Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(18):5424-5431. doi:10.7150/jca.46308 This issue Cite
Review
A brief review concerning Chimeric Antigen Receptors T cell therapy
1. Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Kunming Medical University, Kunming, 650101, Yunnan, China.
2. Department of Nephrology, The First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan, China.
3. Department of Nephrology, The Third People's Hospital of Yunnan Province, Kunming, Yunnan, P.R. China.
#These authors equally contributed to this article as co-first authors.
Received 2020-3-24; Accepted 2020-6-24; Published 2020-7-11
Abstract
The understanding concerning the function of immune system in cancer has achieved considerable advance with time passes by. Manipulating genetically engineered immune cells were investigated as a novel strategy for treating cancer. Chimeric antigen receptors (CARs) are recombinant protein molecules by merging the exquisite targeting the potent cytotoxicity of T cells and specificity of monoclonal antibodies and, which could trigger serial cascades of signal transduction and thereby activate T cells to directly destroy the tumor cells. Manufacturing CAR-modified T lymphocytes were successfully implemented in treating cancer derived from they could specifically retarget tumor-associated antigens, causing effective elimination of tumor cells, which spurred the optimization and development of new CAR-T cell technology. The advancement of synthetic biology methodologies of cell therapy in CAR-T would ultimately provide us with a much safer, reliable and efficient modality to against cancer. This review primarily described the emergence, development and application of cell therapy in CAR-T, then discuss the side effects and the potential factors of tumor reccurrence caused by CAR-T cell therapy, in addition to the corresponding countermeasure concerning complications.
Keywords: Chimeric antigen receptors, Cancer, Complication, Relapse
Introduction
As we all known, the immune system was traditionally divided into two main types consisting of innate and adaptive immunity, which can vigorously identify, guard and defend against foreign invaders [1]. Typically, innate immunity was activated with an instant and non-specific way involved in various cells including innate lymphoid cells neutrophils, mast cells, natural killer (NK) cells and macrophages cells [2], which could be promptly triggered within minutes to hours through rapid defense mechanisms following pathogens invasion [3]. Conversely, the adaptive immune response slowly emerged depending on the initiation and subsequent outcome of innate immune response at several days after undergoing damage. Numerous cells participated in the process of regulating immune response. Dendritic cells (DCs) and macrophages could, as antigen-presenting cells (APCs), secrete cytokines to stimulate immune cells and present T cells with antigens forming major histocompatibility complex (MHC) molecules [4]. Moreover, T cells were swiftly activated so that they can modulate many aspects of the immune response. Activated T cells would acquire memory after proliferating and, and thereafter causing migration of effector T cells to the initial antigens binding site [5]. Research shows that T cells are critical against genesis and tumor development derived from their unparalleled ability comprising responding to antigens and then formulating memory, allowing a rapid robust response upon a similar antigen invasion in future [6]. Accumulated evidences strongly indicated that the crosstalk between T cells and antigen-presenting cells and could effectively protect against the invasion and destroy of malignant cells and foreign pathogens [7]. Generally, T cells could rapidly recognize the tumor-associated antigens then kill tumor cells, and long-term protect against tumor recurrence [8]. Currently, cancer immunotherapy has drawn widespread attention in recent times, with treatment mainly destroyed the tumor and prevented its recurrence by activating the adaptive immune system of cancer patients. With the relentless march of science and technological progress, cancer immunotherapy had achieved a major breakthrough owing to the use of monoclonal antibodies and development of adoptive T cell therapies [9, 10]. Therein, Chimeric antigen receptors (CARs) are synthetic receptors rooted in reprograming T cells by embedding a single chain variable fragment (scFv) ectodomain and an endodomain consisting of CD3 TCR signal in addition to the costimulatory domain [11], which could specifically target antigens and kill tumor cells [12]. To date, the CAR-T cell therapies had been applied for treating certain tumors like acute myeloid leukemia [13], gastric cancer [14], and so on. And CAR-T cell therapy had achieved tremendous success in eradicating hematologic malignancies. Here, we reviewed the great potential and bright prospects, and also noted the current issues induced by employing CAR-T cells therapy in tumors treatment.
