Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(11):2578-2587. doi:10.7150/jca.26961 This issue Cite
Research Paper
Blocking the PD-1/PD-L1 axis in dendritic cell-stimulated Cytokine-Induced Killer Cells with pembrolizumab enhances their therapeutic effects against hepatocellular carcinoma
1. Integrated Hospital of Traditional Chinese Medicine, Southern Medical University Guangzhou, 510315, China
2. Cancer Center, Southern Medical University Guangzhou, 510315, China
3. Department of Physiology, Michigan State University, East Lansing, MI, 48824, USA
*These authors contributed equally to this work
Received 2018-4-30; Accepted 2019-4-25; Published 2019-6-2
Abstract
Immune checkpoint therapies for cancer, like the anti-programmed cell death 1 (PD-1) agent pembrolizumab, have gained considerable attention. However, the use of immune checkpoint inhibitors in the context of adoptive immunotherapy is poorly characterized. We investigated the therapeutic efficacy of dendritic cell-stimulated CIK (DC-CIK) cells pretreated with pembrolizumab against hepatocellular carcinoma (HCC) in cytotoxicity assay in vitro and in a nude mouse xenograft model. We used time-lapse imaging to investigate tumor killing. We also performed a survival analysis based on lymphocyte subpopulation-specific mRNA signatures using The Cancer Genome Atlas (TCGA) HCC cohort (n=371 patients). The results indicated that PD-1 inhibition increased the anti-tumor effects of DC-CIK cells over those of DC-CIK cells alone, resulting in a survival benefit importantly. Time-lapse imaging revealed that DC-CIK cells appeared to be more effective and aggressive after anti-PD-1 treatment than after culture in control conditions. The PD-1 inhibitor also induced more effective immune cell infiltration of the tumor. Our analysis of the TCGA HCC cohort confirmed that a genetic signature consistent with a high degree of intratumoral CD8+ T cell infiltration is associated with good prognosis. These results suggest that blockade of the PD-1/PD-L1 axis in DC-CIK cells with a PD-1 inhibitor prior to infusion is a promising therapeutic strategy against HCC.
Keywords: PD-1, dendritic cells, cytokine-induced killer cells, immunotherapy, hepatocellular carcinoma
Introduction
Although surgery, transarterial chemoembolization (TACE), radiofrequency ablation (RFA), and other curative treatments have been applied as therapies for hepatocellular carcinoma (HCC), the long-term prognosis remains poor, owing to the high frequency of recurrence [1, 2]. Thus, it is imperative to develop additional effective strategies to improve survival for HCC patients. Cancer immunotherapy based on the recruitment of anti-tumor immune cells has been pursued for decades [3-5]. Adoptive immunotherapy, a type of adjuvant therapy, holds great promise for the induction of durable tumor responses and cures [6, 7]. Cytokine-induced killer cells(CIK) that have been co-cultured with tumor lysate-pulsed dendritic cells (DC-CIK cells), used for adoptive cellular immunotherapy, mediate greater cytotoxicity and prevent tumor growth and recurrence in patients with malignant tumors [8, 9].
CIK cells comprise a heterogeneous cell population characterized by the co-expression of CD56 and CD3 or CD3 and CD8. One of the distinguishing features of CD3+ CD56+ CIK cells is their ability to mediate MHC-unrestricted cancer killing [10]. DCs, one of the most powerful antigen-presenting cells, capture antigen and then present on their surfaces to responders, such as CIK cells, thereby activating antigen-specific immune responses and improving the function of effector cells. DC-CIK cells have the potential for both MHC-unrestricted and tumor-specific cytotoxicity. In spite of the progress in DC-CIK cell infusion treatments, their therapeutic efficacy is limited by hypofunction within the solid tumor microenvironment [11]. The upregulation of immune checkpoint molecules, including programmed cell death 1 (PD-1), may leads to exhaustion of the infused effector cells [12, 13].
