Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(12):2635-2642. doi:10.7150/jca.32453 This issue Cite
Research Paper
The impact of Ki-67 in the context of multidisciplinary care in primary inflammatory breast cancer
1. Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
2. Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
3. Department of Medical Oncology, The National Cancer Institute, Cairo University, Cairo, Egypt
4. Department of Pathology, Division of Pathology/Lab Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
5. Department of Breast Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
6. Department of Radiation Oncology, Division of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
7. Morgan Welch Inflammatory Breast Cancer Research Program and Clinic, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Received 2018-12-20; Accepted 2019-4-30; Published 2019-6-2
Abstract
Background: Research on the prognostic or predictive value of Ki-67 among patients with inflammatory breast cancer (IBC) is limited.
Methods: Using the comprehensive database of the Morgan Welch Inflammatory Breast Cancer Research Program at MD Anderson Cancer Center, we identified a cohort of breast cancer patients who were diagnosed with IBC between 1992 and 2012. Distributions of survival outcomes were estimated by the Kaplan-Meier method and compared by log-rank tests and Cox models.
Results: Among a total of 257 patients with stage III IBC, the mean percentage of tumor cells that stained positive for Ki-67 was 48%, (range, 4% to 100%). Using a cutoff of 20% as being Ki-67 positive, this characteristic tended to be associated with worse overall survival (p=0.07) in the univariate analysis. After controlling for hormone receptor (HR) status, human epidermal growth factor receptor 2 (HER2) status and having received trimodality treatment, the association between Ki-67 status and overall survival remained marginally significant (p=0.07). The effects of trimodality treatment on overall survival were statistically significantly different between patients with Ki-67-positive tumors (hazard ratio=0.26, 95% confidence interval [CI]=0.15-0.44, p<0.01) and those with Ki-67-negative tumors (hazard ratio =2.04, 95% CI=0.45-9.29, p=0.36) after adjusting for other tumor characteristics (p=0.01).
Conclusion: IBC patients with Ki-67-positive tumors tended to have worse overall survival, but were more likely to benefit from trimodality treatment, with better overall survival and distant metastasis-free survival. Patients with Ki-67-negative tumors had similar survival distributions, regardless of whether they received trimodality treatment.
Keywords: Inflammatory breast cancer, Ki-67, Metastasis-free survival, Overall survival, Trimodality treatment
Introduction
Inflammatory breast cancer (IBC) is an aggressive and rare form of invasive breast cancer that accounts for <5% of all breast cancer diagnoses [1]. Despite advances in breast cancer treatment and a multidisciplinary approach, patients with IBC continue to have poor prognoses and a 3-year survival rate of less than 40%, compared with 85% for patients with non-inflammatory locally advanced breast cancer [2, 3]. For breast cancer in general, the hormonal receptor (HR) status and the human epidermal growth factor receptor 2 (HER2) status are the most important prognostic factors, as well as the strongest predictors of response to targeted therapies [4]. These molecular biomarkers have been used to classify breast cancer into clinically relevant subtypes. However, molecular markers (or gene signature) that can define IBC or its prognoses are still elusive. Due to the aggressive nature and rarity of IBC, research of this cancer has been insufficient to determine whether IBC is a heterogeneous disease that comprises different subtypes as determined by biomarkers other than HR and HER2, and therefore is associated with different clinical outcomes and treatment benefits according to disease subtype [5].
The use of single-modality treatment to cure IBC has not been successful. Current IBC treatment guidelines published by the National Comprehensive Cancer Network recommend trimodality treatment [6], defined as neoadjuvant chemotherapy followed by modified radical mastectomy and postmastectomy radiation therapy to the chest wall and draining lymphatics. Although a survival advantage with the use of trimodality treatment has been reported for IBC patients [7, 3, 1], the molecular biology of IBC tumors associated with the trimodality treatment effect needs to be furthered identified and evaluated [8].