The emergence and development of CAR-T cell therapy
In order to protect the organism and against pathogens invasion, the immune system evolve gradually to distinguish self from non-self. To eliminate cancerous cells, immune system would patrol and spot these dangerous cells via certain signals in the cell surface. However, cancer cells are stemmed from the normal cell gene mutations, and they have evolved the unusual ability including immune evasion and suppression. Therefore, incurable cancer has become one of the most massive challenges to all human societies in this world. The emergence of genetically modified T cells initiated a new epoch for cancer therapy. CAR-T cell could effectively recognize, target and destroy cancer cells through a MHC-independent mechanism. The early engineered T cell could recognize intracellular and extracellular antigen in perspective that MHC depend on cloned T cell receptors (TCR) expression. Whereas, many tumors downregulated the expression of MHC to hide from the TCR engineered T cell. Consequently, chimeric antigen receptors (CAR) were employed to strengthen T cell specificity by combining B cell receptor and T cell receptor domains. At present, CAR-T cell therapy has yielded stunning outcomes in patients with hematologic malignancies. For instance, CD19 is a marker that is highly expressed in B cells and B cell-derived malignancies cell surface [15]. CD19-directed CAR-T cells (CART19) therapy had contributed to durable and complete remissions in patients with relapsed and refractory B cell malignancies [16, 17]. Nevertheless, CAR-T cell therapy is different from inanimate platforms (antibodies or small molecules) on account of cells capability to sense intelligently and the behaviors of response. Many studies have been conducted regarding how to manufacture, manipulate and control these cellular devices. In future, the advancement of synthetic biology strategies of CAR-T cell therapy would ultimately provide us with a much safer, reliable and efficient modality to against cancer.
Concise overview concerning CAR-T cell therapy
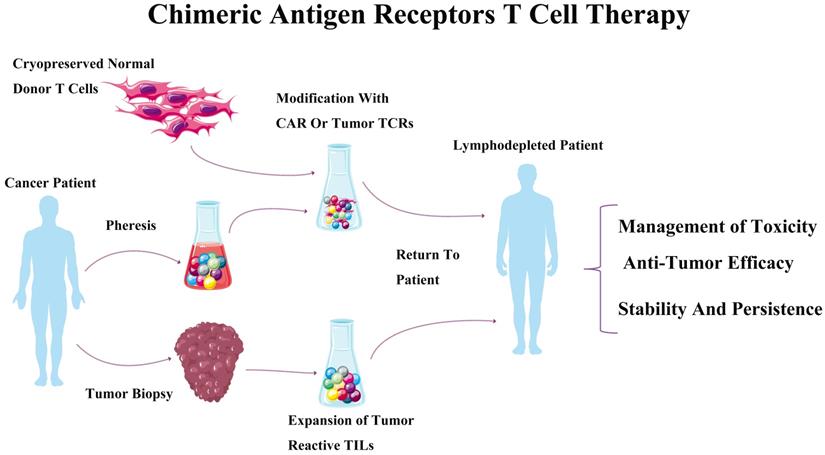
The CAR-T receptor are synthetic transmembrane receptors [18], which are made up of a single chain extracellular antibody fragment (scFv), and intracellular signaling domains from the T Cell Receptor (TCR) and costimulatory molecules [19]. CAR-modified lymphocytes have spurred the rapid development for the therapeutically areas of oncology by directing against a broad range of target antigens. CARs could, as recombinant protein molecules, regulate cell activation and redirect cytotoxic lymphocytes toward target antigens including malignant and other target cells [11]. The antigen-recognition domain of CARs mainly depends on the sequences of monoclonal antibodies (mAbs) to interact with tumor epitopes in an MHC-unrestricted mode. Briefly, CAR-T cell therapy was described as following: a CAR-encoding DNA cassette was constructed and delivered into primary T cells obtained from a patient. Reprogrammed CAR-T cells were abundantly increased in ex vivo environments, and then re-infused into the patient body. Antigen-recognition domain of CAR would activate T cells to destruct the tumor cells when encountered with target tumor cells (Figure 1) [20]. For now, the high feasibility of CAR-T cell technology implementing in treatment of hematologic malignancies [15, 21] indicated that CAR strategy might be a broadly applicable remedy for cancer [22].
The essential properties of CAR-T cell therapy
Currently, the genetically engineered CAR-T cell therapy has drawn increasing public attention as a new paradigm of cancer immunotherapy methods. The efficacy, stability and persistence of CAR-T cell in vivo were crucial for exerting its anti-tumor activities. These essential properties of CAR-T cell were acquired by using genome editing tools consisting of clustered regulatory interspaced short palindromic repeat, zinc-finger nucleases, and CRISPR-associated protein 9 (CRISPR/Cas9) techniques, and so on [23, 24]. These techniques were useful to trace the lineage of CAR-T cell in vivo [25], and the functional activity was enhanced and the side effects were managed via the tetracycline regulatory system [26]. Meanwhile, the CAR-T cell persistence was enhancing by altering the costimulatory domain [27]. Moreover, the anti-tumor efficacy of CAR-T cell was augmented by disrupting programmed cell death protein 1 (PD-1) [28], and the progeny of CAR-T cell through disruption of TET2 also effectively improved the therapeutic efficacy [29].
