Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(8):2465-2471. doi:10.7150/jca.54496 This issue Cite
Research Paper
YTHDF1 gene polymorphisms and neuroblastoma susceptibility in Chinese children: an eight-center case-control study
1. Department of Pediatric Surgery, Guangzhou Institute of Pediatrics, Guangdong Provincial Key Laboratory of Research in Structural Birth Defect Disease, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong, China.
2. Department of Pediatric Surgery, the Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710004, Shaanxi, China.
3. Kunming Key Laboratory of Children Infection and Immunity, Yunnan Key Laboratory of Children's Major Disease Research, Yunnan Institute of Pediatrics Research, Yunnan Medical Center for Pediatric Diseases, Kunming Children's Hospital, Kunming 650228, Yunnan, China.
4. Department of Pediatric Surgery, Hunan Children's Hospital, Changsha 410004, Hunan, China.
5. Department of Hematology, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou 325027, Zhejiang, China
6. Department of Pediatric Surgery, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan, China.
7. Department of Pathology, Children Hospital and Women Health Center of Shanxi, Taiyuan 030013, Shannxi, China.
8. Department of Pediatric Surgery, Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning, China.
* These authors contributed equally to this work.
Received 2020-10-14; Accepted 2021-2-15; Published 2021-3-5
Abstract
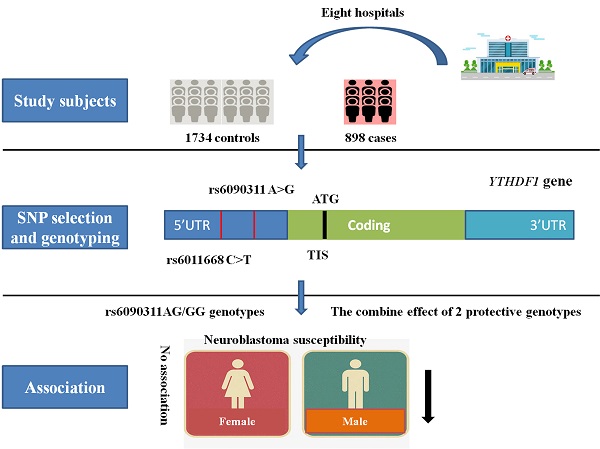
Neuroblastoma is one of the most common life-threatening extracranial tumors that mainly occurs in children, and its genetic etiology remains largely obscure. RNA m6A modification has been thought to play a key role in cancer progression. YTHDF1 is the critical downstream gene by which RNA m6A modification exerts its functions. Single nucleotide polymorphisms in the YTHDF1 gene may affect its expression and biological activity, thereby leading to abnormalities in the regulation of downstream m6A-modified RNA and eventually promoting the initiation and development of tumors. Here, we attempted to evaluate the contributions of two polymorphisms (rs6011668 C>T and rs6090311 A>G) in the YTHDF1 gene to neuroblastoma susceptibility in 898 cases and 1734 controls that originated in China. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated in the logistic regression models to evaluate the associations between selected polymorphisms and neuroblastoma risk. Overall, either in a single locus or combination analysis, no significant association with neuroblastoma risk was found for either of the two selected polymorphisms. However, the stratified analysis showed that rs6090311AG/GG genotypes significantly reduced the neuroblastoma risk in males (adjusted OR=0.77, 95% CI=0.62-0.96, P=0.018). Moreover, we found that subjects with 2 protective genotypes had a lower tumor risk in males than in those with 0-1 protective genotypes (adjusted OR=0.77, 95% CI=0.62-0.96, P=0.018). In summary, our study indicates that YTHDF1 gene polymorphisms may weakly contribute to neuroblastoma susceptibility. Our findings should be further verified by well-designed studies with larger sample sizes.
Keywords: YTHDF1, polymorphism, neuroblastoma susceptibility, m6A modification.
Introduction
Neuroblastoma, one of the most frequent extracranial pediatric tumors, derives from sympathetic neural precursors. It mainly occurs in children younger than 1 year of age, and the average age at diagnosis is approximately 17 months [1]. It accounts for approximately 10% of pediatric malignancies and almost 15% of pediatric oncology deaths [2]. Currently, neuroblastoma is ranked as the third leading cause of cancer-related pediatric deaths around the world [3]. Overall, neuroblastoma can be divided into low-, intermediate- and high-risk subgroups based on clinical phenotypes, pathological features, and prognostic factors [4]. The clinical symptoms of neuroblastoma are very extensive because of its highly heterogeneous nature. For low-risk patients, regardless of whether they are treated with minimal chemotherapy, the survival rate is above 95%; for intermediate-risk patients, which comprise 15% of all neuroblastoma patients, the survival rate is still greater than 80% after multimodal therapy. However, nearly half of patients are categorized as high-risk neuroblastoma, and despite intensive comprehensive treatments, the 5-year survival rate remains less than 40% [5, 6]. The widespread dissemination and metastasis of cancer cells at the time of diagnosis may partially contribute to such poor prognoses [7]. Moreover, survivors have great difficulty marrying and employing due to their lifelong serious coexisting health issues, which impose a great burden on affected families and society [8]. For this reason, it is urgent to identify the risk factors for neuroblastoma.