PD-1 is an immune inhibitory checkpoint molecule expressed on the T-cell surface that limits the responsiveness of activated T cells [14]. The binding of PD-1 with its ligands programmed death ligand 1 (PD-L1) and 2, which are expressed on tumor cells, dendritic cells, and other types of cells, activates the PD-1/PD-L1 pathway. The signaling abrogates T cell responses, weakens anti-tumor immunity, and induces negative regulatory signals [15, 16]. Pembrolizumab, an antibody against PD-1, has shown clinical benefit for multiple tumor types, although it is associated with drug-related adverse events, including pneumonitis, colitis, and hepatitis, in up to 10% of patients [17]. The high cost of the therapy also prevents its wide use.
Therefore, in this study, we systematically explored if pretreatment with a PD-1 blocking agent could enhance the therapeutic effect of DC-CIK cells against HCC in vivo and in vitro.
Materials and methods
Cell culture and PD-1 inhibitor
We obtained human liver cancer cell lines, including SK-HEP.1, HCCLM3, MHCC97H, Huh7, and SMMC-7721, from the Clinical Research Center of Nanfang Hospital. The cells were cultured in DMEM-high glucose medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS). All cell lines were cultured in a humidified incubator containing 5% CO2 at 37°C.
We obtained pembrolizumab (Keytruda), the humanized antibody targeting PD-1, from Merck.
Generation and phenotypic analysis of CIK cells and DCs
Healthy volunteers who had provided informed consent donated human peripheral blood. We get ethical approval of Medical Ethics Committee of Integrated Hospital of Traditional Chinese Medicine of Southern Medical University (No.2015-005). We obtained PBMCs from the blood via Ficoll-Hypaque density gradient centrifugation, and then cultured in RPMI 1640 supplemented with 10% FBS. After 4h of incubation, we used the adherent cells to generate DCs, and collected and resuspended the non-adherent cells in RPMI 1640 with 10% FBS, 500 ng/mL anti-CD3 antibody (Miltenyi), 10 μg/mL polyhydroxyalkanoates (Huizhou Hongyu), and 100 U/mL IFN-γ (PeproTech) to generate CIK cells. We added fresh complete medium containing anti-CD3 monoclonal antibody (50 ng/mL) and IL-2 (1000 U/mL) every 2days.
To obtain DCs, we maintained the adherent cells in RPMI 1640 with 10% FBS plus 100 ng/mL granulocyte-macrophage colony-stimulating factor (PeproTech) and 100 ng/mL IL-4 (PeproTech) for 7 days. We lysed HCCLM3 and SK-HEP.1 cells (1 × 107 cells/mL in phosphate-buffered saline) via 5 cycles of freeze-thaw, alternating between a 37°C environment and liquid nitrogen. Then, the lysed tumor cells were centrifuged at 12,000 rpm for 20 min, and the supernatants were collected for use as tumor antigen.
On day 8, we added the tumor cell lysates along with 10 ng/mL TNF-α and 10 ng/mL IL-1β to the culture media of the DCs and incubated for 48 h. Finally, the DCs were co-cultured with CIK cells at a ratio of 1:10 for 2 days.
On days 7, 14, and 21, we harvested the CIK cells for phenotypic analysis by flow cytometry using anti-CD3-FITC, anti-CD56-PE, and anti-CD8-FITC antibodies (BD Biosciences). On day 12, we detected PD-1 expression on DC-CIK cells by flow cytometry using an anti-PD-1 antibody (BD Biosciences).
On day 12, we added the PD-1 inhibitor (pembrolizumab 20 ug/mL) to DC-CIK cells to mediate PD-1 blockade.
Western blotting
We performed western blotting as previously described [18]. We used antibodies against PD-L1 (Abcam), β-tubulin (Sigma-Aldrich), GAPDH (Sigma-Aldrich), perforin 1(PRF1; Santa Cruz Biotechnology), and granzyme B (GZMB; Santa Cruz Biotechnology). We detected the bands with a Bio-Rad imaging system. We quantified the western blotting results by densitometric analyses using Image J software.