The potential mechanisms of CAR-T cell therapy
To deeply illustrate the intricate relationship between tumor cells and immune system would conduce to accelerate the research and development of anti-tumor consisting of rejuvenation of host immunity, activating the immune cells against the cancer cells, and removing the ageing cells. To date, one of the most difficult problems concerning tumor therapy derived from tumor cells escaped from the surveillance of immune system. Generally, tumor cells avoid from cell mediated immunity by changing surface proteins and intracellular signal molecules [30]. Extensive research showed that antibodies based allogeneic stem cell transplantation, immunotherapies, and CAR-T cell therapy effectively suppressed the proliferation and survival of tumor cells [31, 32]. Epigenetic mechanisms affect the efficacy of CAR-T cell therapy and induce resistance against tumor cells. In addition, a battery of chromatin modifiers were implicated with maintaining proper chromatin structure spatially and temporally. Chromatin states and DNA modifications integrate signal to suppress/active gene expression by acting as the signaling platform [33], and mediate the gene expression pattern [34]. Signal transduction pathways also directly cross talk chromatin to change the epigenetic landscape, and the covalent modifications improve the therapeutic outcomes of CAR-T cell. Epigenetic agent administration prevents immune escape of tumor cells, and enhances the anti-tumor activity of CAR-T cell involving in posttranslational regulation [35]. Therefore, epigenetic mechanism comprising of chromatin/DNA methylation states and non-coding RNA was further investigated to demonstrate the correlation between tumor prognosis and CAR-T cell therapy.
Chimeric antigen receptors T cell therapy. The process of chimeric antigen receptors T cell therapy, which mainly includes tumor biopsy, pheresis and expiation, then modification with CAR or tumor TCRs was transfused to tumor patient.
Combination of CAR-T cell therapy with other therapeutics
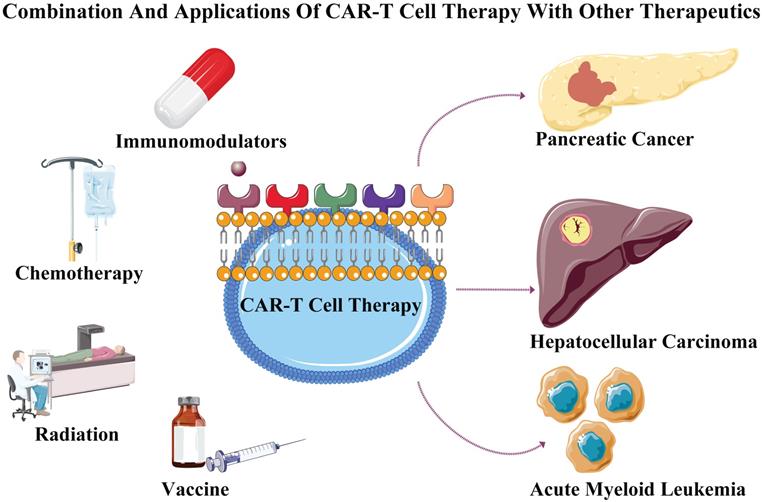
Tumor formation is a gradually evolving process involving in multiple mutations, The CAR-T cell therapy combined with other therapeutics may achieve specific toxicity to effectively inhibit the tumor progression and kill tumor cells [36]. Solid tumors are compact and inaccessible to infiltration, and appropriate heat treatment may increase the drug's permeability. Hence, CAR-T cell combined with photothermal therapy may improve the permeability of CAR-T cells [37]. Meanwhile, the immune-checkpoint inhibitors play an important role in maintaining the antitumor activity through overcoming T-cell dysfunction [38]. The checkpoint receptors including PD-1 and CTLA-4 (cytotoxic T-lymphocyte associated protein 4), are expressed by activating T cells to negatively regulate the cytotoxic activities [39]. Ipilimumab (monoclonal antibody against CTLA-4) promote antitumor activityinvolving in effector T cell exhaustion in patients with metastatic melanoma [40]. The IFNγ increases the expression of the PD-L1 protein on T cells and tumor cells, which promotes the PD-1 receptor of T cells to inhibit the antitumor immunity [41], and the deletion of PD-1 elevated the antitumor efficacy of CAR-T cells [28]. The IFNγ secreted by activated T cell increases the immunosuppressive molecule expression (IDO), whereas IDO inhibitors combined with CAR-T cell enhance its cytotoxic effect [42]. Therefore, CAR-T cell therapy combined with PD-1/CTLA-4 antibodies blockade may achieve objective response in cancer treatment. Recently, CAR-T cell combined with chemotherapeutic agents such as carboplatin significantly improved anti-tumor effect for treating ovarian cancer [43]. Meanwhile, CAR-T cell combined with local subtherapeutic irradiation also enhanced antitumor efficacy [44]. These studies suggested that CAR-T cell therapy combined with chemotherapy or radiation may further improve its treatment efficacy and treating tumor.
