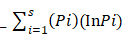Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(6):2014-2028. doi:10.7150/jca.66816 This issue Cite
Research Paper
Gut-Thyroid axis: How gut microbial dysbiosis associated with euthyroid thyroid cancer
1. Department of Microbiology and Immunology, Key Laboratory of Environment and Genes Related to Diseases of Chinese Ministry of Education, School of Medicine, Xi'an Jiaotong University, Xi'an, China
2. Faculty of Veterinary and Animal Sciences, Muhammad Nawaz Shareef University of Agriculture Multan 66000, Pakistan
3. ERKAM-Clinical-Engineering Research and Application Center, Erciyes University, 38039, Kayseri, Turkey
4. ERFARMA-Drug Development and Implementation Center, Erciyes University, 38039, Kayseri, Turkey
5. Department of Pathology, Faculty of Veterinary and Animal Sciences, The Islamia University of Bahawalpur, Pakistan
6. Department of Pharmaceutical Sciences, Superior University, Lahore, Pakistan
7. Department of Pharmaceutics, Faculty of Pharmacy, The Islamia University of Bahawalpur, Pakistan
8. Department of Microbiology, School of Basic Medical Science, Xinxiang Medical University, Xinxiang China
9. Department of Pharmacology, University of Health Sciences, Khyaban-e-Jamia Punjab, Lahore, Pakistan
10. Institute of Pharmaceutical Sciences, University of Veterinary and Animal Sciences, Lahore, Pakistan
11. Xi'an Mental Health Centre, Xi'an China
12. National Institiute for Biotechnology and Genetic Engineering College, Pakistan Institiute Engineering and Applied Sciences (NIBGE-C, PIEAS), Faisalabad 38000, Punjab, Pakistan
13. Department of Zoology Wild-life and Fisheries, University of Agriculture, Faisalabad, Pakistan
14. Department of Pharmaceutical Technology, Faculty of Pharmacy of the University of Coimbra, University of Coimbra, Coimbra, Portugal
15. REQUIMTE/LAQV, Group of Pharmaceutical Technology, Faculty of Pharmacy of the University of Coimbra,University of Coimbra, Coimbra, Portugal
Received 2021-9-5; Accepted 2022-3-3; Published 2022-3-28
Abstract
Thyroid cancer in humans has a fast-growing prevalence, with the most common lethal endocrine malignancy for unknown reasons. The current study was aimed to perform qualitative and quantitative investigation and characterization of the gut bacterial composition of euthyroid thyroid cancer patients. The fecal samples were collected from sixteen euthyroid thyroid cancer patients and ten from healthy subjects. The PCR-DGGE was conducted by targetting the V3 region of 16S rRNA gene, as well as real-time PCR for Bacteroides vulgatus, E.coli Bifidobacterium, Clostridium leptum and Lactobacillus were carried. High-throughput sequencing of V3+V4 region of 16S rRNA gene was performed on Hiseq 2500 platform on 20 (10 healthy & 10 diseased subjects) randomly selected fecal samples. The richness indices and comparative diversity analysis showed significant gut microbial modification in euthyroid thyroid cancer than control. At phylum level, there was significant enrichment of Firmicutes, Verrucomicrobia, while a significant decrease in Bacteroidetes was detected in the experimental group. At family statistics, significant high levels of Ruminococcaceae and Verrucomicrobiaceae, while the significant lower abundance of Bacteroidaceae, Prevotellaceae, Porphyromonadaceae, and Alcaligenaceae was after observed. It also found that the significantly raised level of Escherichia-Shigella, Akkermansia [Eubacterium]_coprostanoligenes, Dorea, Subdoligranulum, and Ruminococcus_2 genera, while significantly lowered genera of the patient group were Prevotella_9, Bacteroides and Klebsiella. The species-level gut microbial composition showed a significantly raised level of Escherichia coli in euthyroid thyroid cancer. Thus, this study reveals that euthyroid thyroid cancer patients have significant gut microbial dysbiosis. Moreover, Statistics (P<0.05) of each gut microbial taxa were significantly changed in euthyroid thyroid cancer patients. Therefore, the current study may propose new approaches to understanding thyroid cancer patients' disease pathways, mechanisms, and treatment.
Keywords: Euthyroid thyroid cancer, DGGE, High-throughput sequencing, Characterization, Gut microbiota.
Introduction
Human gut microbiota is defined as a crucial factor in determining an individual's normal body functioning and health status. The body physiological mechanism depends on the configuration of gut microbiota which remains consistent over the period of time. It may be modulated by different factors like ageing, food, and sickness [1]. The human gut microbiota constitutes approximately 100 trillion bacteria contributing to the immune function, metabolism, nutrition, absorption [2], and defence mechanism against pathogens [3]. The modulation of gut microbial composition act as a predisposing factor for different disease condition like colitis, inflammatory bowel disease, Crohn's disease, metabolic disorders including diabetes mellitus, asthma, smoking and obesity [4-6].
Cancer is characterized by abnormal/uncontrolled growth of cells and is among the deadliest diseases [7-10], with an estimated 11.5 million deaths by 2030 worldwide [11]. The etiological findings of thyroid cancer are commonly manifested with thyroid nodules exhibiting significant clinical signs in diagnosis. Recently, the number of patients with thyroid problems has been observed a sharp rise in worldwide, particularly in women [12]. The recent epidemiological data suggested that thyroid dysfunction is the 5th leading cancer diagnosed in women [13]. If the same developments are continued, thyroid malignancy may perhaps develop into fourth leading carcinoma of the United States in 2030 [15]. The occurrence of thyroid malignancy in several European states also shows a similar tendency to the United States and followed in China [14]. Thyroid cancer is commonly found in malignant endocrine carcinoma classified into 5 distinct types [15]. The most prevailing thyroid cancer is papillary thyroid carcinoma (PTC), which is approximately about (80-85%) thyroid malignant patients in well-advanced nations [16]. In PTC, the genetic modulations predispose the activation of [17] serum thyroid-stimulating hormone (TSH), thus generating malignant thyroid nodules. Many factors may cause many thyroid disorders, including estrogen, BMI ethnicity, abnormal iodine consumption and radioactivity [18-22]. It has been described that the microbial composition of the gut could be altered by abnormal thyroid function [23]. Numerous experimental works have been established that both Graves' disease and Hashimoto's thyroiditis are linked with gut microbiota [24, 25].
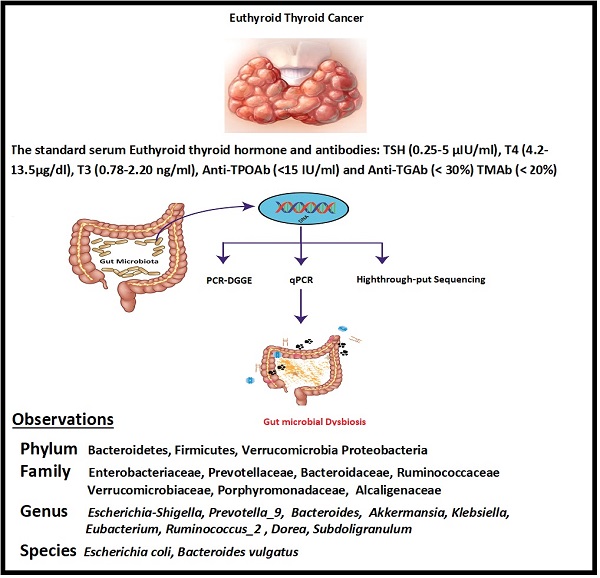
Furthermore, the traditional role of the thyroid gland in the immune system plays a vital role in the development of thyroid cancer. The recent experimental investigations have elaborated on the intestinal microbiota contribution to host immunity and body homeostasis [26]. The current study was planned to determine the modification in diversity and similarity of intestinal microbiota qualitatively and quantitatively in euthyroid thyroid cancer patients with normal circulating antibodies compared to healthy subjects. Gut microbial diversity and similarity of euthyroid thyroid cancer patients were monitored by applying metagenomic High-throughput sequencing, PCR-DGGE, and qPCR. The findings revealed the significant difference in gut microbial composition with some distinctive gut bacteria portraying significantly elevated or lowered richness against healthy subjects. However, the association between gut microbiota and euthyroid thyroid cancer patients with normal level thyroid circulating antibodies has not been reported yet. The current study thus facilitates the explanation of the overall gut microbial composition of euthyroid thyroid cancer patients, as described in Scheme 1.
Methodology
Ethics statement
The volunteer wrote down a semi-structured detailed questionnaire as per the rules of Xi'an Jiaotong University (School of Medicine), the Ethics Committee.
Sample collection
Fecal samples were collected in a sterilized cup from 16 thyroid cancer patients (8 males + 8 females) having euthyroid (aged linking 30 to 50 years) and 10 healthy volunteers (5 males + 5 female) (having the same age between 30 to 50 years). The patients with euthyroid thyroid cancer were diagnosed by set protocols of department of Endocrinology and Metabolic diseases, School of Medicine Xian Jiatong university [27]. The normal standard antibodies and serum thyroid hormone levels are T3 (0.78-2.20 ng/ml), TSH (0.25-5 μIU/ml), T4 (4.2-13.5μg/dl), Anti-TGAb (< 30%) TMAb (< 20%), and Anti-TPOAb (<15 IU/ml). The information sheet for every volunteer was constructed according to their medical status, nutritional habits, lifestyle, and thyroid cancer disease. This inquiry performa also includes individual physical weight, age, and gender. The fecal samples were transported on ice almost four hours after defection. The samples were stored at -80 ⁰C freezer in the lab for further pocess like DNA extraction. In the last 60 days of sample collection, neither of the patients nor the healthy volunteers had taken any antibiotics, probiotics, and prebiotics. Our study subjects were also free of any GIT disease.
