressive malignant biology of PC accompanying with regulating EMT and PI3K/AKT signaling.
Keywords: MSMO1, Pancreatic Cancer, EMT, PI3K-AKT-mTOR, Progression
Introduction
Pancreatic cancer (PC) is one of the most lethal tumors that attributes to aggressive malignant biological behavior [1]. The 5-year survival rate of PC keeps going down and the incidence rate remains continuously rising [2]. Until 2030, PC is forecast for the second leading cause of death associated with cancer in the world [3]. It is urgent to discover the new biomarkers for targeting the malignant biology for PC patients. Epithelial-mesenchymal transition (EMT) is a cellular process initiated from cellular micro environment following the acquisition of mesenchymal phenotypes and the repression of epithelial features, which finally contributes to the cell invasion and metastasis of cancer, including PC [4, 5]. The key characteristic of EMT is the deficiency of epithelial markers, such as E-cadherin and the gain of interstitial markers, such as Vimentin and MMPs [6]. Increasing evidence confirms the significant role of PI3K-AKT-mTOR signaling pathway and it's downstream targets (p-AKT, p-PI3K and p-mTOR) in regulating EMT process [7, 8]. Methylsterol monooxygenase 1 (MSMO1), as a synonym for sterol-C4-methyl oxidase analog (SC4MOL), displays critical function in the normal synthesis of cholesterol [9]. Recently, MSMO1 has been reported to promote the development of various cancers including liver [10], breast [11], and oligodendroglioma [12]. However, the mechanism and function of MSMO1, especially in PC, is still unknown, to our knowledge. In current study, we systematically investigated the potential role and mechanism of MSMO1 in the progression of PC in vitro and vivo.
Materials and methods
Cell Lines and Culture
Capan-2, Panc-1, and SW1990 cell lines were offered by General Surgery Laboratory of China Medical University (Shenyang, China) and Panc02 cell lines was obtained from Cell Biology Institute of China Medical University. All cell lines were cultured in temperate situation according to the recommendation from ATCC, such as suitable culture medium within one percent penicillin-streptomycin combination (HanHeng, China) added ten percent fetal calf serum (Gibco Invitrogen, Carlsbad, CA) and maintained in 37 °C humidified incubators filled with five percent CO2. All cell lines were detected for mycoplasma free before experiments.
Tissue Samples
92 pairs of fresh pancreatic cancer samples and paired adjacent normal pancreatic tissues were collected from surgical treatment patients at the Gastrointestinal Surgery Department of the First Hospital of China Medical University and the General Surgery Department of the People's Hospital of Liaoning Province between 2010/9 and 2020/12. All the patients were with complete follow up data and pathologically diagnosed as PC. 8th AJCC Cancer Staging Manual was utilized to classify staging standard. Meanwhile, 22 pairs of PC fresh samples were collected under -80 °C condition for qRT-PCR assays.
Experimental Animals
All C57BL/6 mice used in this study, weighting 20 to 25 g, were purchased from the company of Beijing Vital River Laboratory Animal Technology (Beijing, China). The animals were kept in the specific pathogens free (SPF) Animal Experimental Ministry of China Medical University with standard conditions (temperature 24±2 °C, humidity 55±10%, and 12-hour day/night cycle). Free access to rodent chow and water were applied for experimental animals. All animal experiments were performed strictly according to institutional regulations in facilities approved by the Animal Care Committee of China Medical University in accordance with Chinese government guidelines for animal experiments.
Immunohistochemistry
Paraffin embedded PC tissues fixed by neutral formaldehyde. Tissues were used to cut 4 µm slices and dewaxing. Antigen retrieval was next done for 3 minutes under high pressure. Three percent H2O2 and goat non-immune serum were utilized to block tissues for 30 minutes. Primary antibody incubated overnight with at 4 °C. Sections were incubated with the secondary antibody for 30 minutes at room temperature and then observed color reaction via using 3,3′ -diaminobenzidine (DAB). Immunohistochemistry (IHC) score was conducted as previously described [13]. Four grades: [0 (negative), 1 (weak), 2 (medium), and 3 (strong)] was described the positive staining intensity. The 5 grades: [0 (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%), and 4 (76-100%)] were used to calculate according to the stained positive areas. The final scores were calculated by three professional pathologists. The final score, ranging 0-12, was the multiplication of two grades and >6 was regarded as high expression.
Hematoxylin-Eosin Staining
Briefly, xylene I and II each for 10 minutes was used to dewax the slices. 100%, 90%, 80%, 70% alcohol for 5 minutes each, hematoxylin for 5 minutes, 5% acetic acid differentiation for 1 minute, tap water for 1 minute, eosin for 3 minutes, 70%, 80%, 90%, 100% alcohol each for 10 seconds, xylene I and II for 5 minutes.