The etiology of neuroblastoma has not yet been fully clarified, and no risk factors have been well documented to influence neuroblastoma susceptibility. Some epidemiological studies have proposed that environmental factors, such as wood dust, radiation sources, and hydrocarbons, may contribute to neuroblastoma susceptibility [9, 10]. However, few children develop neuroblastoma when their parents are exposed to these environmental risk factors, and most children do not [11]. Growing evidence has shown that genetic factors play key roles in predisposing patients to neuroblastoma [12, 13]. For familial neuroblastoma, germline mutations in the PHOX2B [14] and ALK [15] genes are largely attributed to cancer risk. However, regarding sporadic neuroblastoma, the most common type of neuroblastoma, its etiology remains largely unclear. Benefitting from the rapid development of high-throughput sequencing and bioinformatics technology, genome-wide association studies (GWASs) have become a powerful tool to study the possible genetic mechanisms of complex diseases, such as human malignancy [16]. In the past ten years, several GWASs and subsequent replication studies have been conducted. As a result, a series of neuroblastoma susceptibility genes have been identified, including CASC15 [17], BARD1 [18], DUSP12, DDX4, IL31RA, HSD17B12 [19], LMO1 [20], LIN28B, HACE1 [21], CPZ and MLF1 [22]. Furthermore, NEFL [23] and CDKN1B [24] have also been found to be related to neuroblastoma susceptibility by candidate gene approaches.
RNA m6A modification is methylated at the N6 position of adenosine within messenger RNAs (mRNAs), microRNAs (miRNAs), and long noncoding RNAs (lncRNAs), which are considered the most pervasive, abundant and important chemical modifications in eukaryotic RNAs. RNA m6A modification usually occurs in the 3′ untranslated terminal region (UTR) [25], near the stop codon and translated near the 5′ UTR in an independent manner [26], therefore influencing all aspects of RNA metabolism, including RNA transcription, processing, translation and transportation. RNA m6A modification is a dynamic and reversible process that can be installed by the methyltransferase complex ('writers') and removed by demethylases ('erasers') [27]. The m6A sites can be recognized and bound by some RNA binding proteins ('readers'), leading to different destinies of the target RNA [28]. For example, it alters gene expression, which may eventually affect the corresponding cell physiological processes and functions. Mounting evidence proposes that m6A modification is related to tumorigenesis, proliferation, differentiation, invasion and metastasis [29-31]. YTHDF1, a member of the YTH domain family, functions as the 'reader' module for recognizing and binding to m6A-modified RNA [32] and then promotes target mRNA translation and protein synthesis by interacting with initiation factors [33]. In bladder cancer, METTL3 elevates the m6A level of CDCP1, enhancing its translation, which is modulated by YTHDF1, and the upregulation of METTL3 and CDCP1 is correlated with poor prognosis of bladder cancer [34]. In endometrial tumors, m6A methylation of the AKT negative regulator PHLPP2 can facilitate YTHDF1-mediated translation of the PHLPP2 gene, inhibiting the AKT signaling pathway and leading to attenuated cell proliferation, migration and invasion [30]. In liver cancer, high expression of METTL3 and YTHDF1 is associated with worse overall survival, which leads to elevated m6A levels of the Snail gene, a pivotal transcription factor for epithelial-to-mesenchymal transition (EMT), and increases Snail expression through YTHDF1-mediated translation [35]. Thus, it is tempting to speculate that functional single nucleotide polymorphisms (SNPs) in YTHDF1 may influence its expression and binding ability to m6A-modified RNA, which may deregulate downstream target genes, further causing cell dysfunction and eventually tumorigenesis [36]. However, no studies regarding the association between YTHDF1 gene polymorphisms and neuroblastoma susceptibility have been published. Therefore, we conducted this eight-center case-control study to explore the association between SNPs in the YTHDF1 gene and neuroblastoma risk in Chinese children.