CAR-T cell therapy and its applications
CAR-T cell therapy in acute myeloid leukemia
Acute myeloid leukemia (AML) is described when abnormal myeloid progenitors in peripheral blood and bone marrow undergo clonal expansion, which inhibits the creation of normal cells of blood. The statistics suggested that AML patients had a dismal prognosis, and the cure rate was 5-15% for elderly patients, and the patients' average survival span was 5-10 months [45]. Despite improving the understanding about AML, the recurrence rate and mortality still remain high using traditional chemotherapy [46]. The conventional strategies may hit a ceiling for treating AML, and the high toxicity and limited success compel people to exploit new therapeutics. CAR-T cell technology is a promising immunological approach by merging the exquisite aiming at the potent cytotoxicity of T cells and specificity of monoclonal antibodies and, and has been successfully implemented to cure ALL [47, 48]. The CAR-T cell triggered a serial cascades of signal transduction and thereafter activated T cells to directly destroy the tumor cells once recognizing the target antigens [49]. More recently, CAR-T cell therapy was considered as a likely therapy to cure AML owing to its durable and potent anti-tumor activity [50]. Notably, the CAR-T cells could manufacture CAR-T cells with long-term memory following their activation at initial stages, which was conduce to the long-term elimination of tumor cells and prevent tumor relapse. Some clinical trials showed that anti-CD19 CAR-T cells had achieved an impressive outcomes and long-term remissions in patients with AML [21, 51]. In addition to anti-CD19 CAR-T cells, CD123-directed CAR-T cells and CD44v6-directed CAR-T cells also acquired good therapeutic efficacy in AML patients [52]. Collectively, the researches results highlighted that CAR-T cell therapy had extensive application prospect and theory value for AML.
CAR-T cell therapy in solid structured tumors
Enormous hindrances wait beyond CARs safe targets identification and its functional design for treating solid tumors. The efficient retention of viability, maintenance of function and homing of CAR-T cell within the immunosuppressive tumor microenvironment could be a problem to be solved imperatively [53].
Pancreatic cancer
Monoclonal antibodies or checkpoint inhibitors or were utilized to cure prostate cancer derived from it could specifically recognize the prostate stem cell antigen (PSCA) and prostate-specific membrane antigen (PSMA). Therefore, PSCA CAR-T cell was produced in treatment of pancreatic cancer due to the PSCA is overexpressed in initial stages of pancreatic cancer. Meanwhile, 5E5 modified CAR-T-cells administration reduced the growth of tumor and increased the survival rate in mice with pancreatic cancer. Collectively, CAR-T cell therapy had made gratifying achievements in pancreatic cancer.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is among the most serious malignancies threatening human health, besides being the third leading cause of cancer deaths worldwide [54]. Hepatitis B/C (HCV/HBV) viruses are pivotal predisposing factors contributing for approximately 80% of the HCC events. Despite many attempts, the five-year survival rate remained poor, and the majority succumbed to this disease in patients with HCC [55]. According to some studies, infiltrating into the tumor site of T and NK cells was closely related to the high survival rate in HCC patients [56]. Whereas, immunosuppressive tumor microenvironments including regulatory T cells [57], cancer-associated fibroblasts [58], and tumor associated macrophages [59], have come together to gradually suppress and undermine immune function. Previous researches demonstrated that CAR-T cell composed of CD8α, intracellular signaling domains, 4-1BB, and CD3ζ, the anti-GPC3 scFv, hinge and CD28 transmembrane could specifically lyse HCC cell lines, and significantly suppressing growth in immunodeficient mice, of orthotopic xenografts [60]. Furthermore, second-generation CARs (including CD28 costimulation) could specifically target L and HBV S antigens, and thereby enhance the T cells functions to eradicate human hepatoma cells infected with HBV. However, the remedial effect of CAR-T cell was unsatisfactory in HCC, and the inferior efficacy may be due to poor trafficking, hostile tumor microenvironment, penetration of therapeutic cells and target antigen heterogeneity [61]. More efforts and attempts would be paid to further investigate the potential strategy for treating HCC by using engineered CAR-T cell (Figure 2).
Combination and applications of CAR-T cell therapy with other therapeutics. CAR-T cell therapy when separately combined with immunomodulators, chemotherapy, radiation and vaccine to treat tumors consisting of acute myeloid leukemia, pancreatic cancer and hepatocellular carcinoma.