Cytotoxicity assay
We assessed the tumor killing ability of DC-CIK cells against HCCLM3 and SK-HEP.1 with the CytoTox 96® Non-Radioactive Cytotoxicity Assay kit (Promega), which measures lactate dehydrogenase (LDH) release via optical density (OD), according to the manufacturer's protocol. Briefly, we co-cultured effector CIK or DC-CIK cells from each group directly with 8 × 103 HCCLM3 or SK-HEP.1 tumor target cells (40:1, 20:1, 10:1, or 5:1) in triplicate, in U-bottomed, 96-well plates for 6h. We calculated the percentage of cytotoxicity with the following formula:
Percent cytotoxicity = 100 × Experimental LDH Release (OD490)/Maximum LDH Release (OD490)
Carboxyfluorescein succinimidyl ester proliferation assay
After blockade with PD-1 antibody for 48h, DC-CIK cells were labeled with 10 μΜ carboxyfluorescein succinimidyl ester (CFSE), and then incubated for 24 h. Finally, CFSE-labeled cells were analyzed by flow cytometry.
Cell apoptosis analysis
We incubated DC-CIK cells with tumor cells at a ratio 10:1 for 24h. We then measured tumor cell apoptosis using the FITC/PI Annexin V Apoptosis Detection Kit (BD Biosciences), per the staining manual. We analyzed the rate of cell apoptosis by flow cytometry.
Colony formation assay and transwell migration assay
We incubated tumor cells with effector cells (anti-PD-1-treated DC-CIK cells, or non-antibody-treated DC-CIK cells) at 10:1 overnight. Then, we washed 3-4 times with PBS to completely remove the suspended effector cells, and performed colony formation or transwell migration assays, as previously described [19].
Cytokine secretion assay
After we treated DC-CIK cells with PD-1 inhibitor (20 ug/mL) for 48 h, we co-cultured them with cancer cells (10:1) in 24-well plates overnight. Cell-free supernatants from the co-cultures were collected for analysis of TNF-α, IFN-γ, IL-4, IL-6, and IL-10 production using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience).
Time-lapse imaging
To observe the tumor killing by DC-CIK cells, we performed live-cell imaging (YeeSpec). We co-cultured 5-chloromethylfluorescein diacetate-labeled cancer cells with effector cells at a ratio of 1:20 and captured images every 5 min for 24 h.
Xenograft experiments in mice
To establish the HCC mouse model, we subcutaneously injected 1 × 106 HCCLM3 cells in 100 μL PBS into 4-week-old male nude mice. After 7 days, tumor-bearing mice received intravenous infusions of 2 × 107 DC-CIK cells, anti-PD-1-treated DC-CIK cells, or PBS every 2 days. We measured the tumor volume with calipers and the following formula: Tumor volume = 0.5 × length (mm) × width (mm) 2. At day 26, we euthanized the mice and dissected the tumors, which we weighed, then fixed in 4% paraformaldehyde for histological examinations. For survival analyses, we conducted independent survival experiments. We observed the mice every day and recorded their survival times. All animal studies were performed in accordance with the principles and procedures outlined in the Southern Medical University Guide for the Care and Use of Animals.
Immunohistochemical staining
We stained paraffin-embedded sections of tumor tissues from xenograft experiments with hematoxylin and eosin or antibodies against Ki-67, PCNA, and CD8.
Prognostic value of immune infiltration-associated genes in The Cancer Genome Atlas HCC patient dataset
We utilized The Cancer Genome Atlas (TCGA) transcriptome dataset produced by microarray analysis of samples from HCC patients (n=371) to perform a survival analysis. We stratified the patients into high and low expression groups on the basis of their median gene expression profile. We plotted the patient risk groups with respect to the overall survival of the patients to produce Kaplan-Meier curves using GraphPad Prism software.