The whole study methodology and results clearly indicate gut bacterial dysbiosis in euthyroid thyroid cancer patients.
DNA extraction from the fecal sample
DNA was extracted from all thawed fecal samples of diseased and control using the QIAGEN Stool kit (Germany) according to established protocol. The first step of bead-beating was conducted for ½ min with 5000 rpm. Concentration of DNA was evaluated using Nano Photometer TM (IMPLEN, Germany) [28].
PCR Amplification for DGGE
The fecal extracted bacterial DNA was used for analyzing PCR-DGGE. The linkage primers for V3 region were used to emplify the 16S rRNA gene (Table 1). A total of 50 µl PCR mixture (thermocycler ABI2720 USA) was used to amplify the DNA sequence through touchdown PCR programming: Initial start was done with denaturation of PCR mixture at 95 ºC for 5 min that further include 10 extra cycles and followed by a final extension. The gene bands were assessed by using 1.5% agarose gel electrophoresis, and visualized under UV light after dipping in the Ethidium bromide solution [29].
Primers used in Real-time PCR and PCR-DGGE
| Target bacteria | Primer Sequence (5¹-3¹) | |
|---|---|---|
| PCR-DGGE Primer | ||
| 341-F | CCTACGGGAGGCAGCAG | |
| 534-R | ATT ACCGCGGCTGCTGG | |
| 341FG | CGCCCGCCGCGCGCGGCGGCGCGGGGCGGGGGCACGGGGGGCCTACGGGAGGCAGCAG | |
| Real-Time PCR Primer | ||
| Bifidobacterium (550 bp) | Bifid F | CTC CTGGAAACGGGTGG |
| Bifi-R | GGTGTTCTTCCCGATATCTACA | |
| Lactobacillus (250 bp) | Lact F | CTCAAAACTAAACAAAGTTTC |
| Lact R | CTCAAAACT AAACAAAGTTTC | |
| Bacteroides vulgatus (287bp) | BV- F | GCATCATGAGTCCGCATGTTC |
| BV-R | TCCATACCCGACTTTATTCCTT | |
| Escherichia coli (287bp) | E.coli-F | CATTGACGTTACCGCAGAAGAAGC |
| E.coli-R | CTCTACGAGACTCAAGCTTGC | |
| Clostridium leptum (239bp) | C.lep-F | GCACAAGCAGTGGAGT |
| C.lep-R | CTTCCTCCGTTTTGTCAA | |
Denaturing gradient gel electrophoresis (DGGE)
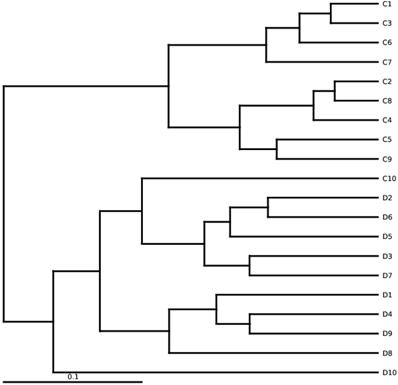
The DGGE was performed by using universal mutational (Bio-Rad, USA) analysis. In brief, Amplified PCR microbial DNA was loaded in 8% acrylamide DGGE gels in a container with 1×TAE buffer solution (linear denaturant grade 30~65%) with the constant optimal temperature at 60 ⁰C. DGGE was run for 13 hours at 90 V. The syngene Genetool (4.3.14) software was used to determine each sample's fecal microbial diversity and intensity strength by analyzing the total number of bands in DGGE. The Dice similarity coefficient was also assessed by using a similarity index. The algorithm clusters and arithmetic means (UPGMA) were applied to develop the unweighted pair group dendrogram [30].
DGGE statisticals analysis with band configuration
Bands intensity of DGGE profiles and bands number were evaluated by computed Syngene software. Bacterial diversity in DGGE profiles was estimated by using (H¹) Shannon Weaver diversity index [31, 32]. Similarity index and DGGE profile's cluster analysis were done through the UPGMA method (band-based Dice similarity coefficient) [33]. GraphPad prism 7 and Microsoft Excel (2010) were used to analyse the Shannon-Weaver and similarity index, where (P<0.05) was considered as statistically significant. Also, the Similarities among DNA samples were indicated by the dendrogram, shown in Figures 1B and 1D.
Shannon Weaver diversity index (H') was calculated by using the following formula.
Shannon-Weaver index (H¹) =
Excision of bands and sequencing
From DGGE gel profile prominent bands were cut with the help of sterile scalpel blade. The excised polyacrylamide DNA gel bands were kept in a 2 ml tube. 50 μl of distelled water was added to the tube and placed at 37 ºC for 30 min. After centrifugation, 8 μl of DNA water was used as a template and similar primers (without GC-clamps) were applied for amplification of V3 region 16S rRNA gene [34]. The sequencing of the amplified PCR-DNA was evaluated through ABI-3500xL. Obtained sequences were carefully analyzed by applying basic local alignment searching tool (BLAST) to identify the species or genus.
Real-time PCR
The Bio-RAD CFX96 (USA) protocol kit was followed for real-time PCR. A total of 20 μl reaction mixture was used for Real-time PCR. The sample includes 2 μl of genomic DNA, 1 μl each of forward and reverse primer along with 10 μl of Sybr green adding in 6 μl of water, thus making the 20 μl of reaction mixture for loading. The details of linkage primers for Real-time PCR are shown in Table 1 [35]. The bacterial species Bifidobacterium (CICC.6186), E.coli, NWS Lactobacillus (taken from our lab), Clostridium leptum (YIT.6169), Bacteroides vulgatus, (CICC.22938) were used as standard bacterial strains. The average mean values were retrieved after performing the Real-time PCR thrice. The result was an estimation of the mean logarithm in the fecal sample of genomic PCR amplicon, where copy number present in 1g of the fecal mass.
High-throughput sequencing and data analysis
The Ilumina based Hiseq protocol was followed for sequencing the paired-end. The data was retrieved and aligned by using QIIME and FLASH software. The QIIME (V1.7.0) software was used for diversity analysis like Simpson and Shannon diversity index and Good's coverage, ACE and chaol. The metagenomic high-throughput sequencing procedure was conducted based on the random reaction of 20 fecal samples. These include 10 samples collected from euthyroid thyroid cancer patients and the other 10 received from healthy subjects. The V3+V4 region of 16S rRNA gene was adjoined with linker primers: 806R (GGACTACHVGGGTWTCTAAT) 515F (GTGCCAGCMGCCGCGGTAA) primers for the construction of amplicon taxonomic libraries [36]. The data was retrieved and aligned by using QIIME [37] and FLASH [38] software. The UCLUST procedure [37] was employed to aggregate the bacterial DNA sequences in operational taxonomic units (OTUs) at the level of 97% identity threshold. The taxonomic position of each OTU was allocated by using the RDP classifier [39]. The QIIME software was used for diversity analysis like Simpson as well as Shannon diversity index along with chaol, Good's coverage and ACE. Moreover, the OTUs data tables retrieved through QIIME pipeline which were incorporated with MEGAN4 softwear and mapped with taxonomic database of NCBI [40]. The gut microbial population composition is witnessing the significant alteration. UniFrac distances were calculated by applying QIIME. PCA (Principal component analysis) and NMDS (non-metric multi-dimensional scaling) estimation were used to observe the dissimilarities and similarities of variables which are represented in paired wise distances between the study and control groups. It showed through stat packages, ggplot2 package and WGCNA packages, in software R (Version 2.15.3). The differences of alpha diversity were compiled by using a nonparametric unpaired t-test by using Graphpad prism 7 (statistic software) and Microsoft Excel (2010).