EMT Construction
We transfected Capan-2, PANC-1 and SW1990 cell lines with negative control and MSMO1 siRNA. Recommended growth media (RPMI 1640 for Capan-2 and SW1990 cells, DMEM for Panc-1 cells) containing 1% FBS was used to better induce EMT phenotype in transfected cell lines. The EMT construction model was confirmed by the alteration involving EMT-like cell morphology (a spindle-shaped and fibroblast-like morphology), EMT enhanced cell motility, and the changes of EMT-related markers, which were regarded to reflect the EMT process.
Western Blot
RIPA lysis buffer containing 1% PMSF (Beyotime, China) was used to collect total protein from cell lines. 10% SDS-PAGE was used to separate protein and PVDF membranes were loaded with separate protein. All membranes were blocked with 5% degreasing milk and incubated with primary antibodies: MSMO1 (Abcam), E-cadherin (Abcam), β-catenin (Proteintech), Vimentin (Proteintech), AKT (Bimake), PI3K (Bimake), m-TOR (Bimake), p-AKT (Ser473) (Cell Signaling Technology), p-PI3K (Tyr458) (Cell Signaling Technology), p-mTOR (Ser2448) (Cell Signaling Technology), GAPDH (Proteintech) overnight at 4 °C. Next day, membranes were incubated with secondary antibodies for two hours. Finally, blots were detected by ECL kit (Beyotime, China). The experiments were done thrice.
Real-time Quantitative PCR
TRIZOL reagent (Takara, Japan) was used to extract total RNA from tissues samples, following the instructions. RNA level was maintained to the same level in each sample by using nucleotide test. GenePharma (Soochow, China) provided the primers for qRT-PCR. The primer sequences were manifested in Table 1. The total RNA and TaKaRa RNAiso Reagent (Takara, Japan) was used to synthesize cDNA. Finally, the QuantStudio 7 Pro Real-Time PCR System (Thermo Fisher Scientific, USA) was used to measure the mRNA expression of MSMO1 and GAPDH under the conditions as below: 95 °C for 30s, 45 cycles of 95 °C for 5s and 60 °C for 45s. The -ΔΔCt method with melt-curve dissociation was used to evaluate the quality of amplification products. The experiments were repeated three times.
The primer sequences
| Gene name | Primer sequences |
|---|---|
| MSMO1 | sense: 5' - TGCTTTGGTTGTGCAGTCAT‐3'; anti-sense: 5' - TTCCAAATGGAGCCTGAAAC-3' |
| GAPDH | sense: 5' - TGACTTCAACAGCGACACCCA-3'; anti-sense: 5' - CACCCTGTTGCTGTAGCCAAA-3' |
RNA Interference
The MSMO1 siRNA and negative control (NC) were designed by OriGene (OriGene Technologies, Inc. USA). The verified sequence (OPF) of MSMO1 siRNA were as followed: Sense: GAAGCCCUUUAUUUUCUUAUTT. Anti-sense: AUAAGAAAUAAAGGGCUUCTT. Capan-2, Panc-1, and SW1990 cells were transiently transfected with MSMO1 siRNA and NC for 24 hours with lipo3000 (Invitrogen, Carlsbad, CA, USA) following the instruction. In the result section, the silencing effect of MSMO1 was testified.
Cell Migration and Invasion Assays
Based on our previous study [14], for cell migration assay, 2×105 cells/ml of Capan-2 cells, 3*10^5 cells/ml of Panc-1 cells and 1.5×106 cells/ml of SW1990 cells were implanted into chamber inserts with serum-free growth media in 24-well plates within growth media containing 10% FBS at the bottom of each well as a stimulus after transfected for 24 hours. 24 hours later, the per-cooling methanol was used to fix the migrated cells and the Crystal Violet (Sigma) was used for dyeing. The invasion assay was similar to migration that the top of membrane coated with Matrigel (BD Biosciences, USA). A microscope (Nikon Microphot-FX, Japan) was used to calculate the final migrating and invading cells at 20× magnification in five random fields each chamber. Each independent experiment was performed thrice.
In vivo Cell-line-Derived Tumor Xenograft
To detect the function of MSMO1 in cell proliferation, total six C57B6 mice were used to construct subcutaneous tumor model by injecting PANC-02 cells resuspended in PBS which were transfected with MSMO1 siRNA and negative control respectively (1×106/ml, 100 ul). The selected mice are generally 4 weeks old and the middle of the armpit was chosen as the planting site. After injection, cotton swab was used to reduce bleeding and the overflow of the cell suspension from the injection site. Four weeks later, the mice were sacrificed and tumors were resected. The longest and shortest parts of the tumor are measured using a vernier caliper with the following formula: V=1/2*a*b2 (a is the long axis, b is the short axis). After the subcutaneous tumor is removed, a part of tumors was used for later extraction of protein and other was used for hematoxylin and eosin (HE) and IHC staining.