Statistical analysis
We performed statistical calculations with GraphPad Prism 5.0 software and the SPSS 19.0 statistical software package. The data are presented as the mean ± standard deviation (SD), and differences with P-values < 0.05(*) or < 0.01(**) were considered statistically significant. The statistical significance of the difference between two groups was analyzed using an independent-sample t-test and the differences among multiple groups were analyzed using one-way analysis of variance. We performed survival analyses with the Kaplan-Meier method, and we used the log-rank test to assess differences in overall survival.
Results
Characteristics of CIK and dendritic cells
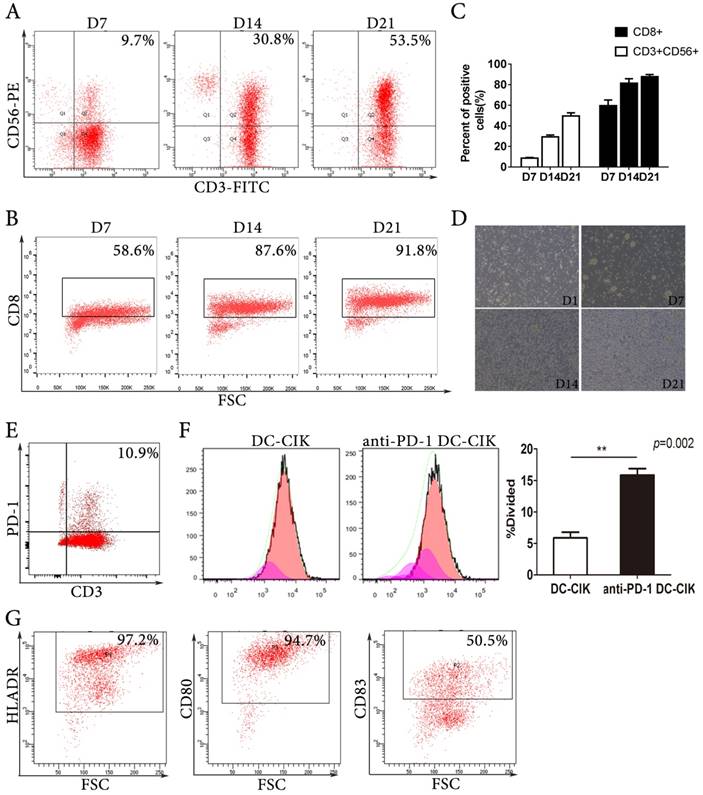
We generated CIK cells by culturing isolated PBMCs with recombinant IL-2, IFN-γ, and anti-CD3. The CIK cells expanded by several hundred-fold and the proportion of effector cells, including CD3+CD56+ cells and CD8+ cells, increased during the culture period (Fig. 1A-C). After 7 days of culture, the subset of CD3+CD56+CIK cells was 9.7%, and it increased to 30.8% and 53.5% after 14 and 21 days of culture, respectively. Morphologically, the expanded CIK cells formed cell colonies. During the cultures, the cell colonies became larger and more numerous (Fig. 1D). We detected PD-1 expression on the DC-CIK cells from all donors; on average 11%, ranging from 4% to 21%, of DC-CIK cells expressed PD-1 (Fig. 1E). We found that anti-PD-1 (20 μg/mL) treatment increased DC-CIK cell proliferation over that observed in the PBS-treated control group (Fig. 1F). Dendritic cells were analysed by flow cytometry with marker of HLADR, CD80 and CD83 (Fig. 1G).
The phenotypic patterns of CIK cells and dendritic cells. (A-B) Representative flow cytometric analysis of the expression of CD3, CD56, and CD8 on CIK cells at days 7, 14, and 21. (C) The quantitative statistics of phenotypes of CIK cells. (D) Representative pictures of the generation and expansion of CIK cells. (E) Representative flow analysis of PD-1 expression on DC-CIK cells. (F) Analysis of divided cell using CFSE staining by flow cytometry. (G) Phenotype analysis of DCs.