Results
Statistics and DGGE profiles analysis in euthyroid thyroid cancer and healthy group
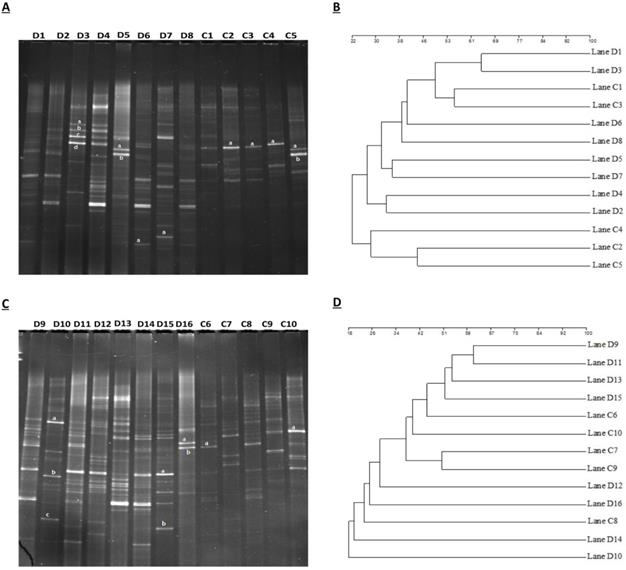
Analytical and experimental process of DGGE (denaturing gradient gel electrophoresis) was performed by using amplified PCR mixture employing universal primers of V3 region of 16S rRNA gene of euthyroid thyroid cancer and healthy control groups. In figure 1A, D1-D8 shows samples of euthyroid thyroid cancer patients and C1-C5 healthy controls. Similarly, in Figure 1C, the D9-D16 shows the samples of euthyroid thyroid cancer patients and C6-C10 healthy controls. Since the position, bands' strength intensity, and numbers were diverse among all fecal DNA samples that depicted the diverse intestinal microbial fingerprints. A total of 225 bands were identified by using the Syngene software [41], in 16 tracks of euthyroid thyroid cancer patients with an average band (14.1 ± 3.21). Moreover, a total of 86 DGGE bands were identified in 10 tracks of healthy subjects averaging (8.6 ± 2.55), which was significantly different (P< 0.001) between euthyroid thyroid cancer and healthy control groups. These results indicate that the increased band numbers demonstrate the increased diversity as well as bacterial overgrowth in euthyroid thyroid cancer patients group. To analyze, the diversity of stool microbiota in euthyroid thyroid cancer patients and healthy group, Shannon weaver (H¹) diversity index showed (3.225 ± 0.422 vs 2.542 ± 0.432) a significant (P< 0.003) in intestinal bacterial diversity alteration between euthyroid thyroid cancer and healthy subjects. (H¹) Shannon Weaver diversity index values were elevated in euthyroid thyroid cancer patients compared to healthy subjects, depicting significant gut bacterial overgrowth in euthyroid thyroid cancer patients. The similarity level of all the gut bacteria in DGGE gel profiles was assessed through the Dice similarity coefficient (UPGMA) dendrogram explained in Figures (1B and 1D). The band intensity-based numeral values of the Dice similarity coefficient between euthyroid thyroid cancer and healthy groups, along with mean similarity index (0.319 ± 0.141) and (0.288 ± 0.130) respectively, are shown in Table 2. When all the statistical values of each sample of euthyroid thyroid cancer and healthy control were calculated and analyzed through mean similarity index and Dice similarity coefficient, they were (0.269 ± 0.125). These findings illustrated that it was exhibited lesser in intergroup compared to intragroup, thus presenting dissimilarity of gut microbial composition in euthyroid thyroid cancer patients in contrast to the control subjects.
Gut microbial similarity and diversity of euthyroid thyroid cancer patients and healthy group
| Groups | Diversity | Similarity | ||
|---|---|---|---|---|
| The number of Bands A | Shannon Index B | Intra-similarity C | Inter-similarity D | |
| Disease group | 14.1 ± 3.21 | 3.225 ± 0.422 | 0.319 ± 0.141 | 0.269 ± 0.125 |
| Control group | 8.6 ± 2.55 | 2.542 ± 0.432 | 0.288 ± 0.130 | |
| P. Value | 0.001 | 0.003 | / | / |
Significantly different results (unpaired t-test), with P<0.05
a. DGGE bands number produced by each sample.
b. Shannon diversity index (H¹) was calculated with the help of all DGGE bands ( relative intensities) in each sample.
c. Comparing DGGE band profiles with Dice similarity coefficients within the individual of a given group.
d. Comparing DGGE band profiles with Dice similarity coefficients between members of euthyroid thyroid cancer and the healthy group.
Sequencing of re-amplified PCR Amplicons excised bands from DGGE gel and identities were checked by BLAST database.
| Selected Excised bands | Bacteria with the highest % homology | Sequence Accession number | Bacterial phyla | Gene bank number |
|---|---|---|---|---|
| D3a | Escherichia coli (93). | IAI39. | Proteobacteria | NZ_JH114216.1 |
| D3b | Proteus mirabilis (94). | HI4320. | Proteobacteria | NC_010554.1 |
| D3c | Pseudomonas cremoricolorata (98). | ND07. | Proteobacteria | NZ_CP009455.1 |
| D3d | Prevotella oulorum (93). | F0390. | Bacteroidetes | NZ_JH114216.1 |
| D5a | Prevotella copri( 96). | DSM 18205. | Bacteroidetes | NZ_GG703862.1 |
| D5b | Faecalibacterium prausnitzii (96). | TDY5834930. | Firmicutes | NZ_CZBH01000014.1 |
| D6a | Phascolarctobacterium sp (94). | YIT 12067. | Firmicutes | NZ_GL830850.1 |
| D7a | Alistipes putredinis (99). | DSM 17216. | Bacteroidetes | NZ_DS499580.1 |
| C2a | Bacteroides oleiciplenus (92). | YIT 12058. | Bacteroidetes | NZ_JH992946.1 |
| C3a | Bacteroides uniformis (90). | CL03T00C23. | Bacteroidetes | NZ_JH724260.1 |
| C4a | Barnesiella intestinihominis (98). | YIT 11860. | Bacteroidetes | NZ_JH815205.1 |
| C5a | Prevotella copri (96). | DSM 18205. | Bacteroidetes | NZ_GG703862.1 |
| C5b | Bacteroides stercoris (90). | ATCC 43183. | Bacteroidetes | NZ_DS499675 |
| D10a | Shigella dysenteriae (98). | Sd197. | Proteobacteria | NC_007606.1 |
| D10b | Bacteroides pyogenes (88). | JCM 10003. | Bacteroidetes | NZ_BAIU01000058.1 |
| D10c | Bacillus sp. (94). | FJAT-25496. | Firmicutes | NZ_LMBY01000086.1 |
| D15a | Klebsiella sp.(94). | NODE14. | Proteobacteria | NZ_LGIT01000014.1 |
| D15b | Enterobacter sacchari (94). | SP1. | Proteobacteria | NZ_CP007215.2 |
| D16a | Parabacteroides distasonis (97). | ATCC 8503 | Bacteroidetes | NZ_JH815205.1 |
| D16b | Bacteroides vulgatus ( 98). | ATCC 8482. | Bacteroidetes | NC_009614.1 |
| C6a | Bacteroides vulgatus ( 98). | ATCC 8482. | Bacteroidetes | NC_009614.1 |
| C10a | Bacteroides paurosaccharolyticus (91). | JCM 15092. | Bacteroidetes | NZ_BAJR01000054.1 |
(A) Assembling the DGGE profile of euthyroid thyroid cancer patients (D1-D8) and control subjects (C1-C5). (B) UPGMA application for Cluster analysis between diseased (D1-D8) and controlled (C1-C5) groups. (C) DGGE depicts constructed between euthyroid thyroid cancer (D9 -D16) and control groups (C6-C10). (D) assembly analysis of euthyroid thyroid cancer(D9 -D16) and control (C6-C10) groups by applying UPGMA.“a” and “b” in figure (A) and (C) constitute the dominant bands of different patients. D or C represents the euthyroid thyroid cancer patients and control subjects, respectively.
Dominant bands sequencing results analysis
The totals of 22 gel bands were excised by two DGGE gel profiles. In Figure 1A, 13 gel bands were cut from the DGGE profile for quantitative analysis. The resolution capability of DGGE gel profile bands was confirmed in different tracks, but in a similar position, the gel bands D5a and C5a were sequenced after excision that was identified as Prevotella copri with 96 % similarity. Furthermore, from Figure 1C, 9 bands were cut and assessed the resolution capability of DGGE gel composition; bands D16b and C6a were sequenced and detected as Bacteroides vulgatus having 98% similarity. The taxonomic identity of other bands of the DGGE profile has depicted in Table 3. Sequencing results were evaluated and analyzed by deploying BLAST software, and findings have confirmed the prevalence of phylum Firmicutes, Proteobacteria, and Bacteroidetes as prominent presence. Sequencing results of excision band from two DGGE gel profiles, also depicted in Table 3, opportunistic bacteria are prevalent (Escherichia coli, Proteus mirabilis, Pseudomonas cremoricolorata, Prevotella oulorum, Faecalibacterium prausnitzii, Phascolarctobacterium sp, Alistipes putredinis, Shigella dysenteriae, Bacteroides pyogenes, Bacillus sp. Klebsiella sp, Enterobacter sacchari, Parabacteroides distasonis) in euthyroid thyroid cancer patients.
Real-time PCR Amplification
The Bacteroides vulgates, Bifidobacterium, Lactobacillus Clostridium leptum, were enumerated by Real-time PCR. The copy numbers of Bifidobacterium (5.75 ± 0.87 vs. 6.73±0.87) were lessened significantly (P < 0.005), also copy numbers of Lactobacillus (6.19 ± 0.98 vs 6.98 ± 0.99) were lowered significantly (P < 0.029) in the disease group. Conversely, Bacteroides vulgatus (5.77 ± 0.86 vs 6.59 ± 0.82) were found significantly (P <0.011) reduced in euthyroid thyroid cancer patients. Copy numbers of Escherichia coli (5.60 ± 0.78 vs. 4.89 ± 0.74) were significantly (P < 0.016) increased in patients. Moreover, the copy numbers of Clostridium leptum (4.05 ± 1.07 vs 3.75 ± 1.11) (P < 0.249) had a non-significant rise in the fecal samples of euthyroid thyroid cancer patients in contrast with healthy subjects. The results are shown in Table 4.
Real-time PCR quantification results in different gut bacteria
| Bacteria | Healthy Subjects | Patients | P value |
|---|---|---|---|
| Bifidobacterium (104) | 6.73±0.87 | 6.30±0.90 | 0.123 |
| Bacteroides vulgatus(109) | 6.59±0.82 | 5.77±0.86 | 0.011 |
| Lactobacillus (105) | 6.98±0.99 | 6.19±0.98 | 0.029 |
| Clostridium leptum (107) | 3.75±1.11 | 4.05±1.07 | 0.249 |
| Escherichia coli (106) | 4.89±0.74 | 5.60±0.78 | 0.016 |
Results were presented as the average estimate of logarithms of fecal PCR target genetic amplicon copy numbers present in 1 g of feces, where (P < 0.05).