Endocrine treatments for breast cancer appear to act largely by inhibiting tumor cell proliferation; hence, tumor cell proliferation markers are candidate markers of treatment efficacy. Immunohistochemical (IHC) evaluation of proliferation markers, such as Ki-67, is a simple method that is widely used in routine clinical practice and has been extensively studied in the last two decades. For early breast cancer, the percentage of tumor cells that stain positive for Ki-67 has been reported as a predictive and prognostic factor [9, 10]. Several studies confirmed that breast cancer patients with high percentages of Ki-67-positive tumor cells tend to respond better to chemotherapy [11, 12]; however, this finding is associated with poor overall prognosis [13]. Thus, the evaluation of Ki-67 has been integrated into emerging prognostic tools such as the Preoperative Endocrine Prognostic Index for estrogen receptor-positive (ER+) breast cancer [14] and the Immunohistochemical 4 score for predicting locoregional disease recurrence in early breast cancer [15]. For IBC patients, it is not clear whether the percentage of Ki-67-positive tumor cells affects the overall survival time (OS) and how it is associated with the trimodality treatment benefit.
In this study, we used an up-to-date IBC database from the Morgan Welch Inflammatory Breast Cancer Research Program and Clinic at MD Anderson Cancer Center (MDA), which is one of the largest cancer institutions in the United States. The database includes detailed information on patients' tumor biology and characteristics at diagnosis, the treatments they received and long-term follow-up information. Our purpose is to evaluate the influence of the proliferation marker Ki-67 on survival outcomes by Ki-67 status and treatment.
Patients and Methods
Data Source and Study Cohort
A comprehensive database from the Morgan Welch Inflammatory Breast Cancer Research Program at MDA contains detailed information on IBC patients who have received their primary treatment at MDA. We focused on a cohort of breast cancer patients who had been diagnosed with non-metastatic IBC between 1992 and 2012, with the follow-up cutoff of 12/31/2012. Following approval by the institutional review board of MDA, we retrieved the records of 1079 patients who were newly diagnosed with primary IBC. All data were de-identified such that no protected health information could be linked to individual patients. After excluding patients who had missing information regarding the Ki-67 status of their tumor, we included a total number of 257 patients with non-metastatic IBC in the analysis.
Definition of outcome
The primary clinical outcomes of interest were OS, defined as the time from IBC diagnosis to death, and distant metastasis-free survival time (DMFS), defined as the time from IBC diagnosis to distant metastasis or death, whichever occurred first. The observed times were censored at the last contact at which the patient was known to be free of any distant metastasis for DMFS or the last contact time the patient was known to be alive for OS.
Demographics, tumor characteristics at diagnosis and treatment received
Demographic data included age at diagnosis, ethnicity and menopausal status. Tumor characteristics included clinical stage, ER status, progesterone receptor (PR) status, HER2 status, and nuclear grade. ER- and PR-positive status was defined as ≥ 10% positivity, and HER2-positive status was defined as an IHC score of 3+ and/or a fluorescence in situ hybridization ratio of ≥ 2.0 [16]. HR status was defined as positive if both or either ER or PR were positive, and defined as negative otherwise. Patients received one or a combination of the following treatments: definitive surgery, adjuvant chemotherapy, neoadjuvant chemotherapy, adjuvant hormonal therapy, adjuvant radiation and neoadjuvant radiation.
Evaluation of Ki-67
Ki-67 scores were retrospectively obtained from pathology reports at initial diagnosis on the primary tumor prior to any treatment. IHC staining on formalin-fixed paraffin-embedded tissue sections was performed. Mind Bomb 1 mouse monoclonal antibody (manufactured by Dako) was used to detect Ki-67. The staining protocol included de-paraffinization (30 minutes at 72°C) and rehydration with antigen retrieval performed at 100°C for 20 minutes with Tris-EDTA buffer, pH 6.0. Endogenous peroxidase was blocked with 3% peroxide for 5 minutes. Primary anti-Ki-67 antibody (Dako, clone MIB-1) was applied at 1:100 dilution for 15 minutes. Post primary antibody detection was carried out using a commercial polymer system (Bond Polymer Refine Detection, Leica), and stain development was achieved by incubation with diaminobenzidine (DAB) and DAB enhancer (Leica). The robust quality control and quality improvement program of the IHC lab at MDA was fully applied to the anti-Ki-67 IHC assay. A positive control was added to every IHC run (reference tonsil tissue, batch control) and was reviewed by a member of the IHC medical directorship team. Records of batch control results were documented daily in internal laboratory records. Stains were evaluated by designated breast pathologists, who visually estimated the percentage of positively staining invasive carcinoma cells.
