3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(6):1734-1744. doi:10.7150/jca.62169 This issue Cite
Research Paper
ACOT12-mediated acetyl-CoA hydrolysis suppresses intrahepatic cholangiocarcinoma metastasis by inhibiting epithelial-mesenchymal transition
1. Department of General Surgery, Huashan Hospital, Fudan University, 12 Urumqi Road (M), Shanghai 200040, China.
2. Department of Infection, Rui'an People's Hospital, 108 WanSong Road, Rui'an, Wenzhou, Zhejiang 325200, China.
3. Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences, Shanghai, 200031, China
#These authors contributed equally to this work.
Abstract
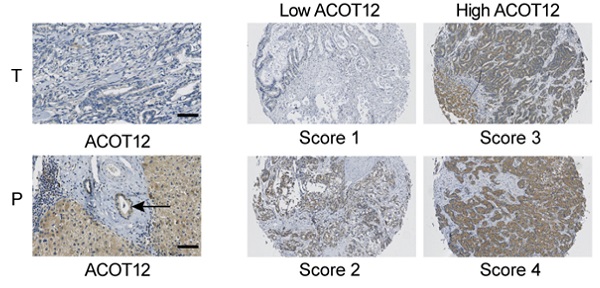
Intrahepatic cholangiocarcinoma (ICC) is the most common malignant bile duct tumor in the liver and the second most common primary liver cancer with increasing morbidity and poor prognosis. Metabolic aberration plays key roles in cancer progression. As a key metabolic intermediate, acetyl-CoA accumulation shows close association with cancer metastasis. However, the role of acetyl-CoA metabolic aberration in ICC is still undetermined. Here, by investigating tissue samples from ICC patients and ICC cell lines, we found that acyl-CoA thioesterase 12 (ACOT12) expression is significantly down-regulated in ICC tissues, and is associated with poor prognosis of ICC. In vitro and in vivo studies demonstrated that ACOT12 suppressed ICC cells metastasis. Further mechanistic studies revealed that down-regulation of ACOT12 promoted ICC metastasis by inducing Slug expression and epithelial-mesenchymal transition (EMT). Our findings link ACOT12-regulated-acetyl-coA metabolic aberration with ICC metastasis and imply that ACOT12 could be a prognostic marker and a potential therapeutic target for ICC metastasis.
Keywords: ICC, ACOT12, cancer metastasis, Acetyl-CoA, histone acetylation, EMT

 Global reach, higher impact
Global reach, higher impact