Statistical Analysis
Statistical analysis was emerged from SPSS 20.0 software (Chicago, IL, USA). Three independent experiments results were presented by mean ± SD. Discrepancises between two groups were compared through independent t test. MSMO1 and clinicopathological parameters were compared by chi-square test. To deal with survival curve, Kaplan-Meier method was utilized and significance was compared via log-rank test. Independent prognostic factors were evaluated by Cox's proportional hazards regression model. P<0.05 was considered as statistically significant.
Results
MSMO1 is Down-Regulated in PC and Associated with The Aggressive Clinical Stage and Prognosis of PC Patients
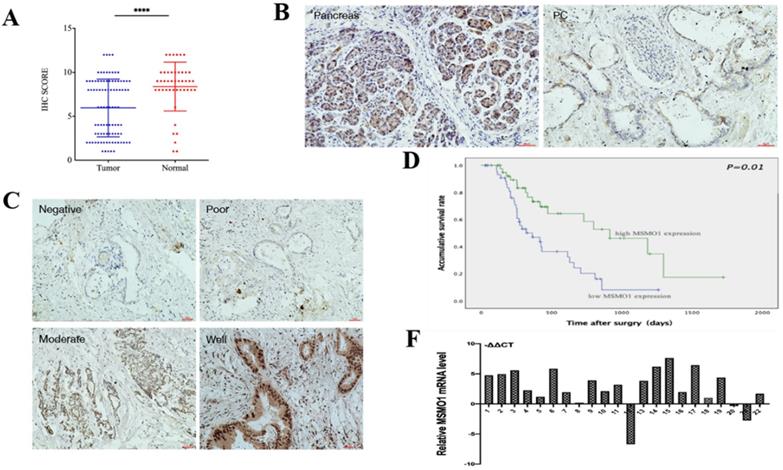
In our study, IHC showed MSMO1 expression in human PC tissues (43/92,46%) was lower compared with normal tissues (38/45, 84%) (P<0.0001) (Figure 1A). MSMO1 was mainly expressed in the cytoplasm with a small amount of nuclear expression in human PC tissues (Figure 1B). Meanwhile, As the degree of tumor differentiation enhancing, the expression of MSMO1 increases (Figure 1C). In current study, high MSMO1 expression was negatively relevant with T stage (P=0.025), lymph-node metastasis (P=0.046) and vascular permeation (P=0.009) of 92 PC patients (Table 2), patients with low MSMO1 expression had a poor prognosis shown by Kaplan-Meier curve (P=0.01) (Figure 1D). In addition, the low MSMO1 expression independently causes poor prognosis was observed by univariate and multivariate Cox's regression analysis results (Table 3). Compared with the PC tissues, higher mRNA expression of MSMO1 in normal pancreatic tissues were shown by qRT-PCR assay (P=0.009) (Figure 1F).
MSMO1 Inhibits Epithelial-Mesenchymal Transition, The Capacities of Cell Malignant Migration and Invasion in vitro
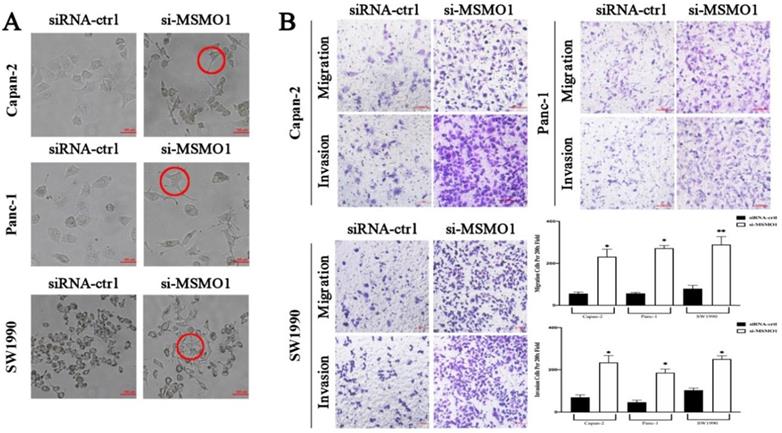
The essential key in cancer metastasis is EMT [15]. To verify the effect of MSMO1 in PC cell function, we transfected RNA interference or negative control (NC) in Capan-2, Panc-1 and SW1990 cells. In results, the EMT-like cell morphology was stimulated by MSMO1 down-regulation, the cells lost their epithelial features and presented a spindle/fibroblast-like morphology, compared with the NC group, (Figure 2A). As demonstrated in Transwell assays, the capacities of invasion and migration were significantly enhanced in MSMO1 siRNA transfected cells group in Capan-2, Panc-1 and SW1990 cells (Figure 2B).