The side effects caused by CAR-T cell therapy
CARs could, redirect antigen specificity as artificial receptors, and enhance T cell function to target an array of tumor cell surface antigens by means of the costimulatory component [62]. CAR-T cell therapy is a novel strategy for treating cancer. Additionally, previous researches indicated that CAR-T cell therapy hold tremendous insights to cancer therapy [63]. CAR-T cells infusion would result in cytokine release syndrome (CRS) as a side effect beside off-tumor/on-target toxicity, and so on. CRS is one of the potentially lethal side effects following CAR-T cell therapy. Proliferation and activation of CAR-T cell in vivo induced a rapid inflammatory systemic response and then caused dramatic increases of inflammatory cytokines [64], which ultimately resulted in high-grade fevers, respiratory insufficiency, hypotension, and neurologic dysfunction [21]. Researches documented that IL-6 participated in constructing a classic feedback loop, with hindrances of the mechanism of IL-6 could halt the toxicity induced by CAR-T cell therapy. CAR-modified T cell derived from murine antibodies provided self-limited expression, while administration by using an intermittent dosing schedule to achieve antitumor effects optimally, ultimately gave raise to anaphylaxis associated with IgE antibody response to CAR [65]. A suicide construct for CAR-T cells ablation is a safe high throughput strategy to control adverse events consisting of engraftment that are prolonged and attenuating severe toxicities (Such as CRS). Moreover, the underlying mechanism concerning the other side effects containing macrophage activation syndrome, hepatosplenomegaly (HSM), and low fibrinogen still need to be further investigated.
Cerebral edema induced by CAR-T cell therapy
In addition to CRS, neurotoxicity characterized by varying the ratios of seizures, cognitive dysfunction and focal neurologic deficits is another obvious side effects following CAR-T cell therapy. Among them, fatal cerebral edema is one of the most serious consequences caused by CAR T-cell therapy. Histopathological findings comprising activated microglia, fragmentation of GFAP and perivascular exudates with fibrin deposition indicated that the secondary cerebral edema induced by CAR-T cell therapy may result from the disruption of the blood-brain barrier (BBB), high cytokine levels and astrocyte dysfunction [66, 67]. The concurrent disseminated intravascular coagulation following cerebral edema may derive from the downregulation of fibrinogen and elevation of D-dimer levels. Moreover, the increase of endothelial cell activation, capillary leak, and microvascular permeability may contribute to the severe neurotoxicity and BBB dysfunction. The accumulation of BBB endothelial cells adhesion molecules in response to cytokine exposure may implicate in BBB dysfunction and edema [68]. The cytokines (such as TNFa, IL-6, IFNc, and IL-1) overexpression, cytokine-mediated endothelial angiopoietin 1/2 (ANG1/2) signaling, activation and increased BBB permeability and aberrant are crucial in the process of cerebral edema formation [66]. Consequently, to further elucidate the underlying mechanism of cerebral edema induced by CAR-T cell therapy is conduced to effectively eliminate tumor cells and minimize the side effects.
Tumor relapse succeeding CAR-T cell therapy
Current perspective argued that tumor relapse may be result from that CAR-T cell could not recognize antigen-negative cancer cells. Multiple mechanisms participated in the antigen escape-caused relapse. Antigen loss and deleterious mutations in the tumor cells may involve in this process of tumor escape [69]. It is feasible by targeting antigens associated to different tumors to elevate the efficiency of CAR-T cell therapy. CARs were redesigned by incorporating costimulatory domains joined to OX-40, CD3ζ, CD137 and CD28, which could effectively enhance cytokine proliferation and production of CAR-T cells, and thereby mediate xenograft models showing tumor regression [70, 71]. Thus promoting survival of CAR-T cells and their augmenting potency by co-expressing additional cytokines, ligands and costimulatory receptors were a valid approach to suppress tumor growth, invasion and recidivism [72]. Moreover, the immunosuppressive microenvironments are important in the tumor relapse. Inhibitory receptors/pathways contributing to the dysfunction and exhaustion of CAR-T cell may be also one of immune escape mechanisms. Gene-editing technology could increase the efficacy of T-cell therapy by permanently disrupting the negative signaling pathways [73]. To solve the relapse cause of antigen escape, CAR-T cell was delicately designed to be triggered by multiple antigens synchronously. For example, a PD-1/TIM-3 receptor segment was incorporated into the CAR-T cell to induce PD-L1/TIM-3 expression within the tumor microenvironment, and thereby augment the granzyme expression, proliferation and cytokine secretion of CAR-T cell [74]. Besides, “TRUCK cells” could shape the tumor environment to boost the CAR-T cell therapy efficacy, thereby averting residual cancer cells causes of tumor relapse. More specifically, “TRUCK cells”, as a novel method, could induce IL-12 release, augment T-cell activation, attract and activate cells of innate immunity to target tumor and then eliminate cancer cells [75] (Table 1).