Materials and Methods
Study population
In the current case-control study, 898 cases with neuroblastoma and 1734 cancer-free controls were included, and the demographic characteristics of all participants are displayed in Table S1. The studied subjects were recruited from eight different regions of China. The criteria of acceptability for the enrolled subjects were described in a previous publication [13]. Written informed consent was provided by all participants or their guardians before the study. The study was approved by the Institutional Review Board of each participating hospital.
SNP selection and genotyping
Two potentially functional polymorphisms (rs6011668 C>T and rs6090311 A>G) in the YTHDF1 gene were chosen through the dbSNP database (http://www.ncbi.nlm.nih.gov/) and SNPinfo (http://snpinfo.niehs.nih.gov/). The selection criteria were described previously in detail [13, 37]. There was no significant LD (R2<0.8) among these two selected SNPs (R2=0.094 between rs6011668 C>T and rs6090311 A>G), which was calculated in our previous publication [38]. Both SNPs were located in the 5' region near the gene, a crucial region for gene expression regulation by numerous transcription factors. Polymorphisms in this region may influence the binding of transcription factors and the gene transcription of YTHDF1. Then, the affected YTHDF1 gene may further influence its downstream genes and ultimately cause a series of abnormalities in downstream biological functions, including cancer susceptibility. For genotyping, we extracted genomic DNA from the peripheral blood of all participants by a TIANamp Blood DNA Kit (TianGen Biotech Co. Ltd., Beijing, China). Then, the purified DNA samples were added to 96-well plates and diluted to 5 ng/μL, and genotyping of all DNA samples for the selected SNPs was performed in 384-well format by standard TaqMan real-time PCR [39-41]. Ten percent of the DNA samples were chosen randomly to genotype again to ensure the authenticity of the results. Two sets of genotype concordance rates reached 100%.
Statistical analysis
The goodness-of-fit χ2 test was applied to check whether the selected SNPs deviated from Hardy-Weinberg equilibrium (HWE) in the controls. The comparisons of demographic distributions and allele frequencies between all cases and controls were conducted by the two-sided chi-square test. The odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess associations between the YTHDF1 polymorphisms and neuroblastoma susceptibility through logistic regression analysis. Furthermore, adjusted ORs and corresponding 95% CIs adjusted for age and sex were calculated by unconditional multivariate logistic regression analysis. Finally, we performed a stratified analysis based on age, sex, tumor origin site, and clinical stage. All statistical analyses were carried out by SAS software (version 9.4 SAS Institute, NC, USA). When the P-value was <0.05, the result was considered to be statistically significant.
Results
Associations between YTHDF1 polymorphisms and neuroblastoma risk
This eight-center case-control study contained 898 cases and 1734 controls, of which genotyping was successfully performed in 896 cases and 1733 controls. As shown in Table 1, the genotype frequencies of both selected SNPs were consistent with HWE among the control subjects (HWE=0.518 for rs6011668 C>T and HWE=0.285 for rs6090311 A>G). In the single locus analysis, no significant association was found between the selected polymorphisms and neuroblastoma risk, and the same result was found in the combined analysis.
Stratification analysis
To evaluate whether the selected YTHDF1 polymorphisms affect neuroblastoma risk among different subgroups, a stratified analysis was carried out according to age, sex, site of tumor origin, and clinical stage (Table 2). We failed to find a significant association between the rs6011668 C>T polymorphism and neuroblastoma risk among subgroups. However, we found that subjects with rs6090311AG/GG genotypes had a lower risk of developing neuroblastoma in the male subgroup (adjusted OR=0.77, 95% CI=0.62-0.96, P=0.018) than in the reference group. Further combined analysis showed that subjects harboring 2 protective genotypes had a significantly reduced neuroblastoma risk compared with those with 0-1 protective genotypes in the male subgroup (adjusted OR=0.77, 95% CI=0.62-0.96, P=0.018).