MSMO1 Silencing Agitated EMT Activation via PI3K-AKT-MTOR Signal Pathway in PC Cells
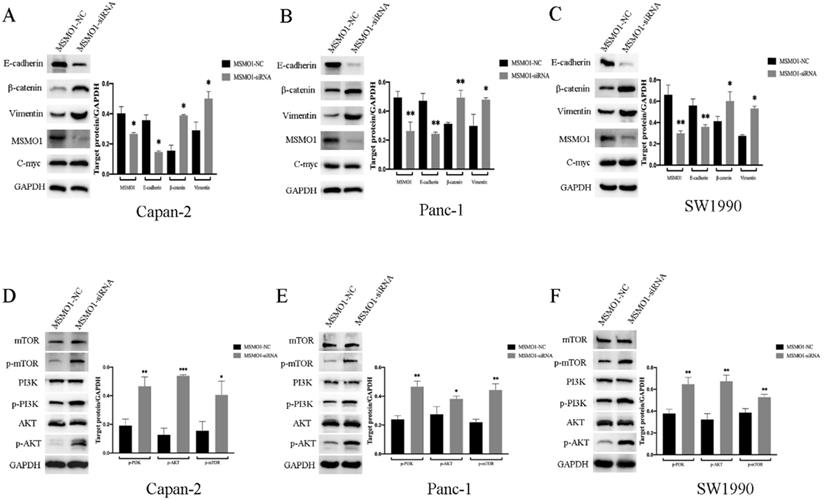
For the purpose of exploring the potential mechanism of MSMO1 in PC cell function. Capan-2 cells, Panc-1 cells and SW1990 cells was used for MSMO1 silencing construction, and protein was then extracted from transfected cells to conduct Western blot. We found MSMO1 silencing inhibited EMT related protein (E-cadherin) and enhanced others (β-catenin and Vimentin). Some EMT markers showed no change, such as C-myc (Figure 3A-C). Simultaneously, several proteins of PI3K-AKT-mTOR signal pathway [p-PI3K (Tyr458), p-AKT (Ser473) and p-mTOR (Ser2448)] was enhanced in MSMO1siRNA group (Figure 3D-F). Based on the above results, the EMT induction and PI3K-AKT-mTOR signal pathway were both stimulated by MSMO1 silencing in PC cells in vitro.
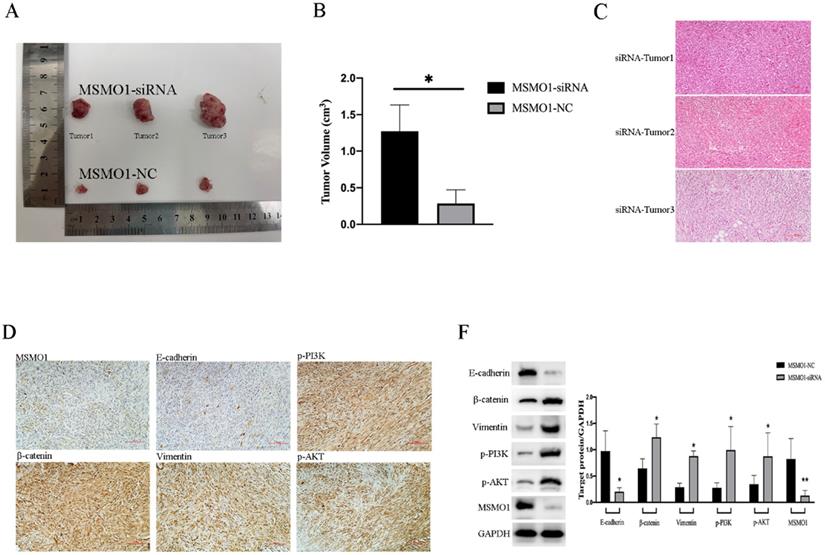
MSMO1 Down-Regulation Promotes Cell Proliferation of Pancreatic Tumors in vivo
We constructed the models of subcutaneous tumor by PANC02 cells which were transfected with MSMO1 siRNA and negative control (NC), respectively. Tumor volumes in MSMO1 siRNA group were much larger (P=0.0138) (Figure 4A, B). In histology, tumor tissues were observed by HE staining (Figure 4C), besides, MSMO1 and E-cadherin expression were decreased but β-catenin, Vimentin, p-PI3K (Tyr458) and p-AKT (Ser473) expression were increased in Tumor was further confirmed by IHC (Figure 4D). By western blot analysis, at protein level, Panc02 cell line exhibited E-cadherin expression was down-regulated, β-catenin, Vimentin, p-PI3K and p-AKT expression was up-regulated (Figure 4F).
The MSMO1 expression in PC tissues. (A) Immunohistochemistry was used to calculate the expression of MSMO1 in the cancer tissues and normal tissues. (B) MSMO1 expression in PC tissues and normal pancreatic tissues using IHC. (C) MSMO1 expression intensity in different Differentiation levels of PC tissues. (D) Kaplan-Meier survival curves in PC patients with different MSMO1 expression. (F) MSMO1 mRNA expression quantities in 22 pairs tissue samples via qRT-PCR. ****P < 0.0001 compared with the control.