The side effects caused by CAR-T cell therapy
| Side Effect | Pathogenesis | Symptom | Reference |
|---|---|---|---|
| Cytokine Release Syndrome | Dramatic increases of inflammatory cytokines | High-grade fevers, Respiratory insufficiency, Hypotension, Neurologic dysfunction | 62-65 |
| Cerebral Edema | Blood-brain barrier, High cytokine levels, Astrocyte dysfunction | Seizures, Cognitive dysfunction, Focal neurologic deficits | 66-68 |
| Tumor Relapse | Antigen loss, Deleterious mutations, Dysfunction and exhaustion of CAR-T cell | Tumor growth, invasion and recidivism | 69-75 |
The corresponding countermeasure concerning complications
Currently, gene-editing technology was employed to further optimize the therapy algorithms for CRS. Gene silencing was performed to effectively disturb the expression of CRS-related cytokines in T cells before undergoing transduction. Furthermore, T cell was designed to express a corresponding single chain variable fragment (ScFv), which could specifically block receptor-mediated signal transduction pathway to actively avoid CRS. Moreover, suicide genes were genetically encoded to selectively ablate the adoptively transferred cells, which could effectively prevent collateral damage to contiguous cells and tissues [76]. These suicide genes comprising a drug-binding domain cloned into human Caspase9 frame may be help to abrogate the on-target and off-tumor toxicities of CAR-T cell [77]. With the rapid development of science and technology, new and greater success will be achieved in overcoming the related complications induced by CAR-T cell therapy.
Conclusion and Future Perspectives
In the past couple of decades, the CAR-T cell therapy has made great achievements, and entered a new and accelerated phase based on the advances in synthetic biology and genetic engineering. Nevertheless, there are lots of problems need to be solved urgently including security, side effects, and so on. The most concerning issue is that there is no convincing evidence to absolutely confirm the security of CAR-T cells. Admirably, some researchers suggested that CAR-T cell therapy had achieved encouraging clinical outcomes in a subset of patients. Moreover, side effects induced by CAR-T cell therapy deserve further attention. Therefore, to further illuminate the underlying mechanism and optimize design scheme of CAR-T cell would extremely minimize bothersome side effects following CAR-T cell therapy. This review provides useful contents regarding the essential information, application areas, tumor relapse, and side effects of CAR-T cell therapy. In conclusion, the CAR-engineered T cells represent a valuable and attractive therapeutic strategy for cancer immunotherapy.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81860423 and 81460649). and The fund of Yun-nan Provincial Clinical Center of Hepato-biliary-pancreatic Diseases, The Medical Leading Talent Project of Yunnan Province (No. L-201606).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yu JC, Khodadadi H, Malik A. et al. Innate immunity of neonates and infants. Front Immunol. 2018;9:1759
2. Arseneau KO, Tamagawa H, Pizarro TT. et al. Innate and adaptive immune responses related to ibd pathogenesis. Curr Gastroenterol Rep. 2007;9(6):508-12
3. Holleran G, Lopetuso L, Petito V. et al. The innate and adaptive immune system as targets for biologic therapies in inflammatory bowel disease. Int J Mol Sci. 2017;18(10):E2020
4. Geremia A, Biancheri P, Allan P. et al. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13(1):3-10
5. Mora JR, Bono MR, Manjunath N. et al. Selective imprinting of gut-homing t cells by peyer's patch dendritic cells. Nature. 2003;424(6944):88-93
6. Dolton G, Zervoudi E, Rius C. et al. Optimized peptide-mhc multimer protocols for detection and isolation of autoimmune t-cells. Front Immunol. 2018;9:1378
7. Knochelmann HM, Smith AS, Dwyer CJ. et al. Car t cells in solid tumors: blueprints for building effective therapies. Front Immunol. 2018;9:1740
8. Vigneron N. Human tumor antigens and cancer immunotherapy. Biomed Res Int. 2015;2015:948501
9. Topalian SL, Drake CG, Pardoll DM. Targeting the pd-1/b7-h1(pd-l1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207-12
10. Topalian SL, Sznol M, McDermott DF. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020-30
11. Kulemzin SV, Kuznetsova VV, Mamonkin M. et al. Engineering chimeric antigen receptors. Acta Naturae. 2017;9(1):6-14
12. Morrissey MA, Williamson AP, Steinbach AM. et al. Chimeric antigen receptors that trigger phagocytosis. Elife. 2018;7:e36688
13. Fan M, Li M, Gao L. et al. Chimeric antigen receptors for adoptive t cell therapy in acute myeloid leukemia. J Hematol Oncol. 2017;10(1):151