Association between YTHDF1 gene polymorphisms and neuroblastoma susceptibility
| Genotype | Cases (N=896) | Controls (N=1733) | P a | Crude OR (95% CI) | P | Adjusted OR (95% CI) b | P b |
|---|---|---|---|---|---|---|---|
| rs6011668 C>T (HWE=0.518) | |||||||
| CC | 647 (72.21) | 1258 (72.59) | 1.00 | 1.00 | |||
| CT | 229 (25.56) | 441 (25.45) | 1.01 (0.84-1.22) | 0.919 | 1.01 (0.84-1.22) | 0.885 | |
| TT | 20 (2.23) | 34 (1.96) | 1.14 (0.65-2.00) | 0.639 | 1.16 (0.66-2.04) | 0.597 | |
| Additive | 0.751 | 1.03 (0.87-1.21) | 0.751 | 1.03 (0.88-1.21) | 0.703 | ||
| Dominant | 249 (27.79) | 475 (27.41) | 0.836 | 1.02 (0.85-1.22) | 0.836 | 1.02 (0.86-1.23) | 0.793 |
| Recessive | 876 (97.77) | 1699 (98.04) | 0.643 | 1.14 (0.65-1.99) | 0.644 | 1.16 (0.66-2.03) | 0.604 |
| rs6090311 A>G (HWE=0.285) | |||||||
| AA | 374 (41.74) | 667 (38.49) | 1.00 | 1.00 | |||
| AG | 411 (45.87) | 833 (48.07) | 0.88 (0.74-1.05) | 0.148 | 0.88 (0.74-1.04) | 0.137 | |
| GG | 111 (12.39) | 233 (13.44) | 0.85 (0.66-1.10) | 0.218 | 0.84 (0.65-1.09) | 0.189 | |
| Additive | 0.121 | 0.91 (0.81-1.03) | 0.121 | 0.91 (0.80-1.02) | 0.103 | ||
| Dominant | 522 (58.26) | 1066 (61.51) | 0.106 | 0.87 (0.74-1.03) | 0.106 | 0.87 (0.74-1.02) | 0.094 |
| Recessive | 785 (87.61) | 1500 (86.56) | 0.446 | 0.91 (0.72-1.16) | 0.447 | 0.90 (0.71-1.15) | 0.405 |
| Combined effect of protective genotypes c | |||||||
| 0 | 20 (2.23) | 34 (1.96) | 1.00 | 1.00 | |||
| 1 | 354 (39.51) | 633 (36.53) | 0.95 (0.54-1.68) | 0.861 | 0.94 (0.53-1.66) | 0.825 | |
| 2 | 522 (58.26) | 1066 (61.51) | 0.83 (0.47-1.46) | 0.523 | 0.82 (0.47-1.44) | 0.483 | |
| 0-1 | 374 (41.74) | 667 (38.49) | 1.00 | 1.00 | |||
| 2 | 522 (58.26) | 1066 (61.51) | 0.106 | 0.87 (0.74-1.03) | 0.106 | 0.87 (0.74-1.02) | 0.094 |
a χ2 test for genotype distributions between neuroblastoma cases and controls. b Adjusted for age and sex. c Protective genotypes were rs6011668 CC/CT and rs6090311 AG/GG.
Stratification analysis for the association between YTHDF1 genotypes and neuroblastoma susceptibility
| Variables | rs6011668 (case/control) | Adjusted OR a | P a | rs6090311 (case/control) | Adjusted OR a | P a | Protective genotypes (case/control) | Adjusted OR a | P a | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT/TT | (95% CI) | AA | AG/GG | (95% CI) | 0-1 | 2 | (95% CI) | ||||
| Age, month | ||||||||||||
| ≤18 | 237/517 | 107/196 | 1.19 (0.90-1.58) | 0.228 | 155/276 | 189/437 | 0.77 (0.59-1.00) | 0.051 | 155/276 | 189/437 | 0.77 (0.59-1.00) | 0.051 |
| >18 | 410/741 | 142/279 | 0.91 (0.72-1.16) | 0.457 | 219/391 | 333/629 | 0.95 (0.76-1.17) | 0.599 | 219/391 | 333/629 | 0.95 (0.76-1.17) | 0.599 |
| Sex | ||||||||||||
| Female | 298/531 | 108/213 | 0.91 (0.69-1.19) | 0.481 | 158/294 | 248/450 | 1.01 (0.79-1.30) | 0.915 | 158/294 | 248/450 | 1.01 (0.79-1.30) | 0.915 |
| Male | 349/727 | 141/262 | 1.13 (0.89-1.44) | 0.333 | 216/373 | 274/616 | 0.77 (0.62-0.96) | 0.018 | 216/373 | 274/616 | 0.77 (0.62-0.96) | 0.018 |
| Sites of origin | ||||||||||||
| Adrenal gland | 184/1258 | 64/475 | 0.92 (0.68-1.25) | 0.606 | 106/667 | 142/1066 | 0.83 (0.63-1.09) | 0.172 | 106/667 | 142/1066 | 0.83 (0.63-1.09) | 0.172 |
| Retroperitoneal | 231/1258 | 87/475 | 1.01 (0.77-1.32) | 0.949 | 129/667 | 189/1066 | 0.91 (0.71-1.16) | 0.437 | 129/667 | 189/1066 | 0.91 (0.71-1.16) | 0.437 |
| Mediastinum | 151/1258 | 62/475 | 1.08 (0.79-1.48) | 0.635 | 93/667 | 120/1066 | 0.81 (0.61-1.08) | 0.153 | 93/667 | 120/1066 | 0.81 (0.61-1.08) | 0.153 |
| Others | 70/1258 | 35/475 | 1.31 (0.86-2.00) | 0.204 | 42/667 | 63/1066 | 0.95 (0.63-1.42) | 0.790 | 42/667 | 63/1066 | 0.95 (0.63-1.42) | 0.790 |
| INSS stage | ||||||||||||