In vitro, MSMO1 silencing promoted PC cells EMT, migration and invasion. (A) The EMT-like cell morphology of Capan-2, PANC-1 and SW1990 cells in MSMO1 siRNA and NC groups. (B) Migration and invasion in Capan-2, Panc-1, and SW1990 cells after MSMO1 silencing. Bars indicate means ± SD, *P < 0.05, **P < 0.01.
The Clinical Significance of MSMO1 Expression in Human PC Samples
| Characteristics | No. of Patients | MSMO1 expression | χ2 | |
|---|---|---|---|---|
| Low | High | |||
| Case | 92 | 49 | 43 | |
| Gender | .910 | |||
| Male | 54 | 33 | 21 | |
| Female | 38 | 16 | 22 | |
| Age (y) | 1.000 | |||
| ≤65 | 23 | 12 | 11 | |
| >65 | 69 | 37 | 32 | |
| Tumor location | .817 | |||
| Bead | 66 | 36 | 30 | |
| Body and tail | 26 | 13 | 13 | |
| Tumor size (cm) | .765 | |||
| <4 | 79 | 43 | 36 | |
| ≥4 | 13 | 6 | 7 | |
| Differentiation | .951 | |||
| Well | 30 | 16 | 14 | |
| Moderate | 57 | 30 | 27 | |
| Poor | 5 | 3 | 2 | |
| T stage | .025* | |||
| T1+T2 | 64 | 29 | 35 | |
| T3+T4 | 28 | 20 | 8 | |
| Lymph nodes metastasis | .046* | |||
| Negative | 63 | 29 | 34 | |
| Positive | 29 | 20 | 9 | |
| UICC staging | .231 | |||
| IA + IIA | 69 | 34 | 35 | |
| IIB + III + IV | 23 | 15 | 8 | |
| Vascular permeation | .009* | |||
| Negative | 59 | 25 | 34 | |
| Positive | 23 | 23 | 9 | |
| Neural permeation | .078 | |||
| Negative | 79 | 39 | 40 | |
| Positive | 13 | 10 | 3 | |
| Pretherapeutic CA19-9 level | .291 | |||
| <37 U/mL | 54 | 26 | 28 | |
| ≥37 U/mL | 38 | 23 | 15 | |
*P<0.05.
The Univariate and Mutivariate Analysis of Clinicopathological factors For Survival
| Characteristics | Median Survival (Days) | Univariate Analysis P (Log Rank) | Multivariate Analysis Hazard Ratio (95% CI) | P |
|---|---|---|---|---|
| Gender (Male /Female) | 708/432 | .910 | - | |
| Age (≤65 />65y) | 474/800 | 1.000 | - | |
| Tumor location (Bead/Body and tail) | 615/317 | .817 | - | |
| Tumor size (<4/≥4 cm) | 629/321 | .765 | - | |
| Differentiation (Well/Moderate/Poor) | 629/474/200 | .951 | - | |
| T stage (T1+T2/T3+T4) | 800/317 | .025 | 2.218 (1.120-4.393) | .022* |
| Lymph nodes metastasis (Negative/Positive) | 660/418 | .046 | 1.212 (0.637-2.305) | .588 |
| UICC staging (IA + IIA/ IIB + III + IV) | 708/275 | .291 | - | |
| Vascular permeation (Negative/Positive) | 184/76 | .009 | 1.223 (0.654-2.290) | .528 |
| Neural permeation (Negative/Positive) | 629/365 | .078 | - | |
| CA19-9 level (<37 U/mL/≥37 U/mL) | 423//615 | .291 | - | |
| MSMO1 expression (low/high) | 356/915 | .001 | 0.431 (0.200-0.931) | .032* |
*P<0.05.
Discussion
MSMO1, as a typical target in cholesterol biosynthesis, catalyzes the demethylation of C4-methylsterol in the cholesterol synthesis pathway and plays a key role in the normal synthesis of cholesterol [9, 16]. Recently, MSMO1 is considered as a novel and critical gene target in various solid cancers, including breast cancer [11], oligodendroglia cells [12], hypopharyngeal cancer [17], gastric cancer [18], uveal melanoma [19], liver cancer [20] and cervical squamous cell carcinoma [21]. Though the role of MSMO1 in PC radio-resistance has been reported in 2014, but the corresponding mechanism remains unclear [22]. In current study, MSMO1 is identified as a novel tumor suppressor in PC development involving a novel signaling axis of EMT and PI3K-AKT-mTOR pathway, which was not reported previously to our knowledge.