High-throughput sequencing of gene analysis
Comparative sequencing amplicons of PCR were computed with 1,731,168 at the position of V3+V4 of the 16SrRNA gene, from 10 euthyroid thyroid cancer and also10 from healthy controls. High-throughput sequencing reads 1, 438,586 (control 736,933 and disease 701,653) were passed, having an average per sample (72,169) for quality assurance and analysis. The taxon tag was estimated (Ave. 68796.2) in both euthyroid thyroid cancer and healthy control groups. Total unique tag counted in the study and healthy groups were 10, 656 and 7,686, respectively (Ave. 917.1 in entire samples). Operational texsonomic unit (OTU) numbers were assigned which is 52,12 (healthy 24,80 and patients 2,732) average/sample (260.6) in current study. The high-throughput unique tag was 18,342 from study and healthy groups, demonstrating the entire phylotypes of current experimental work. The OTU clustering and annotation results of each sample are comprehensively calculated. The results are shown in Figure 2. The average length of the sequence was estimated 418 bp after removal of linkage primers.
Intestinal bacterial diversity analysis
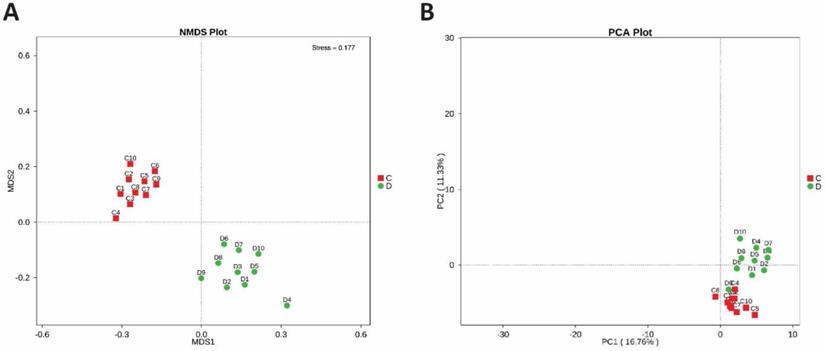
Bacterial community diversity and richness were estimated at a similarity level of 97%. Alpha diversity, as computed by Simpson and Shannon diversity, PD Tree, algorithm ACE, observed species, and Chao1, were found significantly higher in euthyroid thyroid cancer with a comparision of healthy volunteers. The level of bacterial diversity assessment in study and control is described in Table 5. Additionally, the analysis of alpha diversity exhibits an elevated level in euthyroid thyroid cancer patients compared to controls. The elevated diversity depicted a strong intestinal bacterial overgrowth in the patients' group compared to healthy volunteers. Samples of intestinal bacterial DNA in each group were distributed in two distinct clusters, constructed on weighted UniFracs distance shown in Figure 3, which is also analogous to the arrangement of PCR-DGGE of euthyroid thyroid cancer and normal volunteers.
To determine the microbial diversity between the healthy and study groups, the beta diversity was estimated. Non-metric dimensional scaling (NMDS) and OTU number based Principal-component analysis (PCA) were performed, which clearly illustrate the alteration of the intestinal bacterial composition of two groups, shown in Figures 4A and 4B.
Euthyroid thyroid cancer observation of Tag number and OTUs analysis with comparison of control, Tag number and OTUs were estimated at the level of (97 %) similarity
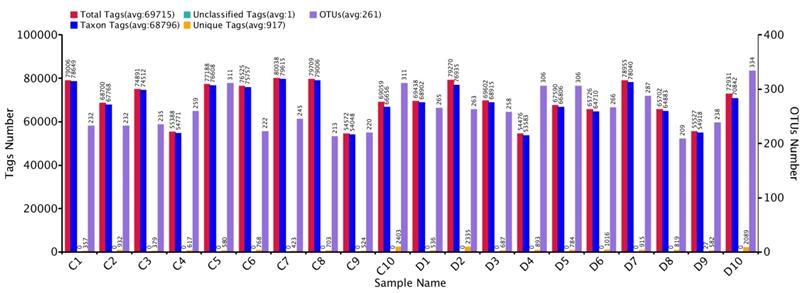
Gut bacterial richness and diversity index, based on 97% similarity through High-throughput analysis.
| Group | Observed Species | OTUs | Shannon | Simpson | Chao1 | ACE | PD Tree | Evenness |
|---|---|---|---|---|---|---|---|---|
| Patients | 255 | 248 | 4.62 | 0.876 | 273.50 | 279.39 | 22.02 | 0.357 |
| Control | 226.5 | 273.2 | 3.85 | 0.770 | 240.24 | 244.54 | 19.44 | 0.296 |
| P. Value | 0.042 | 0.067 | 0.011 | 0.0321 | 0.036 | 0.030 | 0.037 | 0.014 |
Diversification among euthyroid thyroid cancer samples of High-throughput sequencing. UPGMA is based on weighted UniFrac distances. D and C denotes the euthyroid thyroid cancer patients and controlled group, respectively.
Phyla level intestinal bacteria
Intestinal bacterial taxa had a percentage over 0.5% - 1%, considered in the present study, at the phylum, family, genus and species level.
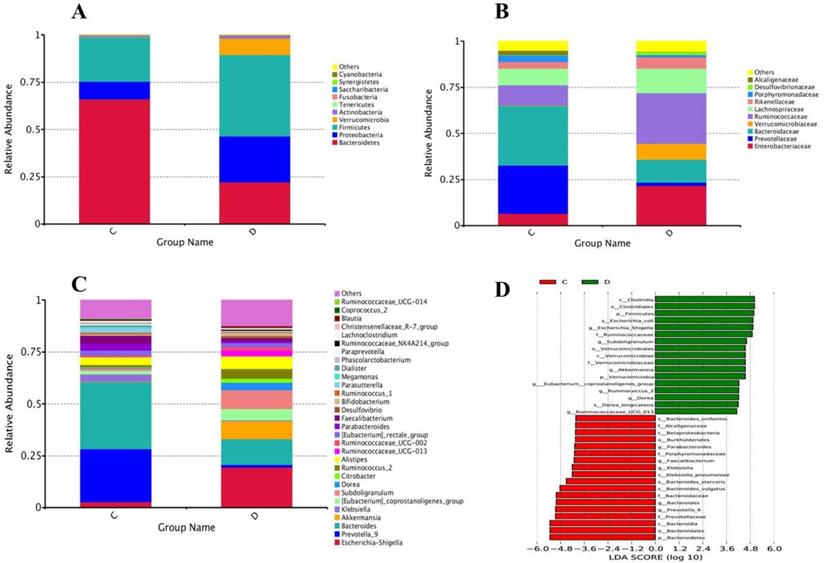
At the level of phylum, a total of 15 phyla were found in sequencing; among the 10 topmost phyla, significantly increased phyla abundance in the diseased group were Firmicutes and Verrucomicrobia while non- significantly raised in Proteobacteria and Actinobacteria. However, Phylum Bacteroidetes in the experimental group was significantly reduced compared to healthy volunteers, depicted in Figure 5A. Statistics of the 10 most prevalent phyla in Table 6A illuminated significant quantitative difference between the two groups.
Intestinal bacterial composition at a family level
Family level sequencing, 75 diverse families were sequenced through Illumina based sequencing, in 10 topmost families, taxa richness of Ruminococcaceae, and Verrucomicrobiaceae were significantly elevated while non-significantly increased in families Enterobacteriaceae, Lachnospiraceae and Rikenellaceae in the study group with the comparison of control, depicted in Figure 5B. Among all these families, the community abundance of Bacteroidaceae and Prevotellacea was significantly reduced in the study group compared to healthy volunteers. Percentage data statistics in euthyroid thyroid cancer group displayed a significant quantitative variation of families shown in Table 6A.