PD-1 blockade enhanced the cytotoxicity of DC-CIK cells against HCC cell lines
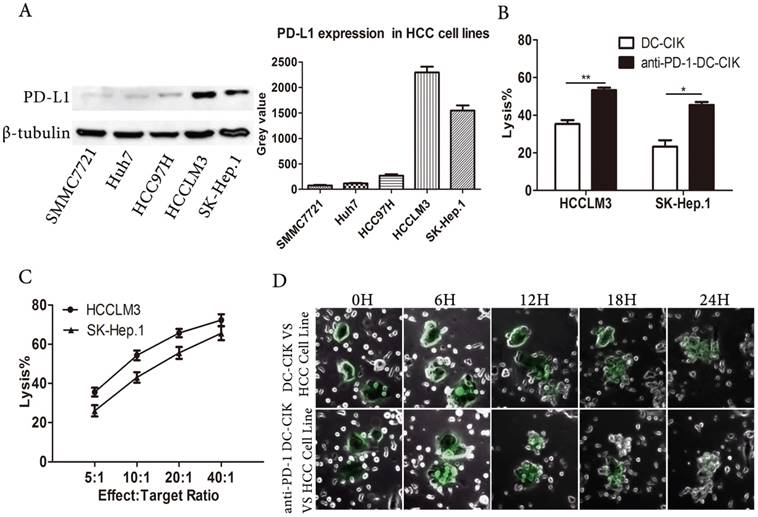
The PD-1/PD-L1 axis negatively regulates immune responses, and PD-L1 expression is a criterion for selecting suitable patients to receive PD-1 inhibitor treatment [20]. We assessed PD-L1 expression in human HCC cell lines. We found that PD-L1 expression varied in the HCC cell lines, but was highest in the HCCLM3 and SK-HEP.1cells (Fig. 2A). Due to their high PD-L1 expression, we selected the HCCLM3 and SK-HEP.1 cell lines as the target cells for our assays.
To enhance the non-specific anti-tumor capacity of CIK cells, we generated antigen-specific DC-CIK cells by co-culturing DCs pulsed with lysates from the target cell lines with CIK cells. In order to investigate the effect of blocking PD-1 signaling on the tumor killing activity of DC-CIK cells, we performed cytotoxicity assays. As expected, anti-PD-1-treated DC-CIK cells mediated greater cytotoxicity against the HCCLM3 and SK-HEP.1 tumor targets than non-antibody-treated DC-CIK cells (Fig. 2B). We next examined the anti-tumor activity of DC-CIK cells treated with anti-PD-1 against SK-HEP.1 and HCCLM3 cells at different effector: target cell ratios (Fig. 2C). The anti-PD-1-treated DC-CIK cells had significantly potentiated anti-tumor activity, in an effector: target cell ratio-dependent manner. To assess the contact between effector and tumor cells, we used time-lapse videos to observe non-antibody-treated and anti-PD-1-treated DC-CIK cell-mediated killing. We found that the tumor killing ability of anti-PD-1-treated DC-CIK cells was stronger than that of the untreated cells, and that the target cells were surrounded by multiple effector cells (Fig. 2D, supplemental videos 1 and 2). As the HCC cells were killed by the DC-CIK cells, they progressively adopted aberrant shapes and broke into small pieces, until killing was complete. Moreover, the anti-PD-1-treated DC-CIK cells appeared to be more effective and aggressive than the non-antibody-treated cells. Collectively, these results suggested that the PD-1inhibitor-pembrolizumab enhanced the cytotoxicity of DC-CIK cells against HCC cell lines.
Anti-PD-1 DC-CIK cells efficiently killed HCC cells. (A) PD-L1 expression was assessed in HCC cell lines. (B) DC-CIK cells exerting cytotoxicity against SK-Hep.1or HCCLM3 with or without PD-1 blockade. (C) Anti-PD-1 DC-CIK cells co-cultured with HCC cell lines at different Effect:Target ratio. (D) Time-lapse imaging (see supplemental video S1and S2) used to observe contact process between HCCLM3 cells and DC-CIK cells with or without antibody. Cancer cells were surrounded by DC-CIK cells.