Several researches have implicated the clinical significance of MSMO1 expression in various cancers. In breast cancer patients, targeting MSMO1 with siRNA increases the resistance to endocrine therapy [11]. Down-regulation of MSMO1 was significantly associated with poor prognosis in liver cancer [20] and worse 5-years overall survival rates in gastric cancer [18]. Currently, we first found that MSMO1 expression in normal pancreatic tissues was much higher than that in paired PC tissues. Meanwhile, low expression of MSMO1 was associated with advanced clinical stage (T stage, vascular permeation, lymphatic metastasis and poor prognosis) of PC patients, which is consistently with previous studies. The close relationship of low MSMO1 expression with the advanced clinical stage of PC patients drives us to investigate the potential role of MSOM1 in PC development in vitro.
EMT (Epithelial-mesenchymal transition) is considered as the initiating factor that converts benign carcinoma to the malignance with the high metastatic growth dissemination involving various signal pathways [23, 24]. Our data implied a novel and specific role of MSMO1 in EMT here. EMT-like cell morphology and cell mobility were stimulated by MSMO1 silencing in vitro. Nowadays, the potential role of MSMO1 in cancer cells is still controversial. MSMO1 overexpression suppressed malignant cytological functional behavior in oligodendroglia cells [12]. The high propensity for metastatic spread of uveal melanoma is the results of the transformation of MSMO1 [19]. Bioinformatics analysis presents the same trend in hypopharyngeal cancer [17] and cervical squamous cell carcinoma [21]. However, MAGEA6 positively regulates MSMO1 and facilities the capacity of migration and invasion in esophageal cancer cells [25]. Due to the different kinds of cancer and the related cellular environment, the function and mechanism of MSMO1 would be different and even contrary in various cancers.
Discrepancy of EMT-induced targets and PI3K-AKT-mTOR signal pathway in vitro. (A) The protein level of MSMO1 and EMT features in Capna-2 cells. (B) The protein level of MSMO1 and EMT features in Panc-1 cells. (C) The protein level of MSMO1 and EMT features in SW1990 cells. (D) The changes of PI3K-AKT-mTOR signal pathway in Capan-2 cells. (E) The changes of PI3K-AKT-mTOR signal pathway in Panc-1 cells. (F) The changes of PI3K-AKT-mTOR signal pathway in SW1990 cells. Bars indicate means ± SD, *P < 0.05, **P < 0.01, ***P < 0.001.
MSMO1 silencing stimulated cell proliferation in subcutaneous tumor model in vivo. (A) Subcutaneous tumor in MSMO1 siRNA and NC group. (B) The tumor volumes between MSMO1 siRNA and NC groups were evaluated by statistical analysis. (C) HE staining for subcutaneous tumor in MSMO1 siRNA group. (D) Target protein expressed in MSMO1 siRNA group detected by IHC. (F) The protein levels of MSMO1, E-cadherin, β-catenin Vimentin, p-PI3K and p-AKT in MSMO1 siRNA and NC transfected PANC02 cells detected by western blot. Bars indicate ± SD. *P<0.05, **P<0.01.
Numerous evidences reveal E-cadherin is a critical EMT-related element [26]. As previously, Numb-PRRL amplifies a set of EMT activators in PC, including E-cad and Vimentin [27]. GATA6, the down-stream of Wnt-β-catenin signaling pathway, promotes EMT associated with E-cadherin downregulation [28]. At the same time, we found MSMO1 down-regulation inhibited E-cadherin but enhanced β-catenin and Vimentin expression. To this context, the down-regulation of MSMO1 provided a potential unique point to influence EMT.
PI3K-AKT pathway plays a significant role in the malignant cellular function and the initiation of EMT [29]. AKT regulates cell-cycle following the down-regulation of E-cadherin in EMT transmission [30, 31]. mTOR, as the downstream target of PI3K-AKT signal pathway, also relates to regulate EMT[32]. Besides, AKT is the key down-stream effector of MSMO1 and inter-crossing protein between EGFR and PI3K/AKT/mTOR signaling pathway [33]. However, MSMO1 has not been previously analyzed in the context of PI3K/AKT/mTOR signaling. In our study, WB shown that MSMO1 silencing increased p-PI3K (Tyr458), p-AKT (Ser473) and p-mTOR (Ser2448) expression in vitro. Thus, MSMO1 down-regulation facilitated EMT processing by mediating the PI3K/AKT/mTOR signaling pathway in PC, which is not reported yet. It is worthy noted that mTOR catalyze two multiprotein complexes, mTORC1 and mTORC2 [34]. mTORC1 promotes biosynthesis [35], while mTORC2 plays seminal functions in cell metabolism and survival through the p-AKT (Ser473) [36]. In current study, we did not distinguish these two multiprotein complexes and the mTOR complexes will be a worthwhile point for us to explore in the future. Finally, MSMO1 down-regulation promoted the formulation of subcutaneous tumors accompanied with the promotion of EMT and PI3K/AKT/mTOR signaling pathway in vivo.