Statistical methods
We calculated the summary statistics, including the means, medians, and ranges, for the continuous variables, such as age at diagnosis. We summarized the values for the categorical variables, such as sex and ethnicity, as counts and frequencies. We used chi-squared tests or Fisher's exact tests to compare the distributions of the tumor characteristics at diagnosis according to whether the patient received or did not receive trimodality treatment. We applied the Kaplan-Meier method and log-rank tests to estimate and compare the distributions of OS and DMFS by treatment arms. We used Cox proportional hazards (PH) models to evaluate the risk factors associated with the survival outcomes and checked the PH assumption. We dichotomized the continuous variables such as Ki-67, which violated the PH assumption. We obtained a multivariate Cox PH model by first including the variables with p-value < 0.10 in the univariate analysis, except for the variables of interest (Ki-67 and trimodality treatment). We then performed backward elimination using 0.05 for the significance level of the Wald chi-squared test for a variable to remain in the model. Once the list of variables in our final model was selected, we further assessed the interaction effects of Ki-67 status and trimodality treatment on OS and DMFS. The purpose of the interaction analyses was to evaluate whether or not the magnitude or direction of the association between trimodality treatment and the survival outcomes differed by Ki-67 status. All p-values are two-sided. We used SAS (v9.3; SAS Institute, Cary, NC, USA) and S-Plus (version 8.2, TIBCO, Palo Alto, CA, USA) to conduct all analyses.
Results
Among the 257 IBC patients, the mean age at diagnosis was 50 years (standard deviation, 11; median (range), 50 (19 to 83) years). The mean percentage for Ki-67-positive tumor cells was 48% and the median was 46% (range, 4% to 100%). More detailed information on Ki67 status in relation to patients' demographic and tumor characteristics is summarized in Supplemental Table S1. Other patient characteristics and treatments received are detailed in Table 1. The median follow-up for this cohort was 62 months (range, 0.8-228 months). During the follow-up, 109 patients died and 127 patients experienced distant metastasis. Among the 257 patients in the cohort, 191 (74%) had received trimodality treatment. There were no statistically significant differences in the distributions of patients' demographic and tumor characteristics at diagnosis between patients who received and did not receive trimodality treatment (Supplemental Table S2).
Overall Survival
Supplemental Table S3 presents the results of univariate Cox PH models for OS, which we used to guide variable selection in the multivariate analyses. The factors independently associated with worse OS were having HR-negative disease and/or HER2-negative disease, and not receiving trimodality treatment. When treating Ki-67 status as a continuous variable, a higher percentage of Ki-67-positive tumor cells was associated with worse OS (hazard ratio =1.01, 95% CI= 1.00-1.02, p=0.01), but the PH assumption was violated (p = 0.01). Conventionally, after dichotomizing Ki-67 using a cutoff of 20% to represent a high proliferative tumor [17], having a high proliferative tumor tended to be associated with worse OS (hazard ratio=1.65, 95% CI=0.97-2.81, p=0.07). Table 2 lists the results of the multivariable Cox PH model for OS. After controlling for the other risk factors, the association between Ki-67 status and OS remained marginally significant. Specifically, patients with high proliferative tumors tended to have a higher risk of death, with hazard ratio =1.76 (95% CI=0.95-3.28, p=0.07). The use of trimodality treatment decreased the risk of death significantly: hazard ratio =0.36 (95% CI=0.22-0.6, p<0.01). The patients with triple-negative IBC had the highest risk of death: hazard ratio =2.67 (95% CI=1.62-4.38, p<0.01) when compared with patients with HR+/HER2- IBC; hazard ratio =4.7 (95% CI=2.29-9.64, p<0.01) when compared with patients with HR-/HER2+ IBC; and hazard ratio =2.71 (95% CI=1.31-5.6, p=0.01) when compared with patients with HR+/HER2+ IBC.