Gut microbial taxa at phyla and families level from High-throughput results
| Taxa | Mean D | Mean C | p.value | q value | %D | % C |
|---|---|---|---|---|---|---|
| Phylum | ||||||
| Bacteroidetes | 0.2224 | 0.6605 | 0.0010 | 0.0040 | 22.24 | 66.05 |
| Proteobacteria | 0.2420 | 0.0941 | 0.1039 | 0.1662 | 24.20 | 9.41 |
| Firmicutes | 0.4305 | 0.2345 | 0.0050 | 0.0160 | 43.05 | 23.45 |
| Verrucomicrobia | 0.0862 | 0.0009 | 0.0010 | 0.0040 | 8.62 | 0.09 |
| Actinobacteria | 0.0167 | 0.0092 | 0.2947 | 0.3929 | 1.67 | 0.92 |
| Tenericutes | 0.0015 | 0.0000 | 0.0060 | 0.0160 | 0.15 | 0.00 |
| Fusobacteria | 0.0000 | 0.0008 | 0.0290 | 0.0515 | 0.00 | 0.08 |
| Saccharibacteria | 0.0002 | 0.0000 | 0.0070 | 0.0160 | 0.02 | 0.00 |
| Synergistetes | 0.0001 | 0.0000 | 0.0010 | 0.0040 | 0.01 | 0.00 |
| Cyanobacteria | 0.0002 | 0.0000 | 0.0250 | 0.0500 | 0.02 | 0.00 |
| Others | 0.01 | 0.00 | ||||
| Family | ||||||
| Enterobacteriaceae | 0.2169 | 0.0650 | 0.0679 | 0.2323 | 21.69 | 6.50 |
| Prevotellaceae | 0.0167 | 0.2624 | 0.0030 | 0.0325 | 1.67 | 26.24 |
| Bacteroidaceae | 0.1260 | 0.3238 | 0.0150 | 0.0949 | 12.60 | 32.38 |
| Verrucomicrobiaceae | 0.0862 | 0.0009 | 0.0010 | 0.0127 | 8.62 | 0.09 |
| Ruminococcaceae | 0.2750 | 0.1113 | 0.0050 | 0.0475 | 27.50 | 11.13 |
| Lachnospiraceae | 0.1323 | 0.0889 | 0.1259 | 0.3417 | 13.23 | 8.89 |
| Rikenellaceae | 0.0621 | 0.0378 | 0.2478 | 0.5717 | 6.21 | 3.78 |
| Porphyromonadaceae | 0.0125 | 0.0351 | 0.0070 | 0.0591 | 1.25 | 3.51 |
| Desulfovibrionaceae | 0.0144 | 0.0011 | 0.0010 | 0.0127 | 1.44 | 0.11 |
| Alcaligenaceae | 0.0020 | 0.0237 | 0.0010 | 0.0127 | 0.20 | 2.37 |
| Others | 5.58 | 5.02 | ||||
Euthyroid thyroid cancer D and Control C, P<0.05
Genus level intestinal bacterial distribution
Genera level highthrough-put sequencing characterized the abundance of 211 diverse genera. Among 30 topmost sequenced genera, there was significantly raised genera prevalence of Escherichia-Shigella, [Eubacterium]_coprostanoligenes, Subdoligranulum, and Ruminococcus_2 in the study group with the comparison of healthy group depicted in Figure 5C. Conversely, significantly lowered genera in the patients' group were Bacteroides, Klebsiella and Prevotella_9. Genera abundance statistics in two groups were gathered in Table 6B. Euthyroid thyroid cancer has a specific effect on intestinal bacteria, in particular, Phylum Firmicutes Verrucomicrobia, Proteobacteria, Bacteroidetes and Actinobacteria, families Ruminococcaceae, Verrucomicrobiaceae, Prevotellacea and Bacteroidaceae genera Escherichia-Shigella, [Eubacterium]_coprostanoligenes, Subdoligranulum Ruminococcus_2, Prevotella_9, Bacteroides and Klebsiella. The disease also dramatically influences the intestinal bacteria, which may change the health status of an individual due to the alteration of intestinal bacterial composition.
Gut bacterial composition at the species level
Species-level intestinal bacterial community patterns are demonstrated in Table 7. The species finding illustrate the significant differences between euthyroid thyroid cancer and healthy volunteers. However, the levels of Escherichia coli in euthyroid thyroid cancer patients have been raised significantly when compared to healthy volunteers.
The linear discriminant analysis (LDA) value distribution histogram demonstrates the taxa differences between the two groups. Taxa with significant differences in abundance in euthyroid thyroid cancer and control groups, shown in Figure 5D, and the length of the histogram represents the size and impact of the different Taxa.
(A) Beta diversity between diseased and healthy subjects. PCA plots which are obtained from Highthrough-put sequencing of fecal microbial DNA samples. (B) NMDS plot between study and control bacterial DNA samples. Each dot in the plot indicates an individual fecal bacterial DNA Samples of patient and control group
Gut microbial phylotypes at genus level from High-throughput results
| Taxa | Mean D | Mean C | p.value | q value | % D | % C |
|---|---|---|---|---|---|---|
| Genus | ||||||
| Escherichia-Shigella | 0.1941 | 0.0265 | 0.0140 | 0.0824 | 19.41 | 2.65 |
| Prevotella_9 | 0.0125 | 0.2568 | 0.0020 | 0.0193 | 1.25 | 25.68 |
| Bacteroides | 0.1260 | 0.3238 | 0.0160 | 0.0869 | 12.60 | 32.38 |
| Akkermansia | 0.0862 | 0.0009 | 0.0010 | 0.0125 | 8.62 | 0.09 |
| Klebsiella | 0.0031 | 0.0351 | 0.0340 | 0.1412 | 0.31 | 3.51 |
| [Eubacterium]_coprostanoligenes | 0.0564 | 0.0178 | 0.0290 | 0.1253 | 5.64 | 1.78 |
| Subdoligranulum | 0.0888 | 0.0105 | 0.0010 | 0.0125 | 8.88 | 1.05 |
| Dorea | 0.0377 | 0.0038 | 0.0010 | 0.0125 | 3.77 | 0.38 |
| Citrobacter | 0.0179 | 0.0020 | 0.4525 | 0.7260 | 1.79 | 0.20 |
| Ruminococcus_2 | 0.0459 | 0.0109 | 0.0180 | 0.0953 | 4.59 | 1.09 |
| Alistipes | 0.0619 | 0.0377 | 0.2388 | 0.4907 | 6.19 | 3.77 |
| Ruminococcaceae_UCG-013 | 0.0280 | 0.0024 | 0.0020 | 0.0193 | 2.8016 | 0.2437 |
| Ruminococcaceae_UCG-002 | 0.0168 | 0.0060 | 0.1948 | 0.4302 | 1.6804 | 0.5981 |
| [Eubacterium]_rectale_group | 0.0181 | 0.0248 | 0.5335 | 0.7440 | 1.8073 | 2.4769 |
| Parabacteroides | 0.0092 | 0.0314 | 0.0080 | 0.0529 | 0.9193 | 3.1430 |
| Faecalibacterium | 0.0137 | 0.0385 | 0.0030 | 0.0254 | 1.3730 | 3.8462 |
| Desulfovibrio | 0.0112 | 0.0004 | 0.0010 | 0.0125 | 1.1250 | 0.0424 |
| Bifidobacterium | 0.0074 | 0.0143 | 0.2358 | 0.4900 | 0.7390 | 1.4255 |
| Ruminococcus_1 | 0.0041 | 0.0082 | 0.5654 | 0.7684 | 0.4091 | 0.8187 |
| Parasutterella | 0.0018 | 0.0203 | 0.0240 | 0.1105 | 0.1752 | 2.0260 |
| Megamonas | 0.0003 | 0.0079 | 0.0010 | 0.0125 | 0.0338 | 0.7905 |
| Dialister | 0.0006 | 0.0061 | 0.0999 | 0.2750 | 0.0586 | 0.6062 |
| Phascolarctobacterium | 0.0021 | 0.0044 | 0.5604 | 0.7665 | 0.2124 | 0.4408 |
| Paraprevotella | 0.0026 | 0.0053 | 0.3516 | 0.6318 | 0.2646 | 0.5263 |
| Ruminococcaceae_NK4A214_group | 0.0051 | 0.0020 | 0.1369 | 0.3297 | 0.5058 | 0.1965 |
| Lachnoclostridium | 0.0052 | 0.0098 | 0.0420 | 0.1435 | 0.5250 | 0.9817 |
| Christensenellaceae_R-7_group | 0.0035 | 0.0009 | 0.1129 | 0.2919 | 0.3494 | 0.0879 |
| Blautia | 0.0081 | 0.0039 | 0.0619 | 0.1960 | 0.8060 | 0.3923 |
| Coprococcus_2 | 0.0012 | 0.0043 | 0.0519 | 0.1721 | 0.1230 | 0.4276 |
| Ruminococcaceae_UCG-014 | 0.0005 | 0.0026 | 0.2238 | 0.4697 | 0.0476 | 0.2559 |
| Others | 12.3011 | 8.7739 | ||||
Euthyroid thyroid cancer D and Control C, (P < 0.05)
(A) Configuration of Gut microbiota at phyla levels from High-throughput sequencing results. The excessive occurrence of the most prevalent phyla in euthyroid thyroid cancer and control D and C designated as euthyroid thyroid cancer patients and control group, respectively, (B) High-throughput sequencing findings of gut bacterial conformation at family level. The relative plentiful of the most profoundly found families in euthyroid thyroid cancer and healthy controls. D and C represent euthyroid thyroid cancer and control group, respectively, (C) The genera levels gut bacterial compositions from High-throughput sequencing results. The relative abundance of the most prevalent genera in euthyroid thyroid cancer and healthy control. D and C represent euthyroid thyroid cancer and control group, respectively, (D) LDA (linear discriminant analysis) value distribution histogram was applied to find the most altered gut bacterial taxa abundance between euthyroid thyroid cancer patients D and control subjects C.
The final data obtained from the results analysis of metagenomic DGGE and High-throughput sequencing confirms the similar bacterial taxa prevalence. However, High-throughput sequencing is a highly sensitive, authentic, and much reliable technique than DGGE to study the intestinal bacterial taxa. In conclusion, the results agree and affiliate the intestinal bacterial data generated by three molecular procedures.
Discussion
Thyroid cancer is an endocrine system malignancy which is progressively rising in the last few decade that is to be believed due to better diagnostic facilities.[42]. Recent studies have been illustrated that gut microbiome has great importance and role in driving the different types of malignancies, including lung, breast, intestine and esophageal cancer [43, 44]. The human gut microbiota role is also critical in protecting the body's defense mechanism by employing trophic activity [45]. The gut microbiota and its metabolites like short-chain fatty acids have great impact in normal functioning of thyroid gland. It shows an existence of gut-thyroid axis and gut bacterial dysbiosis in autoimmune thyroid diseases such as Hashiomot's thyroiditis and Graves' disease [24, 25, 46].
