3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(9):2922-2932. doi:10.7150/jca.74617 This issue Cite
Research Paper
Clinical outcomes of Atezolizumab Therapy for Previously-Treated Advanced-Stage Non-Small Cell Lung Cancer: A Real-World Study in Taiwan
1. Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University, Taipei, Taiwan.
2. Department of Internal Medicine, National Taiwan University Cancer Center, National Taiwan University, Taipei, Taiwan.
3. Department of Chest Medicine, Taipei Veterans General Hospital, Taipei, Taiwan.
4. School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
5. Institute of Clinical Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
6. Division of Pulmonary and Critical Care Medicine, Department of Medicine, Chang Gung Memorial Hospital-Kaohsiung Medical Center, Chang Gung University College of Medicine, Kaohsiung, Taiwan.
7. Department of Internal Medicine, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
8. Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
9. Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
10. Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, China Medical University and China Medical University Hospital, Taichung, Taiwan.
11. Division of Lung Cancer and Interventional Bronchoscopy, Department of Thoracic Medicine, College of Medicine, Chang Gung Memorial Hospital, Chang Gung University, Taipei, Taiwan.
Abstract
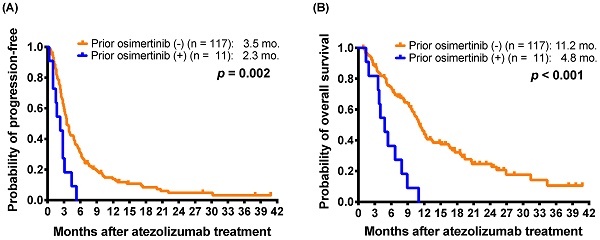
Immune checkpoint inhibitors (ICIs) are the standard treatment for non-small-cell lung cancer (NSCLC). We assessed the clinical prognostic factors in NSCLC patients receiving atezolizumab as a second- or later-line (2L+) treatment. Data were retrospectively collected for NSCLC patients treated with atezolizumab from July 2017 to June 2019 at six medical centers in Taiwan. Clinical characteristics, treatment course and responses of patients were recorded. A total of 128 NSCLC patients received 2L+ atezolizumab, and the outcomes included a response rate of 10.2%, median progression-free survival (mPFS) of 3.5 months, and median overall survival (mOS) of 10.7 months. Eleven patients who had received osimertinib treatment before atezolizumab had a shorter mPFS (2.3 versus 3.5 months; p = 0.002) and mOS (4.8 versus 11.2 months; p < 0.001) than those without prior osimertinib treatment. Even for the subgroup of patients with EGFR-mutant non-squamous NSCLC, prior osimertinib was still associated with shorter PFS (2.3 versus 4.1 months; p = 0.006) and OS (4.8 versus 11.7 months; p < 0.001). Multivariate analysis revealed that prior osimertinib treatment correlated with not only shorter PFS (hazard ratio [HR]: 2.94; 95% confidence interval [CI], 1.34-6.47; p = 0.007) but also shorter OS (HR, 3.55; 95% CI, 1.57-8.03; p = 0.002). Patients with prior ICIs treatment (HR, 3.18; p = 0.002) or poor performance status (HR, 2.70; p = 0.001) had shorter OS. In conclusion, osimertinib treatment before atezolizumab therapy was associated with a shorter PFS and a poor prognosis in NSCLC patients in real-world settings. Further studies with larger sample sizes are needed to validate these observations.
Keywords: Atezolizumab, Epidermal growth factor receptor mutation, Non-small-cell lung cancer, Tyrosine kinase inhibitor, Osimertinib, Immune checkpoint inhibitor

 Global reach, higher impact
Global reach, higher impact