Patients' characteristics and treatments received among the entire study cohort
| Variable | Category | Frequency Count | Percentage of Total Frequency* |
|---|---|---|---|
| Ethnicity | Black | 27 | 10.51 |
| Other | 39 | 15.18 | |
| White | 191 | 74.32 | |
| Menopausal status | Missing | 2 | 0.78 |
| Post | 139 | 54.09 | |
| Pre | 116 | 45.14 | |
| Grade | Missing | 6 | 2.33 |
| I/II | 46 | 17.90 | |
| III | 205 | 79.77 | |
| Clinical stage | IIIB | 186 | 72.37 |
| IIIC | 71 | 27.63 | |
| Ki-67 | Low proliferative (<20%) | 41 | 15.95 |
| High proliferative (≥20%) | 216 | 84.05 | |
| HR | Missing | 2 | 0.78 |
| Negative | 116 | 45.14 | |
| Positive | 139 | 54.09 | |
| HER2 | Missing | 31 | 12.06 |
| Negative | 150 | 58.37 | |
| Positive | 76 | 29.57 | |
| Subtype | Missing | 31 | 12.06 |
| HR+/HER2- | 96 | 37.35 | |
| HER2+/HR+ | 32 | 12.45 | |
| HER2+/HR- | 44 | 17.12 | |
| HER2-/HR- | 54 | 21.01 | |
| Definitive surgery | No | 24 | 9.34 |
| Yes | 233 | 90.66 | |
| Neoadjuvant chemotherapy | No | 11 | 4.28 |
| Yes | 246 | 95.72 | |
| Neoadjuvant hormonal therapy | No | 250 | 97.28 |
| Yes | 7 | 2.72 | |
| Adjuvant chemotherapy | No | 154 | 59.92 |
| Yes | 103 | 40.08 | |
| Adjuvant hormonal therapy | No | 163 | 63.42 |
| Yes | 94 | 36.58 | |
| Neoadjuvant radiation therapy | No | 244 | 94.94 |
| Yes | 13 | 5.06 | |
| Adjuvant radiation therapy | No | 59 | 22.96 |
| Yes | 198 | 77.04 | |
| Trimodality treatment | No | 66 | 25.68 |
| Yes | 191 | 74.32 |
HR: hormone receptor; HER2: human epidermal growth factor receptor 2
* Percentages may not add up to 100% due to rounding off decimals.
To evaluate whether or not the magnitude or direction of the effect of trimodality treatment on OS differed by Ki-67 status, we included an interaction term between the use of trimodality treatment and Ki-67 status in the multivariate model (see Table 2). While adjusting for the HR and HER2 status, the interaction term was statistically significant (p=0.01), which indicates that the trimodality treatment significantly improved the OS for patients with high proliferative tumors (hazard ratio =0.26, 95% CI=0.15-0.44, p<0.01), but showed no benefit for patients with low proliferative tumors (hazard ratio =2.04, 95% CI=0.45-9.29, p=0.36). The estimated OS curves according to the trimodality treatment and Ki-67 status are presented in Figure 1; patients with high proliferative tumors were more likely to benefit from trimodality treatment; whereas patients with low proliferative tumors had similar survival distributions, regardless of the use of trimodality treatment.