The intestinal bacterial confirmation has been underlined in various disease situations, i.e., melanoma and diabetes too [4]. The DGGE gel profile findings were elucidated by illuminating the sequencing of prominent bands, High-throughput sequencing, and Real-time PCR. The statistical data in α diversity, nonparametric Simpson, Shannon, Chao1, observed species, and ACE algorithm were found significantly elevated in the patients' group compared to the control group [47]. Likewise, the diversity of gut bacterial population assessment in DGGE banding profiles and High-throughput sequencing analysis were found elevated in euthyroid thyroid cancer patients. Therefore, this increased level of gut microbiota evident the notable overgrowth in patients compared to the control group. However, these raised findings and interpersonal variations parallel to microbial results of skin, vagina, and gastrointestinal tract [48, 49].
The statistical data interpretation of intestinal microbial similarity index of euthyroid thyroid carcinoma group in DGGE banding profile configuration of intra-groups was measured to be significantly elevated; this indicated the bacterial overgrowth in the gut of study group. The comparative data analysis of diversity and similarity index found lesser in intergroup with the comparison of intra-group that are aligned with preceding research literature [50], indicating the variation in the composition of intestinal microbiota in euthyroid thyroid cancer patients with comparison of normal control. Hence, the diversity as mentioned above, findings elucidate a significant dissimilarity in the composition of intestinal bacteria between patients and healthy control groups. The statistical data showed significant quantitative and qualitative alteration between study and healthy groups.
At phylum level study, Firmicutes exhibited a significantly higher trend while low in Bacteroidetes in euthyroid thyroid cancer patients with comparison of control that is compatible with reported research in gut microbial alteration, endocannabinoid tone system and chronic pain with vitamin D mediated deficiency [51]. The reported meta-analysis of intestinal bacteria related to IBD and obesity showed that the percentage of Firmicutes to Bacteroidetes is not a constant feature that is distinct between lean and obese intestinal bacteria [52]. The current study illustrates a higher level of Proteobacteria, which is in accordance with prior work of proteobacteria as risk-factor in abdominal pain patients of the post-cholecystectomy syndrome [53]. It has also been reported that Proteobacteria has a crucial role in inflammatory bowel disease, metabolic disorders, asthma, and obstructive pulmonary diseases [54]. Our work indicates the significant increased abundance of phylum Verrucomicrobia which agrees with the reported literature of abnormalities of blood pressure associated and guts microbiota of children with anomalies of the urinary tract and kidney [55]. Furthermore, Verrucomicrobia is related to blood pressure abnormalities in the initial stage of CKD children [55].
At the family level, our study showed an increased level of Enterobacteriaceae, agreeing with the reported work of altered gut microbiota in vitamin D deficiency-mediated chronic pain [56]. It has been documented that pathogens of family Enterobacteriaceae involve in nosocomial pneumonia which is approximately1/3 of reported cases [57]. Our work showed a significant decrease of family Bacteroidaceae which is aligned with published literature of intestinal microecology in primary Sjogren's Syndrome patients [58]. The current study showed a significantly higher level abundance of family Ruminococcaceae, while significantly decreased family Prevotellaceae, which is parallel with reported work of diabetes type 2 and changes in intestinal bacteria with supplementation of diet [59].
Family Verrucomicrobiaceae is significantly enriched in study group, which is aligned with previous work of variation of the intestinal microbiota of the Chinese population with Parkinson's disease [60]. Our study depicted a significant abundance of Eubacterium and Akkermansia, which aligns with the existing work of altered intestinal microbiota in chronic pain endocannabinoid tone systems with vitamin D deficiency [56]. However, In advanced periodontal disease, Eubacterium may contribute to making about half of the microbiota and express a significant relationship with the disease [61]. It has been published in numerous studies of humans and mice that the increased abundances of Akkermansia are linked with patients of post-RYGB [62].
High-throughput differential intestinal bacterial phylotypes between euthyroid thyroid cancer D and Control C at the species level
| Taxa | Mean C | Mean D | p value | q value |
|---|---|---|---|---|
| Escherichia coli | 0.026521 | 0.194062 | 0.016983 | 0.079437 |
| Bacteroides vulgatus | 0.166316 | 0.033518 | 0.004995 | 0.03812 |
| Klebsiella_pneumoniae | 0.03508 | 0.003094 | 0.022977 | 0.104115 |
| Dorea longicatena | 0.003479 | 0.035789 | 0.000999 | 0.013169 |
| Bacteroides stercoris | 0.065741 | 0.005255 | 0.001998 | 0.019314 |
| Bacteroides uniformis | 0.028511 | 0.006969 | 0.00999 | 0.060356 |
| Akkermansia muciniphila | 0.000379 | 0.01261 | 0.001998 | 0.019314 |
| Parabacteroides distasonis | 0.018982 | 0.004001 | 0.015984 | 0.079437 |
| Bacteroides cellulosilyticus | 0.004009 | 0.015244 | 0.032967 | 0.119505 |
| Dialistersuccinatiphilus | 0.003622 | 0.000106 | 0.042957 | 0.144855 |
| Bacteroides coprophilus; | 0.0039 | 0.000248 | 0.008991 | 0.059259 |
| Bacteroides plebeius | 0.006049 | 0.000388 | 0.002997 | 0.025563 |
| Bacteroides massiliensis | 0.006224 | 0.000758 | 0.002997 | 0.025563 |
| Roseburia inulinivorans; | 0.010472 | 0.002868 | 0.001998 | 0.019314 |
| Bacteroides thetaiotaomicron; | 0.007045 | 0.002454 | 0.026973 | 0.111745 |
| Subdoligranulum_sp._4_3_54A2FAA | 0.00044 | 0.002646 | 0.001998 | 0.019314 |
| Lactobacillus mucosae | 0.000586 | 1.87E-05 | 0.030969 | 0.115141 |
| Bacteroides salyersiae | 0.000504 | 3.17E-05 | 0.015984 | 0.079437 |
| Fusobacterium varium; | 0.00078 | 2.43E-05 | 0.030969 | 0.115141 |
| Bacteroides eggerthii | 0.000621 | 8.40E-05 | 0.02997 | 0.115141 |
| Dorea formicigenerans | 0.000351 | 0.001935 | 0.000999 | 0.013169 |
| [Clostridium] leptum | 6.91E-05 | 0.000674 | 0.090439 | 0.113169 |
| Faecalibacterium_prausnitzii | 7.09E-05 | 0.001133 | 0.00999 | 0.013169 |
| Lactobacillusruminis | 0.000215 | 1.87E-06 | 0.011988 | 0.06953 |
| Ruminococcus_sp._16442; | 1.49E-05 | 0.000155 | 0.023976 | 0.105349 |
| [Clostridium] scindens | 0.000101 | 0.000317 | 0.030969 | 0.115141 |
| Paraprevotella xylaniphila; | 0.000106 | 3.73E-06 | 0.016983 | 0.079437 |
| Pseudomonas caeni | 0.000105 | 3.73E-06 | 0.008991 | 0.059259 |
| Pyramidobacter piscolens | 0 | 9.52E-05 | 0.000999 | 0.013169 |
| Acinetobacter_lwoffii | 3.73E-06 | 8.21E-05 | 0.026973 | 0.111745 |
P<0.05.
Moreover, genera Prevotella_9 were significantly diminished in the study group. Decreased level of Prevotella has been publicized in types1 diabetes and autism with intestinal bacteria [63, 64], while Escherichia-Shigella genera were increased in the study group, which agrees with reported preliminary research of gut bacterial relationship with autism problems [65]. Current study results depicted the lowered level of the Prevotella genus; however, the existing research literature shows the Prevotella dominance in intestinal microbial composition, which has a positive effect on the host's metabolism [66]. Prevalence of the Prevotella genus is considered a useful gut bacteria which help in digestion of plant-based food material. Also, the gut microbiota has been associated with numerous inflammatory conditions and diseases [67, 68]. Our results exhibited a significant raised level of Escherichia-Shigella in euthyridthyroid cancer patients with a comparison of healthy control. Previously, it was documented that Escherichia-Shigella can produce Shiga-toxin, which may cause thrombocytopenia septicaemia, hemorrhagic colitis, gastrointestinal inflammations, particularly in the ileocolonic region, hemolytic uremic syndrome (HUS), problems in urinary duct track [69]. The current study indicated a significantly higher prevalence of genus Escherichia-Shigella, particularly Escherichia coli species, which could be the strongest contributing agent in intestinal bacteria of euthyroid thyroid cancer patients. Besides, Escherichia coli (ubiquitous) is responsible for triggering predominant infections, i.e. urinary tract infections (UTIs) and foodborne illnesses [70].
Current research work described the significan alteration in the abundance of the phylum to genus and species-level in experimental samples, which demonstrated the clear disparity between euthyroid thyroid cancer patients and control groups. Furthermore, the bacterial community and species-level comparison also unveiled a significant variation of gut bacterial composition in study group as compared of healthy control [71]. These research findings further elaborate that euthyroid thyroid cancer plays a critical role in altering intestinal physiology that may cause the modification in the composition of gut microbiota. Likewise, such variations in intestinal microbial composition may trigger the disease complications [72].