In conclusion, down-regulation of MSMO1 takes an important part in the aggressive clinical stage of PC patients. MSMO1 mediates EMT through activating PI3K-AKT-mTOR pathway in vitro and in vivo, which facilitates a novel diagnostic and therapeutic facility for PC. However, the corresponding mechanism is not further investigated in current study, which would be investigated in future.
Abbreviations
MSMO1: Methylsterol monooxygenase 1; PC: Pancreatic cancer; EMT: Epithelial-mesenchymal transition; qRT-PCR: Quantitative real-time polymerase chain reaction; IHC: Immunohistochemistry; HE: Hematoxylin-Eosin Staining; RIPA: Radio Immunoprecipitation Assay; PMSF: Phenylmethylsulfonyl Fluoride; PVDF: Poly(vinylidene fluoride); ECL: Enhanced Chemiluminescence; FBS: Fetal Bovine Serum.
Acknowledgements
Thanks for the technical supports offered by Central Laboratory and General Surgery Laboratory from the First Hospital of China Medical University.
Funding
This work was supported by the Social Development Program from Shenyang Science and Technology Bureau, China (No. F20-205-4-033) and (No. F20-205-4-061).
Ethics statement
Human participants and animal study in this research was authorized by The Ethics Committee of The First Hospital of China Medical University (No. AF-SOP-07-1.1-01). Informed consent was accepted by each participating patient for this essay.
Author contributions
All authors contributed to conception and design, data, statistical analysis and interpretation; participated in drafting and revising the article; allowed to submit for publication and be responsible to all aspects of the work.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sheng W, Dong M, Wang G, Shi X, Gao W, Wang K. et al. The diversity between curatively resected pancreatic head and body-tail cancers based on the 8th edition of AJCC staging system: a multicenter cohort study. BMC cancer. 2019;19:981
2. Siegel R, Miller K, Fuchs H, Jemal A. Cancer Statistics, 2021. CA: a cancer journal for clinicians. 2021;71:7-33
3. Rahib L, Smith B, Aizenberg R, Rosenzweig A, Fleshman J, Matrisian L. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014;74:2913-21
4. Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M. et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nature reviews Molecular cell biology. 2020;21:341-52
5. Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu Y. et al. The epithelial to mesenchymal transition (EMT) and cancer stem cells: implication for treatment resistance in pancreatic cancer. Molecular cancer. 2017;16:52
6. Sheng W, Wang G, Tang J, Shi X, Cao R, Sun J. et al. Calreticulin promotes EMT in pancreatic cancer via mediating Ca dependent acute and chronic endoplasmic reticulum stress. Journal of experimental & clinical cancer research: CR. 2020;39:209
7. Rahmani F, Ziaeemehr A, Shahidsales S, Gharib M, Khazaei M, Ferns G. et al. Role of regulatory miRNAs of the PI3K/AKT/mTOR signaling in the pathogenesis of hepatocellular carcinoma. Journal of cellular physiology. 2020;235:4146-52
8. Tan A. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thoracic cancer. 2020;11:511-8
9. Kalay Yildizhan I, Gökpınar İli E, Onoufriadis A, Kocyigit P, Kesidou E, Simpson M. et al. New Homozygous Missense MSMO1 Mutation in Two Siblings with SC4MOL Deficiency Presenting with Psoriasiform Dermatitis. Cytogenetic and genome research. 2020;160:523-30
10. Xu P, Wu M, Chen H, Xu J, Wu M, Li M. et al. Bioinformatics analysis of hepatitis C virus genotype 2a-induced human hepatocellular carcinoma in Huh7 cells. OncoTargets and therapy. 2016;9:191-202
11. Simigdala N, Gao Q, Pancholi S, Roberg-Larsen H, Zvelebil M, Ribas R. et al. Cholesterol biosynthesis pathway as a novel mechanism of resistance to estrogen deprivation in estrogen receptor-positive breast cancer. Breast cancer research: BCR. 2016;18:58
12. He P, Sun L, Zhu D, Zhang H, Zhang L, Guo Y. et al. Knock-Down of Endogenous Bornavirus-Like Nucleoprotein 1 Inhibits Cell Growth and Induces Apoptosis in Human Oligodendroglia Cells. International journal of molecular sciences. 2016;17:435
13. Sheng W, Dong M, Chen C, Wang Z, Li Y, Wang K. et al. Cooperation of Musashi-2, Numb, MDM2, and P53 in drug resistance and malignant biology of pancreatic cancer. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2017;31:2429-38
14. Sheng W, Shi X, Lin Y, Tang J, Jia C, Cao R. et al. Musashi2 promotes EGF-induced EMT in pancreatic cancer via ZEB1-ERK/MAPK signaling. Journal of experimental & clinical cancer research: CR. 2020;39:16