| I+II+4s | 341/1258 | 128/475 | 0.99 (0.79-1.24) | 0.926 | 203/667 | 266/1066 | 0.82 (0.67-1.01) | 0.064 | 203/667 | 266/1066 | 0.82 (0.67-1.01) | 0.064 |
| III+IV | 284/1258 | 110/475 | 1.04 (0.81-1.33) | 0.768 | 155/667 | 239/1066 | 0.95 (0.75-1.19) | 0.627 | 155/667 | 239/1066 | 0.95 (0.75-1.19) | 0.627 |
a Adjusted for age and sex, omitting the corresponding stratification factor.
Discussion
To explore the correlation between YTHDF1 gene polymorphisms and neuroblastoma susceptibility, we conducted the present eight-center case-control study in a Chinese population. To the best of our knowledge, this is the first study to evaluate the association between SNPs within the YTHDF1 gene and neuroblastoma risk. However, neither of the two studied SNPs was correlated with neuroblastoma risk.
YTHDF1, which is localized on chromosome 20q11, plays a “reader” role in the m6A modification pathway. As an RNA-binding protein, it functions as a translation regulator by specifically binding to m6A-modified mRNA and then promoting cap-dependent translation [26], enhancing ribosome loading on m6A-containing mRNA and recruiting translation initiation factors, such as eIF3A or eIF3B, to promote the translation efficiency of targeted mRNA [33]. Aberrant expression of YTHDF1 may change the translation efficiency and the expression level of downstream targeted genes, alter the biological functions of cells, and eventually induce oncogenesis. Dysregulation of m6A modification has been shown to be closely related to the initiation and progression of various cancers, such as hepatocellular carcinoma, lung cancer, and acute myeloid leukemia. Numerous studies have shown that YTHDF1 is overexpressed in various cancers, such as colorectal cancer [42], hepatocellular carcinoma [43], breast cancer [44], Merkel cell carcinoma [45], non-small cell lung cancer [46], and ovarian cancer [47], which are closely associated with an increased risk of these cancers. In colorectal cancer, upregulated YTHDF1 could stabilize transcripts of the oncogene C-MYC and then promote tumor cell proliferation [48]. Furthermore, key Wnt signaling components, such as TCF4, DVL3, and FZD7, and the β-catenin major transcriptional effector TCF7L2 are direct targets of YTHDF1, and YTHDF1 can activate the Wnt/β-catenin signaling pathway by regulating these targeted genes and then promote intestinal stemness and tumorigenesis of colorectal cancer [49]. Moreover, YTHDF1 can directly target EIF3C and augment EIF3C translation in an m6A-dependent manner, facilitating the tumorigenesis and metastasis of ovarian cancer [47].
Increasing evidence has indicated that genetic variations, such as copy number variation (CNV) and SNPs, which are related to m6A modification modulators, are closely correlated with the malignant progression of various cancers [42, 50, 51]. DNA copy number gain is a key cause of aberrant overexpression of oncogenes in cancer [52]. There is a certain association between DNA copy number amplification and YTHDF1 overexpression. Bai et al. found that YTHDF1 is upregulated in colorectal cancer, and the gain of copy number may be a major mechanism driving the overexpression of YTHDF1 [42]. One study performed by Liu et al. showed that the YTHDF1 gene was upregulated by frequent amplification in high-grade serous ovarian cancer [47]. Importantly, oncogene overexpression driven by gene amplification may be a crucial event during cancer evolution. Furthermore, functional SNPs in gene regulatory regions may also alter gene expression rather than contributing to cancer risk. Numerous studies have reported that SNPs in oncogenes or tumor suppressor genes can modify cancer susceptibility [21-24, 53]. However, few studies have been conducted regarding YTHDF1 gene polymorphisms and cancer risk.