The clinical signs of thyroid cancer manifested with thyroid nodules. However, Serum circulating antibodies, i.e., anti-thyroglobulin antibodies, anti-thyroid peroxidase and thyroid hormones in euthyroid thyroid cancer patients and healthy volunteers, are shown in (Table S1 and Table S2). The normal serum results of thyroid cancer patients in Table S1 showed euthyroid in thyroid cancer patients. So, it may be hypothesized that euthyroid thyroid cancer might alter the intestinal bacterial composition, in particular, Phylum Bacteroidetes, Firmicutes, Verrucomicrobia, family Enterobacteriaceae, Prevotellaceae, Bacteroidaceae, Verrucomicrobiaceae Ruminococcaceae, genera Escherichia-Shigella, Prevotella_9, Bacteroides, Akkermansia, Klebsiella, Eubacterium and Escherichia coli species, also largely disturb the intestinal bacteria. The current study on bacterial intestinal alterations between the euthyroid thyroid cancer group and healthy counterparts was very interesting because there is no direct connection and the straight relationship between euthyroid thyroid cancer and gut bacteria. Thus, the current study results further intricate the diverse intestinal bacterial composition between euthyroid thyroid cancer and normal healthy counterparts. These bacterial alterations may disturb the host's health status. Nevertheless, disease progress has no direct linkage with the gastrointestinal tract [73].
The Real-time PCR experiment was performed to investigate the quantitative gut microbial changes [74]. Statistical data showed a significant reduction of Lactobacillus in the study group, hence parallel with previously reported research [75]. The food supplement probiotics belong to genera Lactobacillus and incorporate physiological health benefits in the body [76]. Moreover, Lactobacillus has been observed a reduced trend in diseases like colorectal cancer [77]. In the human gut, Lactobacillus has great importance in the maintenance of selenium levels inside the cell. Selenium plays a crucial role in producing thyroid hormone and avoiding the oxidative destruction of the thyroid gland [78, 79]. Many studies also exhibited good effects against anti-atherogenic, anti-obesity and anti-inflammatory body response [80]. Different strains of lactobacillus have excellent antimicrobial characteristics in the human body to protect against uropathogens [81].
There was a significant decreased level of Bacteroides vulgates in the study group, which is steady with the reported literature of viral diarrhea with intestinal bacteria, while a significant increased level of Escherichia coli [82, 83]. Numerically Bacteroides vulgatus species is the predominant Bacteroides of human intestinal bacteria, which comprise a beneficial but complex association with its host and the avoidance of gut colonization [84, 85]. Data generated from PCR-DGGE and Illumina-based High-throughput sequencing analysis is suitable for characterizing intestinal bacteria. However, PCR-DGGE has been observed as a semi-quantitative technique; banding profile assessment which may not accurately illustrate the targeting taxa abundance of intestinal bacterial community [32]. Moreover, Illumina-based High-throughput sequencing is a highly sensitive, most advanced, and pretty much reliable technique to study and investigate the intestinal bacterial ecology [73]. Furthermore, the technique like PCR-DGGE might be applied as a routine basic laboratory technique to detect the significant modulation of gut bacterial taxa because it is lesst time-taking and low-cost experimental method.
Conclusion
A difference in composition of gut microbiota was found between euthyroid thyroid cancer patients and healthy counterparts. More precisely, there is a significant alteration in intestinal bacterial taxa abundance of the study group compared to healthy groups. The bacterial estimation analysis of taxa diversity exhibits a higher level of presence of intestinal bacteria in the euthyroid thyroid cancer group than controls, which shows the bacterial dysbiosis and overgrowth in the euthyroid thyroid cancer patients group. Consequently, the additional multicentre study approach has been needed to apprehend the basic underlying process and mechanism of bacterial dysbiosis in the intestine of euthyroid thyroid cancer patients.
Supplementary Material
Supplementary tables.
Acknowledgements
This study was supported by National Natural Science Foundation of China (NSFC 81730056).
The authors pay thanks to Dr. Hui Guo (Department of Endocrinology 1st affiliated Hospital Xi'an Jiaotong University, China) for helping in sample collection for current experimental study. The authors also thankful to NCBI for sequence analysis.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Power SE, O'Toole PW, Stanton C, Ross RP, Fitzgerald GF. Intestinal microbiota, diet and health. British Journal of Nutrition. 2014;111:387-402
2. Walsh CJ, Guinane CM, O'Toole PW, Cotter PD. Beneficial modulation of the gut microbiota. FEBS Letters. 2014;588:4120-4130
3. Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nature immunology. 2013;14:685-690
4. Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. Journal of clinical gastroenterology. 2010;44:354-360
5. Ishaq HM, Shahzad M, Wu X, Ma C, Xu J. Gut Microbe Analysis between Asthma Patients and Healthy Volunteers in Shaanxi Province, Xi'an, China. Pakistan Journal of Zoology. 2018;50:165-173
6. Ishaq HM, Shahzad M, Wu X, Ma C, Xu J. Molecular Characterization Of Fecal Microbiota Of Healthy Chinese Tobacco Smoker Subjects In Shaanxi Province, Xi'an China. Journal of Ayub Medical College Abbottabad Jamc. 2017;29:3-7
7. Hameed A, Ijaz S, Mohammad IS, Muhammad KS, Akhtar N, Khan HMS. Aglycone solanidine and solasodine derivatives: A natural approach towards cancer. Biomedicine & Pharmacotherapy. 2017;94:446-457
8. Shair Mohammad I, Chaurasiya B, Yang X, Lin C, Rong H, He W. Homotype-Targeted Biogenic Nanoparticles to Kill Multidrug-Resistant Cancer Cells. Pharmaceutics. 2020;12:950-968
9. Lin C, Yang X, Li H, Zou Y, Mohammad IS, Rong H. et al. Self-assembled nanomedicine combining a berberine derivative and doxorubicin for enhanced antitumor and antimetastatic efficacy via mitochondrial pathways. Nanoscale. 2021;13:6605-6623
10. Mohammad IS, He W, Yin L. A smart paclitaxel-disulfiram nanococrystals for efficient MDR reversal and enhanced apoptosis. Pharmaceutical research. 2018;35:1-18
11. Mohammad IS, He W, Yin L. Insight on multidrug resistance and nanomedicine approaches to overcome MDR. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2020;37:473-509
12. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. Journal of cancer epidemiology. 2013;2013:965212
13. Md HB, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE. et al. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133
14. Parkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR. et al. Fifty years of cancer incidence: CI5 I-IX. International Journal of Cancer. 2010;127:2918-2927
15. Gonçalves CF, De Freitas ML, Ferreira AC. Flavonoids, thyroid iodide uptake and thyroid cancer—a review. International journal of molecular sciences. 2017;18:1247
16. Carling T, Udelsman R. Thyroid cancer. Annual review of medicine. 2014;65:125-137
17. Vuong HG, Altibi AM, Abdelhamid AH, Ngoc PU, Quan VD, Tantawi MY. et al. The changing characteristics and molecular profiles of papillary thyroid carcinoma over time: a systematic review. Oncotarget. 2017;8:10637-10649
18. Clavel-Chapelon F, Guillas G, Tondeur L, Kernaleguen C, Boutron-Ruault MC. Risk of differentiated thyroid cancer in relation to adult weight, height and body shape over life: the French E3N cohort. International journal of cancer. 2010;126:2984-2990
19. Magreni A, Bann DV, Schubart JR, Goldenberg D. The effects of race and ethnicity on thyroid cancer incidence. JAMA Otolaryngology-Head & Neck Surgery. 2015;141:319-23
20. Sokouti M, Montazeri V, Fakhrjou A, Samankan S, Goldust M. 25 Years in Tabriz, Iran (2000-2012). Pakistan Journal of Biological Sciences. 2013;16:2003-2008
21. Veiga LH, Holmberg E, Anderson H, Pottern L, Sadetzki S, Adams MJ. et al. Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiation research. 2016;185:473-484
22. Zane M, Parello C, Pennelli G, Townsend DM, Merigliano S, Boscaro M. et al. Estrogen and thyroid cancer is a stem affair: a preliminary study. Biomedicine & Pharmacotherapy. 2017;85:399-411
23. Virili C, Centanni M. “With a little help from my friends”-the role of microbiota in thyroid hormone metabolism and enterohepatic recycling. Molecular and cellular endocrinology. 2017;458:39-43
24. Ishaq HM, Mohammad IS, Guo H, Shahzad M, Hou YJ, Ma C. et al. Molecular estimation of alteration in intestinal microbial composition in Hashimoto's thyroiditis patients. Biomedicine & Pharmacotherapy. 2017;95:865-874
25. Ishaq HM, Mohammad IS, Shahzad M, Ma C, Raza MA, Wu X. et al. Molecular Alteration Analysis of Human Gut Microbial Composition in Graves' disease Patients. International journal of biological sciences. 2018;14:1558-1570
26. Tan J, McKenzie C, Potamitis M, Thorburn A, Mackay C, Macia L. The role of short-chain Fatty acids in health and disease. Advances in immunology. 2013;121:91-119
27. Giannini AJ. The Biological foundations of clinical psychiatry: Medical Examination Pub. Co. 1986
28. Scanlan PD, Shanahan F, O'Mahony C, Marchesi JR. Culture-independent analyses of temporal variation of the dominant fecal microbiota and targeted bacterial subgroups in Crohn's disease. Journal of clinical microbiology. 2006;44:3980-3988
29. Muyzer G, De Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and environmental microbiology. 1993;59:695-700
30. Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang Y. et al. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC genomics. 2010;11:488