Multivariate Cox PH models for overall survival
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Parameter | Hazard Ratio | Lower Limit | Upper Limit | P-Value | ||
| Model without trimodality treatment by Ki-67 interaction term | Subtype | TN vs HR+/HER2- | 2.67 | 1.62 | 4.38 | 0.00 |
| TN vs HER2+/HR+ | 2.71 | 1.31 | 5.60 | 0.01 | ||
| TN vs HER2+/HR- | 4.70 | 2.29 | 9.64 | <.01 | ||
| Ki-67 | high proliferative vs low proliferative | 1.76 | 0.95 | 3.28 | 0.07 | |
| Trimodality | Yes vs No | 0.36 | 0.22 | 0.60 | <.01 | |
| Model with trimodality treatment by Ki-67 interaction term | Subtype | TN vs HR+/HER2- | 2.64 | 1.61 | 4.34 | 0.00 |
| TN vs HER2+/HR+ | 3.21 | 1.53 | 6.75 | 0.00 | ||
| TN vs HER2+/HR- | 5.45 | 2.62 | 11.32 | <.01 | ||
| Ki-67 within patients treated with trimodality | high proliferative vs low proliferative | 0.94 | 0.48 | 1.84 | 0.86 | |
| Ki-67 within patients not treated with trimodality | high proliferative vs low proliferative | 7.52 | 1.73 | 32.57 | 0.01 | |
| Trimodality within patients with high proliferative tumors | Trimodality vs No Trimodality | 0.26 | 0.15 | 0.44 | <.01 | |
| Trimodality within patients with low proliferative tumors | Trimodality vs No Trimodality | 2.04 | 0.45 | 9.29 | 0.36 | |
Distant Metastasis-free Survival (DMFS)
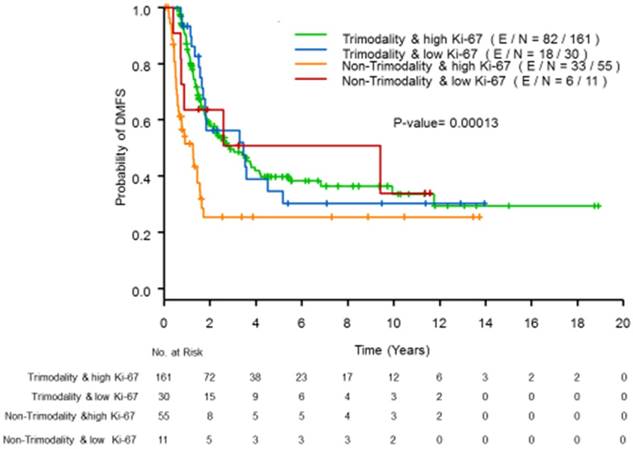
Supplemental Table S3 provides the results of the univariate PH models, and Table 3 lists the results of the multivariable PH models for DMFS. In the univariate analyses, trimodality treatment and tumor subtypes determined by HR and HER2 status were independently associated with DMFS. Being high proliferative IBC was not statistically significantly associated with DFMS (p=0.49). In multivariate analyses, being high proliferative IBC remained insignificant after adjusting for the use of trimodality treatment and for tumor subtypes (p=0.34). Similar to the OS outcome, we found that the trimodality treatment effects on DMFS were significantly different between patients with high proliferative tumors (hazard ratio=0.33, 95% CI=0.21-0.53, p <0.01) and those with low proliferative tumors (hazard ratio=1.23, 95% CI=0.45-3.38, p=0.69); (p=0.02 for the interaction term). Figure 2 presents the estimated DMFS curves according to trimodality treatment and Ki-67 status.
Multivariate Cox PH models of DMFS
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Parameter | Hazard Ratio | Lower Limit | Upper Limit | P-Value | ||
| Model without trimodality treatment by Ki-67 interaction term | Subtype | TN vs HR+/HER2- | 2.34 | 1.51 | 3.60 | 0.00 |
| TN vs HER2+/HR+ | 1.92 | 1.07 | 3.47 | 0.03 | ||
| TN vs HER2+/HR- | 3.47 | 1.89 | 6.36 | <.01 | ||
| Ki-67 | high proliferative vs low proliferative | 1.27 | 0.78 | 2.09 | 0.34 | |
| Trimodality | Yes vs No | 0.44 | 0.29 | 0.67 | <.0001 | |
| Model with trimodality treatment by Ki-67 interaction term | Subtype | TN vs HR+/HER2- | 2.32 | 1.50 | 3.58 | 0.00 |
| TN vs HER2+/HR+ | 2.12 | 1.17 | 3.85 | 0.01 | ||
| TN vs HER2+/HR- | 3.88 | 2.10 | 7.17 | <.01 | ||
| Ki-67 within patients treated with trimodality | high proliferative vs low proliferative | 0.84 | 0.48 | 1.46 | 0.53 | |
| Ki-67 within patients not treated with trimodality | high proliferative vs low proliferative | 3.11 | 1.17 | 8.26 | 0.02 | |
| Trimodality within patients with high proliferative tumors | Trimodality vs No Trimodality | 0.33 | 0.21 | 0.53 | <.01 | |
| Trimodality within patients with low proliferative tumors | Trimodality vs No Trimodality | 1.23 | 0.45 | 3.38 | 0.69 | |
Discussion
In the era of precision medicine, clinical management of cancer depends on all the available information on the tumor's molecular biology at diagnosis. Although Ki-67 tumor status is recognized as being associated with clinical outcomes of breast cancer, and trimodality treatment has been shown to effectively treat IBC, it is unclear whether there is any subgroup of IBC patients who are most likely to benefit from trimodality treatment. To the best of our knowledge, our study is the first to investigate the usage of Ki-67 status as a predictive marker for the effects of trimodality treatment in IBC patients.