To date, only one other study, performed by Meng et al. [54], has assessed the association between SNPs in the YTHDF1 gene and cancer susceptibility. However, they failed to find any relationship between SNPs rs2024768 and rs6090289 in the YTHDF1 gene and colorectal cancer risk, but they revealed that SNP rs118049207 located in the SND1 gene could modify the mRNA expression of SND1 and then change the m6A level. Furthermore, the SNP rs118049207 was shown to be associated with colorectal cancer susceptibility. In our recent study, we evaluated the association between two SNPs (rs6011668 C>T and rs6090311 A>G) in the YTHDF1 gene and hepatoblastoma susceptibility. The results showed that rs6011668 C>T was not associated with hepatoblastoma susceptibility; however, participants with the rs6090311 G allele had a significantly decreased risk of hepatoblastoma. In subsequent expression quantitative trait locus (eQTL) analysis, we found that the rs6090311 G allele was related to reduced expression of the BIRC7, RP5-963E22.4 and NKAIN4 genes [38].
To the best of our knowledge, this current study is the first to explore the correlation between YTHDF1 gene polymorphisms and neuroblastoma susceptibility. In this eight-center case-control study, we assessed whether two SNPs (rs6011668 C>T and rs6090311 A>G) affect neuroblastoma susceptibility, which is located in the 5' region of YTHDF1, a vital region for regulating gene expression. We failed to find any relationships between the selected SNPs and neuroblastoma risk in the single locus or combination analysis. The paradoxical results may be attributed to different types of cancer. However, in the stratified analysis, we found that the rs6090311AG/GG genotype significantly decreased the neuroblastoma risk in males, and the participants with 2 protective genotypes had a reduced tumor risk in males when compared to those with 0-1 protective genotypes. It should be noted that these positive results may have been due to the relatively small sample size in the stratification analysis.
Several limitations of this study should be mentioned. First, the sample size remained relatively moderate even though the subjects in this study were recruited from eight independent hospitals, especially for the stratification analysis. Second, only two SNPs in the YTHDF1 gene were evaluated, and more potentially functional SNPs in the YTHDF1 gene should be investigated. Third, the participants involved in this study were of Chinese origin; therefore, the conclusions obtained from this study may not be suitable for other ethnicities. Fourth, only genetic analysis was conducted on neuroblastoma risk, incorporating analysis on environmental factors and genetic-environmental factors. Neuroblastoma is a heterogeneous disease with complicated etiologies.
In conclusion, our present results indicate that YTHDF1 polymorphisms (rs6090311 A>G) may affect neuroblastoma susceptibility in a low-penetrance and sex-dependent manner. Well-designed studies with a larger sample size are needed to verify our conclusion. Furthermore, mechanistic research should be carried out to expound on the underlying mechanisms by which YTHDF1 gene polymorphisms affect neuroblastoma susceptibility.
Supplementary Material
Supplementary table.
Acknowledgements
This study was supported by grants from the Natural Science Foundation of Guangdong Province (No: 2019A1515010360), National Natural Science Foundation of China (No: 81960294, 81560262), Yunnan Key Laboratory of Research on Children's Critical Diseases (No: 202005AG070073); the Basic Applied Study Planning Projects of Yunnan Province (No: 2018FB130), and the Guangdong Provincial Key Laboratory of Research in Structural Birth Defect Disease (No: 2019B030301004).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13:397-411
2. Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L. et al. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078
3. Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M. et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28:2625-34
4. Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B. et al. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer. 1999;86:364-72
5. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106-20
6. Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202-11
7. Esposito MR, Aveic S, Seydel A, Tonini GP. Neuroblastoma treatment in the post-genomic era. J Biomed Sci. 2017;24:14