31. Gafan GP, Lucas VS, Roberts GJ, Petrie A, Wilson M, Spratt DA. Statistical analyses of complex denaturing gradient gel electrophoresis profiles. Journal of clinical microbiology. 2005;43:3971-3978
32. Ledder RG, Gilbert P, Huws SA, Aarons L, Ashley MP, Hull PS. et al. Molecular analysis of the subgingival microbiota in health and disease. Applied and Environmental Microbiology. 2007;73:516-523
33. Fromin N, Hamelin J, Tarnawski S, Roesti D, Jourdain-Miserez K, Forestier N. et al. Statistical analysis of denaturing gel electrophoresis (DGE) fingerprinting patterns. Environmental Microbiology. 2002;4:634-643
34. Green SJ, Leigh MB, Neufeld JD. Denaturing gradient gel electrophoresis (DGGE) for microbial community analysis. Handbook of hydrocarbon and lipid microbiology: Springer. 2010:4137-4158
35. Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Applied and Environmental Microbiology. 2004;70:7220-7228
36. Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL. et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6548-6553
37. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK. et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335-336
38. Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957-2963
39. Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Applied and Environmental Microbiology. 2007;73:5261-5267
40. Huson DH, Mitra S, Ruscheweyh HJ, Weber N, Schuster SC. Integrative analysis of environmental sequences using MEGAN4. Genome Research. 2011;21:1552-1560
41. Van Der Gucht K, Sabbe K, De Meester L, Vloemans N, Zwart G, Gillis M. et al. Contrasting bacterioplankton community composition and seasonal dynamics in two neighbouring hypertrophic freshwater lakes. Environmental Microbiology. 2001;3:680-690
42. R FY, M RT, A AO, MR MT, AR S. The metabolomics and lipidomics window into thyroid cancer research. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals. 2017;22:595-603
43. Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A. et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. The lancet. 2016;388:1459-1544
44. Ishaq HM, Mohammad IS, Sher Muhammad K, Li H, Abbas RZ, Ullah S. et al. Gut microbial dysbiosis and its association with esophageal cancer. Journal of Applied Biomedicine. 2021;19:1-13
45. Guarner F, Malagelada J-R. Gut flora in health and disease. The Lancet. 2003;361:512-519
46. Bargiel P, Szczuko M, Stachowska L, Prowans P, Czapla N, Markowska M. et al. Microbiome Metabolites and Thyroid Dysfunction. Journal of Clinical Medicine. 2021;10:3609
47. Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MMP. et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Scientific Reports. 2016;6:28484
48. Hummelen R, Fernandes AD, Macklaim JM, Dickson RJ, Changalucha J, Gloor GB. et al. Deep Sequencing of the Vaginal Microbiota of Women with HIV. PloS one. 2010;5:12078
49. Ling Z, Kong J, Jia P, Wei C, Wang Y, Pan Z. et al. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microbial Ecology. 2010;60:677-690
50. Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X. et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Current microbiology. 2010;61:69-78
51. Guida F, Boccella S, Belardo C, Iannotta M, Piscitelli F, De Filippis F. et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain, Behavior, and Immunity. 2020;85:128-141
52. Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS letters. 2014;588:4223-4233
53. Kang Z, Lu M, Jiang M, Zhou D, Huang H. Proteobacteria Acts as a Pathogenic Risk-Factor for Chronic Abdominal Pain and Diarrhea in Post-Cholecystectomy Syndrome Patients: A Gut Microbiome Metabolomics Study. Med Sci Monit. 2019;25:7312-7320
54. Rizzatti G, Lopetuso L, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: a common factor in human diseases. BioMed research international. 2017;2017:9351507
55. Hsu C-N, Lu P-C, Hou C-Y, Tain Y-L. Blood Pressure Abnormalities Associated with Gut Microbiota-Derived Short Chain Fatty Acids in Children with Congenital Anomalies of the Kidney and Urinary Tract. Journal of clinical medicine. 2019;8:1090
56. Guida F, Boccella S, Belardo C, Iannotta M, Piscitelli F, De Filippis F. et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain, Behavior, and Immunity. 2020;85:128-141
57. Hornick D, Allen B, Horn M, Clegg S. Fimbrial types among respiratory isolates belonging to the family Enterobacteriaceae. Journal of clinical microbiology. 1991;29:1795-1800
58. Wu G-l, Lu H-f, Chen Y-l, Wang Q, Cao H, Li T-y. Changes of Intestinal Microecology in Patients with Primary Sjogren's Syndrome after Therapy of Yangyin Yiqi Huoxue Recipe. Chinese journal of integrative medicine. 2019;25:654-662
59. Zhang H-h, Liu J, Lv Y-j, Jiang Y-l, Pan J-x, Zhu Y-j. et al. Changes in Intestinal Microbiota of Type 2 Diabetes in Mice in Response to Dietary Supplementation with Instant Tea or Matcha. Canadian journal of diabetes. 2020;44:44-52
60. Li F, Wang P, Chen Z, Sui X, Xie X, Zhang J. Alteration of the fecal microbiota in North-Eastern Han Chinese population with sporadic Parkinson's disease. Neuroscience letters. 2019;707:134297
61. Wade W. The role of Eubacterium species in periodontal disease and other oral infections. Microbial Ecology in Health and Disease. 1996;9:367-370
62. Floch N. The Influence of Microbiota on Mechanisms of Bariatric Surgery. The Microbiota in Gastrointestinal Pathophysiology: Elsevier. 2017:267-281
63. Kang DW, Jin GP, Ilhan ZE, Wallstrom G, Labaer J, Adams JB. et al. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PloS one. 2013;8:68322
64. Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F, Soriguer F. et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Medicine. 2013;11:46
65. Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J. et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5:24
66. Kovatchevadatchary P, Nilsson A, Akrami R, Lee YS, De VF, Arora T. et al. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metabolism. 2015;22:971-982
67. Scher J. Scher, J.U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:01202
68. Dillon SM, Lee EJ, Kotter CV, Austin GL, Gianella S, Siewe B. et al. Gut Dendritic Cell Activation Links an Altered Colonic Microbiome to Mucosal and Systemic T Cell Activation in Untreated HIV-1 infection. Mucosal Immunology. 2016;9:24-37
69. Amani J, Ahmadpour A, Imani Fooladi AA, Nazarian S. Detection of E. coli O157:H7 and Shigella dysenteriae toxins in clinical samples by PCR-ELISA. The Brazilian journal of infectious diseases: an official publication of the Brazilian Society of Infectious Diseases. 2015;19:278-284
70. Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: Interplay between uropathogenic Escherichia coli and innate host defenses. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8829-8835
71. Manichanh C, Varela E, Martinez C, Antolin M, Llopis M, Doré J. et al. The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. American Journal of Gastroenterology. 2008;103:1754-1761
72. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65
73. Nam YD, Kim HJ, Seo JG, Kang SW, Bae JW. Impact of Pelvic Radiotherapy on Gut Microbiota of Gynecological Cancer Patients Revealed by Massive Pyrosequencing. PLoS One. 2013;8:82659
74. Lyons SR, Griffen AL, Leys EJ. Quantitative real-time PCR forPorphyromonas gingivalis and total bacteria. Journal of clinical microbiology. 2000;38:2362-2365
75. Zhou L, Li X, Ahmed A, Wu D, Liu L, Qiu J. et al. Gut microbe analysis between hyperthyroid and healthy individuals. Current microbiology. 2014;69:675-680
76. Butel MJ. Probiotics, gut microbiota and health. Medecine et maladies infectieuses. 2014;44:1-8
77. Borges-Canha M, Portela-Cidade JP, Dinis-Ribeiro M, Leite-Moreira AF, Pimentel-Nunes P. Role of colonic microbiota in colorectal carcinogenesis: a systematic review. Revista Española de Enfermedades Digestivas. 2015;107:659-671
78. Pessione E. Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Frontiers in cellular and infection microbiology. 2012;2:86
79. Danzi S, Klein I. Thyroid hormone and the cardiovascular system. Minerva endocrinologica. 2004;29:139-150
80. Nova E, Perez de Heredia F, Gomez-Martinez S, Marcos A. The Role of Probiotics on the Microbiota: Effect on Obesity. Nutrition in clinical practice: official publication of the American Society for Parenteral and Enteral Nutrition. 2016;31:387-400
81. Shim YH, Lee SJ, Lee JW. Antimicrobial activities of Lactobacillus Strains against Uropathogens. Pediatrics international: official journal of the Japan Pediatric Society. 2016;58:1009-1013
82. Ma C, Wu X, Nawaz M, Li J, Yu P, Moore JE. et al. Molecular characterization of fecal microbiota in patients with viral diarrhea. Current microbiology. 2011;63:259-266
83. Mushtaq N, Hussain S, Zhang S, Yuan L, Li H, Ullah S. et al. Molecular characterization of alterations in the intestinal microbiota of patients with grade 3 hypertension. International journal of molecular medicine. 2019;44:513-522
84. Wells C, Maddaus MA, Jechorek R, Simmons R. Role of intestinal anaerobic bacteria in colonization resistance. European Journal of Clinical Microbiology and Infectious Diseases. 1988;7:107-113
85. Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clinical microbiology reviews. 2007;20:593-621
Author contact
![]() Corresponding author: Prof. Dr. Jiru Xu, PhD. Department of Microbiology and Immunology, Key Laboratory of Environment and Genes Related to Diseases of Chinese Ministry of Education, School of Medicine, Xi'an Jiaotong University, Xi'an, China. Email: xujiruedu.cn
Corresponding author: Prof. Dr. Jiru Xu, PhD. Department of Microbiology and Immunology, Key Laboratory of Environment and Genes Related to Diseases of Chinese Ministry of Education, School of Medicine, Xi'an Jiaotong University, Xi'an, China. Email: xujiruedu.cn

 Global reach, higher impact
Global reach, higher impact