15. Cao H, Xu E, Liu H, Wan L, Lai M. Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review. Pathology, research and practice. 2015;211:557-69
16. He M, Smith L, Chang R, Li X, Vockley J. The role of sterol-C4-methyl oxidase in epidermal biology. Biochimica et biophysica acta. 2014;1841:331-5
17. Xu C, Shi R, Chen D, Sun Y, Wu Q, Wang T. et al. Potential biomarkers for paclitaxel sensitivity in hypopharynx cancer cell. International journal of clinical and experimental pathology. 2013;6:2745-56
18. Chang W, Cheng W, Cheng B, Chen L, Ju L, Ou Y. et al. Mitochondrial Acetyl-CoA Synthetase 3 is Biosignature of Gastric Cancer Progression. Cancer medicine. 2018;7:1240-52
19. Ness C, Garred Ø, Eide N, Kumar T, Olstad O, Bærland T. et al. Multicellular tumor spheroids of human uveal melanoma induce genes associated with anoikis resistance, lipogenesis, and SSXs. Molecular vision. 2017;23:680-94
20. van der Meer D, Degenhardt T, Väisänen S, de Groot P, Heinäniemi M, de Vries S. et al. Profiling of promoter occupancy by PPARalpha in human hepatoma cells via ChIP-chip analysis. Nucleic acids research. 2010;38:2839-50
21. Zheng G, Wang Z, Fan Y, Wang T, Zhang L, Wang M. et al. The Clinical Significance and Immunization of MSMO1 in Cervical Squamous Cell Carcinoma Based on Bioinformatics Analysis. Frontiers in genetics. 2021;12:705851
22. Souchek J, Baine M, Lin C, Rachagani S, Gupta S, Kaur S. et al. Unbiased analysis of pancreatic cancer radiation resistance reveals cholesterol biosynthesis as a novel target for radiosensitisation. British journal of cancer. 2014;111:1139-49
23. Campbell K, Casanova J. A common framework for EMT and collective cell migration. Development (Cambridge, England). 2016;143:4291-300
24. Sheng W, Chen C, Dong M, Wang G, Zhou J, Song H. et al. Calreticulin promotes EGF-induced EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling pathway. Cell death & disease. 2017;8:e3147
25. Liu M, Li J, Wang Y, Ghaffar M, Yang Y, Wang M. et al. MAGEA6 positively regulates MSMO1 and promotes the migration and invasion of oesophageal cancer cells. Experimental and therapeutic medicine. 2022;23:204
26. Monteith G, Davis F, Roberts-Thomson S. Calcium channels and pumps in cancer: changes and consequences. The Journal of biological chemistry. 2012;287:31666-73
27. Sheng W, Tang J, Cao R, Shi X, Ma Y, Dong M. Numb-PRRL promotes TGF-β1- and EGF-induced epithelial-to-mesenchymal transition in pancreatic cancer. Cell death & disease. 2022;13:173
28. Tang J, Gao W, Liu G, Sheng W, Zhou J, Dong Q. et al. miR-944 Suppresses EGF-Induced EMT in Colorectal Cancer Cells by Directly Targeting GATA6. OncoTargets and therapy. 2021;14:2311-25
29. Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell adhesion & migration. 2015;9:317-24
30. Tang H, Massi D, Hemmings B, Mandalà M, Hu Z, Wicki A. et al. AKT-ions with a TWIST between EMT and MET. Oncotarget. 2016;7:62767-77
31. Ding Y, Zhou Z, Ha C, Zhang X, Pan S, He Z. et al. Alisertib, an Aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells. Drug design, development and therapy. 2015;9:425-64
32. Karimi Roshan M, Soltani A, Soleimani A, Rezaie Kahkhaie K, Afshari A, Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. 2019;165:229-34
33. Sukhanova A, Gorin A, Serebriiskii I, Gabitova L, Zheng H, Restifo D. et al. Targeting C4-demethylating genes in the cholesterol pathway sensitizes cancer cells to EGF receptor inhibitors via increased EGF receptor degradation. Cancer discovery. 2013;3:96-111
34. Mukhopadhyay S, Frias M, Chatterjee A, Yellen P, Foster D. The Enigma of Rapamycin Dosage. Molecular cancer therapeutics. 2016;15:347-53
35. Dibble C, Manning B. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nature cell biology. 2013;15:555-64
36. Sarbassov D, Guertin D, Ali S, Sabatini D. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science (New York, NY). 2005;307:1098-101
Author contact
![]() Corresponding author: Ming Dong, Department of Gastrointestinal Surgery, The First Hospital of China Medical University, 110001, Shenyang, China. E-mail: dongmingedu.cn; ORCID: 0000-0003-2685-1420.
Corresponding author: Ming Dong, Department of Gastrointestinal Surgery, The First Hospital of China Medical University, 110001, Shenyang, China. E-mail: dongmingedu.cn; ORCID: 0000-0003-2685-1420.

 Global reach, higher impact
Global reach, higher impact