8. Laverdiere C, Liu Q, Yasui Y, Nathan PC, Gurney JG, Stovall M. et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:1131-40
9. De Roos AJ, Olshan AF, Teschke K, Poole C, Savitz DA, Blatt J. et al. Parental occupational exposures to chemicals and incidence of neuroblastoma in offspring. Am J Epidemiol. 2001;154:106-14
10. De Roos AJ, Teschke K, Savitz DA, Poole C, Grufferman S, Pollock BH. et al. Parental occupational exposures to electromagnetic fields and radiation and the incidence of neuroblastoma in offspring. Epidemiology. 2001;12:508-17
11. Patton T, Olshan AF, Neglia JP, Castleberry RP, Smith J. Parental exposure to medical radiation and neuroblastoma in offspring. Paediatr Perinat Epidemiol. 2004;18:178-85
12. Capasso M, Diskin SJ. Genetics and genomics of neuroblastoma. Cancer Treat Res. 2010;155:65-84
13. He J, Wang F, Zhu J, Zhang R, Yang T, Zou Y. et al. Association of potentially functional variants in the XPG gene with neuroblastoma risk in a Chinese population. J Cell Mol Med. 2016;20:1481-90
14. Mosse YP, Laudenslager M, Khazi D, Carlisle AJ, Winter CL, Rappaport E. et al. Germline PHOX2B mutation in hereditary neuroblastoma. Am J Hum Genet. 2004;75:727-30
15. Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF. et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930-5
16. Stadler ZK, Thom P, Robson ME, Weitzel JN, Kauff ND, Hurley KE. et al. Genome-wide association studies of cancer. J Clin Oncol. 2010;28:4255-67
17. Maris JM, Mosse YP, Bradfield JP, Hou C, Monni S, Scott RH. et al. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N Engl J Med. 2008;358:2585-93
18. Capasso M, Devoto M, Hou C, Asgharzadeh S, Glessner JT, Attiyeh EF. et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat Genet. 2009;41:718-23
19. Nguyen le B, Diskin SJ, Capasso M, Wang K, Diamond MA, Glessner J. et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility Loci. PLoS Genet. 2011;7:e1002026
20. Wang K, Diskin SJ, Zhang H, Attiyeh EF, Winter C, Hou C. et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature. 2011;469:216-20
21. Diskin SJ, Capasso M, Schnepp RW, Cole KA, Attiyeh EF, Hou C. et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat Genet. 2012;44:1126-30
22. McDaniel LD, Conkrite KL, Chang X, Capasso M, Vaksman Z, Oldridge DA. et al. Common variants upstream of MLF1 at 3q25 and within CPZ at 4p16 associated with neuroblastoma. PLoS Genet. 2017;13:e1006787
23. Capasso M, Diskin S, Cimmino F, Acierno G, Totaro F, Petrosino G. et al. Common genetic variants in NEFL influence gene expression and neuroblastoma risk. Cancer Res. 2014;74:6913-24
24. Capasso M, McDaniel LD, Cimmino F, Cirino A, Formicola D, Russell MR. et al. The functional variant rs34330 of CDKN1B is associated with risk of neuroblastoma. J Cell Mol Med. 2017;21:3224-30
25. Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A. et al. A majority of m6A residues are in the last exons, allowing the potential for 3' UTR regulation. Genes Dev. 2015;29:2037-53
26. Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O. et al. 5' UTR m(6)A Promotes Cap-Independent Translation. Cell. 2015;163:999-1010
27. Dai D, Wang H, Zhu L, Jin H, Wang X. N6-methyladenosine links RNA metabolism to cancer progression. Cell Death Dis. 2018;9:124
28. Casella G, Tsitsipatis D, Abdelmohsen K, Gorospe M. mRNA methylation in cell senescence. Wiley Interdiscip Rev RNA. 2019;10:e1547
29. Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol Cell. 2016;62:335-45
30. Liu J, Eckert MA, Harada BT, Liu SM, Lu Z, Yu K. et al. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20:1074-83
31. Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH, Wang F. et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology. 2017;65:529-43
32. Xu C, Wang X, Liu K, Roundtree IA, Tempel W, Li Y. et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10:927-9
33. Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H. et al. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161:1388-99
34. Yang F, Jin H, Que B, Chao Y, Zhang H, Ying X. et al. Dynamic m(6)A mRNA methylation reveals the role of METTL3-m(6)A-CDCP1 signaling axis in chemical carcinogenesis. Oncogene. 2019;38:4755-72
35. Lin X, Chai G, Wu Y, Li J, Chen F, Liu J. et al. RNA m(6)A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat Commun. 2019;10:2065
36. Tan H. The association between gene SNPs and cancer predisposition: Correlation or causality? EBioMedicine. 2017;16:8-9