Kaplan-Meier curves of OS by Ki-67 status using a cutoff of 20% and trimodality treatment
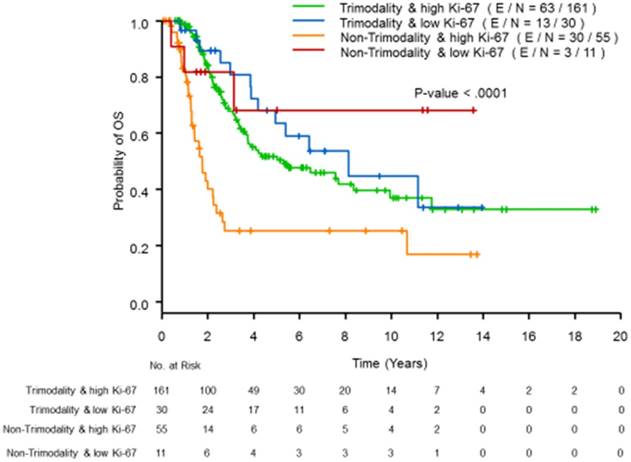
Kaplan-Meier curves of DMFS by Ki-67 status using a cutoff of 20% and trimodality treatment
The rarity of IBC brings challenges when identifying potential biomarkers associated with the optimal treatment selection. MDA has one of the largest multidisciplinary clinics for treating IBC in the United States. Compared to the studies based on single hospitals or cancer centers, our study cohort has a reasonably large sample size with sufficient follow-up to evaluate the prognostic and predictive value of Ki-67 status by treatment scheme. Overall, Ki-67 status was marginally associated with OS for IBC patients, after adjusting for the use of trimodality treatment and tumor subtypes as determined by HR and HER2 status. However, we note that the Ki-67 status significantly modified the effect of trimodality treatment on both OS and DMFS. The overall effect of trimodality treatment was attenuated among patients with low proliferative tumors; whereas the trimodality treatment effect on OS among the patients with high proliferative tumors was significant, with a hazard ratio of 0.26 (95% CI=0.15-0.44), which was lower than the overall hazard ratio (0.36, 95% CI=0.22-0.60).
There is no consensus in clinical decision making regarding a meaningful cutoff point for the percentage of tumor cells that stain high proliferative for Ki-67, although this measure is used in the distinction between luminal A and B breast cancer subtypes, and was recently used in prognosis at baseline and after exposure to endocrine therapy for ER/PR+ and HER2-negative cases [18]. The Ki-67-percentage cutoffs most commonly used for breast cancer have been between 10% and 20% [17]. IBC patients generally have had higher levels of Ki-67 tumor cells compared to patients with non-IBC due to the invasive biological behavior of IBC [19]. Accordingly, we used a cutoff value of 20% in our analysis, which was also recommended at the Thirteenth St. Gallen Conference [20].