14. Han H, Wang S, Hu Y. et al. Monoclonal antibody 3h11 chimeric antigen receptors enhance t cell effector function and exhibit efficacy against gastric cancer. Oncol Lett. 2018;15(5):6887-94
15. Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168(4):724-40
16. Davila ML, Riviere I, Wang X. et al. Efficacy and toxicity management of 19-28z car t cell therapy in b cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224r-225r
17. Maude SL, Frey N, Shaw PA. et al. Chimeric antigen receptor t cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-17
18. Fesnak AD, June CH, Levine BL. Engineered t cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16(9):566-81
19. Kochenderfer JN, Feldman SA, Zhao Y. et al. Construction and preclinical evaluation of an anti-cd19 chimeric antigen receptor. J Immunother. 2009;32(7):689-702
20. Barrett DM, Grupp SA, June CH. Chimeric antigen receptor- and tcr-modified t cells enter main street and wall street. J Immunol. 2015;195(3):755-61
21. Grupp SA, Kalos M, Barrett D. et al. Chimeric antigen receptor-modified t cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509-18
22. Gill S, June CH. Going viral: chimeric antigen receptor t-cell therapy for hematological malignancies. Immunol Rev. 2015;263(1):68-89
23. Dong MB, Wang G, Chow RD. et al. Systematic immunotherapy target discovery using genome-scale in vivo crispr screens in cd8 t cells. Cell. 2019;178(5):1189-204
24. Mirzaei HR, Pourghadamyari H, Rahmati M. et al. Gene-knocked out chimeric antigen receptor (car) t cells: tuning up for the next generation cancer immunotherapy. Cancer Lett. 2018;423:95-104
25. Roszik J, Rabinovich B, Cooper LJ. Imaging of t cells expressing chimeric antigen receptors. Immunotherapy. 2011;3(12):1411-4
26. Gu X, He D, Li C. et al. Development of inducible cd19-car t cells with a tet-on system for controlled activity and enhanced clinical safety. Int J Mol Sci. 2018;19(11):3455
27. Hsieh EM, Scherer LD, Rouce RH. Replacing car-t cell resistance with persistence by changing a single residue. J Clin Invest. 2020: 136872.
28. Rupp LJ, Schumann K, Roybal KT. et al. Crispr/cas9-mediated pd-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor t cells. Sci Rep. 2017;7(1):737
29. Fraietta JA, Nobles CL, Sammons MA. et al. Disruption of tet2 promotes the therapeutic efficacy of cd19-targeted t cells. Nature. 2018;558(7709):307-12
30. Vitale I, Sistigu A, Manic G. et al. Mutational and antigenic landscape in tumor progression and cancer immunotherapy. Trends Cell Biol. 2019;29(5):396-416
31. Maris MB, Sandmaier BM, Storer BE. et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104(12):3535-42
32. Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell. 2012;148(6):1081-4
33. Badeaux AI, Shi Y. Emerging roles for chromatin as a signal integration and storage platform. Nat Rev Mol Cell Biol. 2013;14(4):211-24
34. Kagoya Y, Nakatsugawa M, Yamashita Y. et al. Bet bromodomain inhibition enhances t cell persistence and function in adoptive immunotherapy models. J Clin Invest. 2016;126(9):3479-94
35. Ramakrishna S, Highfill SL, Walsh Z. et al. Modulation of target antigen density improves car t-cell functionality and persistence. Clin Cancer Res. 2019;25(17):5329-41
36. Morgan MA, Shilatifard A. Chromatin signatures of cancer. Genes Dev. 2015;29(3):238-49
37. Chen Q, Hu Q, Dukhovlinova E. et al. Photothermal therapy promotes tumor infiltration and antitumor activity of car t cells. Adv Mater. 2019;31(23):e1900192
38. Grywalska E, Pasiarski M, Gozdz S. et al. Immune-checkpoint inhibitors for combating t-cell dysfunction in cancer. Onco Targets Ther. 2018;11:6505-24
39. Sharma P, Hu-Lieskovan S, Wargo JA. et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707-23
40. Hodi FS, O'Day SJ, McDermott DF. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-23
41. Dong H, Strome SE, Salomao DR. et al. Tumor-associated b7-h1 promotes t-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793-800
42. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014-22
43. Parente-Pereira AC, Whilding LM, Brewig N. et al. Synergistic chemoimmunotherapy of epithelial ovarian cancer using erbb-retargeted t cells combined with carboplatin. J Immunol. 2013;191(5):2437-45
44. Weiss T, Weller M, Guckenberger M. et al. Nkg2d-based car t cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res. 2018;78(4):1031-43
45. Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-52
46. Kantarjian H, O'Brien S. Questions regarding frontline therapy of acute myeloid leukemia. Cancer. 2010;116(21):4896-901
47. Kalos M, Levine BL, Porter DL. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):73r-95r