37. He J, Yang T, Zhang R, Zhu J, Wang F, Zou Y. et al. Potentially functional polymorphisms in the LIN28B gene contribute to neuroblastoma susceptibility in Chinese children. J Cell Mol Med. 2016;20:1534-41
38. Luo Z, Li G, Wang M, Zhu J, Yang Z, Li Y. et al. YTHDF1 rs6090311 A>G polymorphism reduces Hepatoblastoma risk: Evidence from a seven-center case-control study. J Cancer. 2020;11:5129-34
39. He J, Qiu LX, Wang MY, Hua RX, Zhang RX, Yu HP. et al. Polymorphisms in the XPG gene and risk of gastric cancer in Chinese populations. Hum Genet. 2012;131:1235-44
40. Lou J, Gong J, Ke J, Tian J, Zhang Y, Li J. et al. A functional polymorphism located at transcription factor binding sites, rs6695837 near LAMC1 gene, confers risk of colorectal cancer in Chinese populations. Carcinogenesis. 2017;38:177-83
41. Chang J, Tian J, Yang Y, Zhong R, Li J, Zhai K. et al. A Rare Missense Variant in TCF7L2 Associates with Colorectal Cancer Risk by Interacting with a GWAS-Identified Regulatory Variant in the MYC Enhancer. Cancer Res. 2018;78:5164-72
42. Bai Y, Yang C, Wu R, Huang L, Song S, Li W. et al. YTHDF1 Regulates Tumorigenicity and Cancer Stem Cell-Like Activity in Human Colorectal Carcinoma. Front Oncol. 2019;9:332
43. Zhao X, Chen Y, Mao Q, Jiang X, Jiang W, Chen J. et al. Overexpression of YTHDF1 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Biomark. 2018;21:859-68
44. Liu L, Liu X, Dong Z, Li J, Yu Y, Chen X. et al. N6-methyladenosine-related Genomic Targets are Altered in Breast Cancer Tissue and Associated with Poor Survival. J Cancer. 2019;10:5447-59
45. Orouji E, Peitsch WK, Orouji A, Houben R, Utikal J. Oncogenic Role of an Epigenetic Reader of m(6)A RNA Modification: YTHDF1 in Merkel Cell Carcinoma. Cancers (Basel). 2020;12:202
46. Shi Y, Fan S, Wu M, Zuo Z, Li X, Jiang L. et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat Commun. 2019;10:4892
47. Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y. et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48:3816-31
48. Nishizawa Y, Konno M, Asai A, Koseki J, Kawamoto K, Miyoshi N. et al. Oncogene c-Myc promotes epitranscriptome m(6)A reader YTHDF1 expression in colorectal cancer. Oncotarget. 2018;9:7476-86
49. Han B, Yan S, Wei S, Xiang J, Liu K, Chen Z. et al. YTHDF1-mediated translation amplifies Wnt-driven intestinal stemness. EMBO Rep. 2020;21:e49229
50. Li Z, Qian P, Shao W, Shi H, He XC, Gogol M. et al. Suppression of m(6)A reader Ythdf2 promotes hematopoietic stem cell expansion. Cell Res. 2018;28:904-17
51. Qian JY, Gao J, Sun X, Cao MD, Shi L, Xia TS. et al. KIAA1429 acts as an oncogenic factor in breast cancer by regulating CDK1 in an N6-methyladenosine-independent manner. Oncogene. 2019;38:6123-41
52. Tang J, Chen H, Wong CC, Liu D, Li T, Wang X. et al. DEAD-box helicase 27 promotes colorectal cancer growth and metastasis and predicts poor survival in CRC patients. Oncogene. 2018;37:3006-21
53. Zhuo Z, Zhou C, Fang Y, Zhu J, Lu H, Zhou H. et al. Correlation between the genetic variants of base excision repair (BER) pathway genes and neuroblastoma susceptibility in eastern Chinese children. Cancer Commun (Lond). 2020;40:641-6
54. Meng Y, Li S, Gu D, Xu K, Du M, Zhu L. et al. Genetic variants in m6A modification genes are associated with colorectal cancer risk. Carcinogenesis. 2020;41:8-17
Author contact
![]() Corresponding authors: Jing He, Department of Pediatric Surgery, Guangzhou Institute of Pediatrics, Guangdong Provincial Key Laboratory of Research in Structural Birth Defect Disease, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, 9 Jinsui Road, Guangzhou 510623, Guangdong, China, Email: hejing198374com; or Zhonghua Yang, Department of Pediatric Surgery, Shengjing Hospital of China Medical University, 36 Sanhao Street, Heping District, Shenyang 110004, Liaoning, China, Email: yangzh5283com.
Corresponding authors: Jing He, Department of Pediatric Surgery, Guangzhou Institute of Pediatrics, Guangdong Provincial Key Laboratory of Research in Structural Birth Defect Disease, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, 9 Jinsui Road, Guangzhou 510623, Guangdong, China, Email: hejing198374com; or Zhonghua Yang, Department of Pediatric Surgery, Shengjing Hospital of China Medical University, 36 Sanhao Street, Heping District, Shenyang 110004, Liaoning, China, Email: yangzh5283com.

 Global reach, higher impact
Global reach, higher impact