Some recent studies have partially demonstrated the validity of Ki-67 status for breast cancer patients as a predictive marker in terms of neoadjuvant chemotherapy [21]. The American College of Surgeons Oncology Group Z1031B trial evaluated the Ki-67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant treatment of breast cancer with aromatase inhibitor. That study concluded that the analysis of post-neoadjuvant endocrine therapy surgical samples with Ki-67 and ER status provides additional useful prognostic information. In our study, we did not have such information available to evaluate whether the change in the percentage of Ki-67 cells or postoperative level of Ki-67 cells can better predict the clinical prognosis.
Our study was an observational study, and the use of trimodality treatment was not randomly assigned. When evaluating the predictive value of Ki-67 status, the potential imbalance in the patients' other characteristics is a threat to our conclusions. Accordingly, we have carefully compared the distributions of patient and tumor characteristics at diagnosis between patients who received trimodality treatment and those who did not. We did not find any statistically significant differences between the two groups (see Supplemental Table S2). Further, because trimodality treatment is a standard form of breast cancer care, we do not expect that randomization is feasible.
There are some limitations to this study. First, although a change in Ki-67 status with endocrine and HER2-targeted therapy has also been shown to be prognostic for ER/PR+ and HER2+ early breast cancer [22], we did not include the change in Ki-67 status before and after treatment in our analysis. The database we used recorded only the Ki-67 status prior to the systemic treatments. Second, because of the low incidence of IBC, this study included a limited number of patients with low proliferative tumors; more of these cases are needed to further confirm the effect on different IBC subtypes as determined by the association between Ki-67 status and therapeutic response and prognosis. Last, we excluded from our cohort the observations with missing values of Ki-67. We have compared the distributions of patients' characteristics at diagnosis, and did not found any significant difference between patients with and without values of Ki-67 observed (see Supplemental Table S4).
Supplementary Material
Supplementary tables.
Acknowledgements
This work was partially supported by grants from the National Institutes of Health (R01CA193878 and P30CA016672).
Funding
This work was partially supported by grants from the National Institutes of Health (R01CA193878 and P30CA016672).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Rueth NM, Lin HY, Bedrosian I, Shaitelman SF, Ueno NT, Shen Y, Babiera G. Underuse of trimodality treatment affects survival for patients with inflammatory breast cancer: an analysis of treatment and survival trends from the National Cancer Database. Journal of Clinical Oncology. 2014;32(19):2018-2024
2. Robertson FM, Bondy M, Yang W, Yamauchi H, Wiggins S, Kamrudin S, Krishnamurthy S, Le-Petross H, Bidaut L, Player AN, Barsky SH. Inflammatory breast cancer: the disease, the biology, the treatment. CA: a cancer journal for clinicians. 2010;60(6):351-375
3. Masuda H, Brewer TM, Liu DD, Iwamoto T, Shen Y, Hsu L, Willey JS, Gonzalez-Angulo AM, Chavez-MacGregor M, Fouad TM, Woodward WA. Long-term treatment efficacy in primary inflammatory breast cancer by hormonal receptor-and HER2-defined subtypes. Annals of oncology. 2014;25(2):384-391
4. Ding J, Yang Y, Jiang L, Wu W, Shao Z. Predictive factors of pathologic complete response in HER2-positive and axillary lymph node positive breast cancer after neoadjuvant paclitaxel, carboplatin plus with trastuzumab. Oncotarget. 2017;8(34):56626-56634
5. Li J, Xia Y, Wu Q, Zhu S, Chen C, Yang W, Wei W, Sun S. Outcomes of patients with inflammatory breast cancer by hormone receptor-and HER2-defined molecular subtypes: A population-based study from the SEER program. Oncotarget. 2017;8(30):49370-49379