48. Cruz CR, Micklethwaite KP, Savoldo B. et al. Infusion of donor-derived cd19-redirected virus-specific t cells for b-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965-73
49. Davila ML, Bouhassira DC, Park JH. et al. Chimeric antigen receptors for the adoptive t cell therapy of hematologic malignancies. Int J Hematol. 2014;99(4):361-71
50. Kochenderfer JN, Dudley ME, Feldman SA. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-cd19 chimeric-antigen-receptor-transduced t cells. Blood. 2012;119(12):2709-20
51. Kochenderfer JN, Dudley ME, Kassim SH. et al. Chemotherapy-refractory diffuse large b-cell lymphoma and indolent b-cell malignancies can be effectively treated with autologous t cells expressing an anti-cd19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540-9
52. Casucci M, Nicolis DRB, Falcone L. et al. Cd44v6-targeted t cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122(20):3461-72
53. Moon EK, Wang LC, Dolfi DV. et al. Multifactorial t-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human t cells in solid tumors. Clin Cancer Res. 2014;20(16):4262-73
54. Collaborators GMAC. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385(9963):117-71
55. Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28(5):753-70
56. Chuang WL, Liu HW, Chang WY. Natural killer cell activity in patients with hepatocellular carcinoma relative to early development and tumor invasion. Cancer. 1990;65(4):926-30
57. Tu JF, Ding YH, Ying XH. et al. Regulatory t cells, especially icos(+) foxp3(+) regulatory t cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci Rep. 2016;6:35056
58. Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol. 2017;12:153-86
59. Ding T, Xu J, Wang F. et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009;40(3):381-9
60. Gao H, Li K, Tu H. et al. Development of t cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014;20(24):6418-28
61. Newick K, O'Brien S, Moon E. et al. Car t cell therapy for solid tumors. Annu Rev Med. 2017;68:139-52
62. Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388-98
63. Brentjens RJ, Davila ML, Riviere I. et al. Cd19-targeted t cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):138r-177r
64. Lee DW, Gardner R, Porter DL. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188-95
65. Maus MV, Haas AR, Beatty GL. et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1(1):26-31
66. Gust J, Hay KA, Hanafi LA. et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with cd19 car-t cells. Cancer Discov. 2017;7(12):1404-19
67. Jcar015 in all. a root-cause investigation. Cancer Discov. 2018;8(1):4-5
68. Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1-12
69. Wang Z, Wu Z, Liu Y. et al. New development in car-t cell therapy. J Hematol Oncol. 2017;10(1):53
70. Zhong XS, Matsushita M, Plotkin J. et al. Chimeric antigen receptors combining 4-1bb and cd28 signaling domains augment pi3kinase/akt/bcl-xl activation and cd8+ t cell-mediated tumor eradication. Mol Ther. 2010;18(2):413-20
71. Carpenito C, Milone MC, Hassan R. et al. Control of large, established tumor xenografts with genetically retargeted human t cells containing cd28 and cd137 domains. Proc Natl Acad Sci U S a. 2009;106(9):3360-5
72. Chmielewski M, Kopecky C, Hombach AA. et al. Il-12 release by engineered t cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71(17):5697-706
73. Su S, Hu B, Shao J. et al. Crispr-cas9 mediated efficient pd-1 disruption on human primary t cells from cancer patients. Sci Rep. 2016;6:20070
74. Liu X, Ranganathan R, Jiang S. et al. A chimeric switch-receptor targeting pd1 augments the efficacy of second-generation car t cells in advanced solid tumors. Cancer Res. 2016;76(6):1578-90
75. Chmielewski M, Abken H. Trucks: the fourth generation of cars. Expert Opin Biol Ther. 2015;15(8):1145-54
76. Minagawa K, Zhou X, Mineishi S. et al. Seatbelts in car therapy: how safe are cars? Pharmaceuticals (Basel). 2015;8(2):230-49
77. Mardiros A, Forman SJ, Budde LE. T cells expressing cd123 chimeric antigen receptors for treatment of acute myeloid leukemia. Curr Opin Hematol. 2015;22(6):484-8
Author contact
![]() Corresponding authors: Lin Wang (E-mail: wanglinfeycom) & Ren-Chao Zou (E-mail: 1275523786com); Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Kunming Medical University, No. 374 Dianmian Avenue, Kunming 650032, Yunnan, China. Fax number: +86-0871-63402704; Phone number: +86-0871-63402704.
Corresponding authors: Lin Wang (E-mail: wanglinfeycom) & Ren-Chao Zou (E-mail: 1275523786com); Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Kunming Medical University, No. 374 Dianmian Avenue, Kunming 650032, Yunnan, China. Fax number: +86-0871-63402704; Phone number: +86-0871-63402704.

 Global reach, higher impact
Global reach, higher impact