6. Surveillance, Epidemiology, and End Results (SEER) Program SEER Database: Incidence—SEER Regs Public-Use 2007 National Cancer Institute. www.seer.cancer.gov
7. Ueno NT, Buzdar AU, Singletary SE, Ames FC, McNeese MD, Holmes FA, Theriault RL, Strom EA, Wasaff BJ, Asmar L, Frye D. Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at MD Anderson Cancer Center. Cancer chemotherapy and pharmacology. 1997;40(4):321-329
8. Yamauchi H, Woodward WA, Valero V, Alvarez RH, Lucci A, Buchholz TA, Iwamoto T, Krishnamurthy S, Yang W, Reuben JM, Hortobágyi GN. Inflammatory breast cancer: what we know and what we need to learn. Oncologist. 2012;17(7):891-899
9. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. Journal of clinical oncology. 2005;23(28):7212-7220
10. Abubakar M, Orr N, Daley F, Coulson P, Ali HR, Blows F, Benitez J, Milne R, Brenner H, Stegmaier C. et al. Prognostic value of automated KI67 scoring in breast cancer: a centralised evaluation of 8088 patients from 10 study groups. Breast Cancer Res. 2016;18:104
11. Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ, DeSchryver K, Crouch E, Brink A, Watson M, Luo J. Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). Journal of Clinical Oncology. 2017;35(10):1061-1069
12. Jones RL, Salter J, A'Hern R, Nerurkar A, Parton M, Reis-Filho JS, Smith IE, Dowsett M. Relationship between oestrogen receptor status and proliferation in predicting response and long-term outcome to neoadjuvant chemotherapy for breast cancer. Breast cancer research and treatment. 2010;119(2):315
13. Jung SY, Han W, Lee JW, Ko E, Kim E, Yu JH, Moon HG, Park IA, Oh DY, Im SA, Kim TY. Ki-67 expression gives additional prognostic information on St. Gallen 2007 and Adjuvant! Online risk categories in early breast cancer. Annals of Surgical Oncology. 2009;16(5):1112-1121
14. Ellis MJ, Tao Y, Luo J, A'hern R, Evans DB, Bhatnagar AS, Chaudri Ross HA, von Kameke A, Miller WR, Smith I, Eiermann W. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. Journal of the National Cancer Institute. 2008;100(19):1380-1388
15. Lakhanpal R, Sestak I, Shadbolt B, Bennett GM, Brown M, Phillips T, Zhang Y, Bullman A, Rezo A. IHC4 score plus clinical treatment score predicts locoregional recurrence in early breast cancer. The Breast. 2016;29:147-152
16. Hammond ME, Hayes DF, Dowsett M. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784-2795
17. Bustreo S, Osella-Abate S, Cassoni P, Donadio M, Airoldi M, Pedani F, Papotti M, Sapino A, Castellano I. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: a large case series study with a long-term follow-up. Breast cancer research and treatment. 2016;157(2):363-371
18. Robertson J. F. R, M. Dowsett, J. M. Bliss, J. P. Morden, M. Wilcox, A. Evans, C. Holcombe et al. Perioperative aromatase inhibitor treatment in determining or predicting long-term outcome in early breast cancer-The POETIC* Trial (CRUK/07/015). Cancer research. 2018;48(4):GS1-03
19. Nguyen DM, Sam K, Tsimelzon A, Li X, Wong H, Mohsin S, Clark GM, Hilsenbeck SG, Elledge RM, Allred DC, O'Connell P. Molecular heterogeneity of inflammatory breast cancer: a hyperproliferative phenotype. Clinical cancer research. 2006;12(17):5047-5054
20. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Panel members, Albain KS, André F, Bergh J. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Annals of oncology. 2013;24(9):2206-2223
21. Ács B, Zámbó V, Vízkeleti L, Szász AM, Madaras L, Szentmártoni G, Tőkés T, Molnár BÁ, Molnár IA, Vári-Kakas S, Kulka J. Ki-67 as a controversial predictive and prognostic marker in breast cancer patients treated with neoadjuvant chemotherapy. Diagnostic pathology. 2017;12(1):20
22. Gianni L, Bisagni G, Colleoni M, Del Mastro L, Zamagni C, Mansutti M, Zambetti M, Frassoldati A, De Fato R, Valagussa P, Viale G. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): an exploratory, open-label, phase 2 study. The Lancet Oncology. 2018;19(2):249-256
Author contact
![]() Corresponding authors: Dr. Naoto T. Ueno (nuenoorg) and Dr. Yu Shen (yshenorg).
Corresponding authors: Dr. Naoto T. Ueno (nuenoorg) and Dr. Yu Shen (yshenorg).

 Global reach, higher impact
Global reach, higher impact