PD-1 blockade promoted the anti-tumor activity of DC-CIK cells in vitro
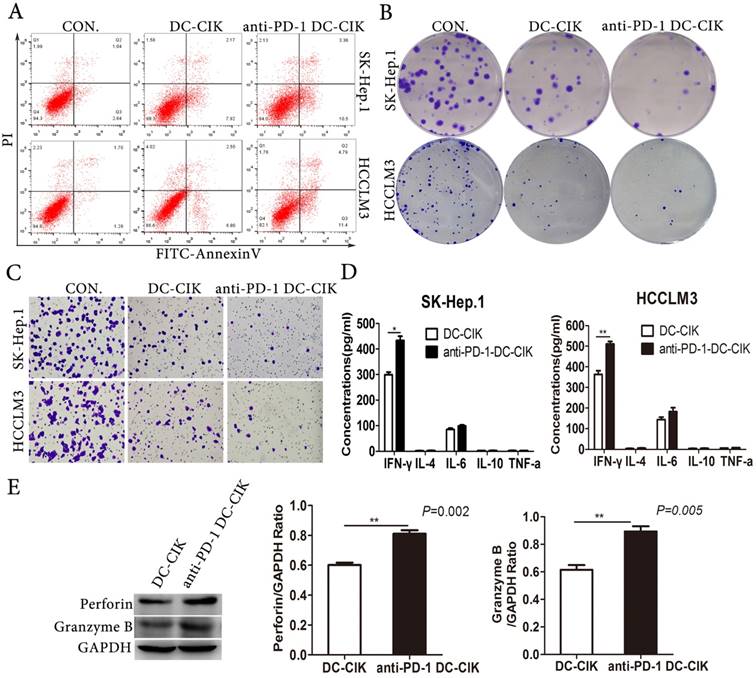
Because DC-CIK cells could prolong the survival of advanced cancer patient in clinical studies, we presumed that DC-CIK cells could not only kill the tumor cells but also affect the malignant biological behavior of tumor cells.
We performed a variety of assays in vitro including apoptosis, proliferation, and migration to evaluate the effect of blocking PD-1 of DC-CIK cells on tumor cells functions. We found that PD-1 blockade of DC-CIK cells enhanced their suppression of tumor cell growth and migration (Fig. 3A-C). Cytokine secretion is one of the most important ways that DC-CIK cells kill tumor cells, so we examined the production of a panel of cytokines produced by non-antibody-treated and anti-PD-1 treated CIK cells. After co-cultivation of effector cells and target cells at a ratio of 10:1 for 24 h, we collected the cell-free supernatants to analyze the concentrations of IL-4, IL-6, IL-10, TNF-α and IFN-γ using ELISA kits. Among the cytokines tested in the co-culture supernatants, only IFN-γ was more highly expressed in the cultures with anti-PD-1-treated DC-CIK cells than in those with non-antibody-treated DC-CIK cells (Fig. 3D). In addition, we explored the expression of the cytotoxic molecules granzyme B and perforin1, which play crucial roles in the cytotoxicity of DC-CIK cells. The cytotoxic molecules granzyme B and perforin1 were more highly expressed on anti-PD-1-treated DC-CIK cells than on non-antibody-treated DC-CIK cells (Fig. 3E). Collectively, our data suggest that PD-1 inhibition enhanced the anti-tumor activity of DC-CIK cells.
PD-1 blockade promoted the antitumor activity of DC-CIK in vitro. (A-C) The apoptosis (A), proliferation (B) and migration (C) of HCCLM3 and SK-Hep.1 cells after incubation with anti-PD-1 DC-CIK cells/DC-CIK cells were evaluated by apoptosis assay, colony formation assay, transwell migration assay. (D) Cytokine secretion assay determined by ELISA. (E) Expression of perforin1 and granzyme B were detected in anti-PD-1 DC-CIK cells and DC-CIK cells.
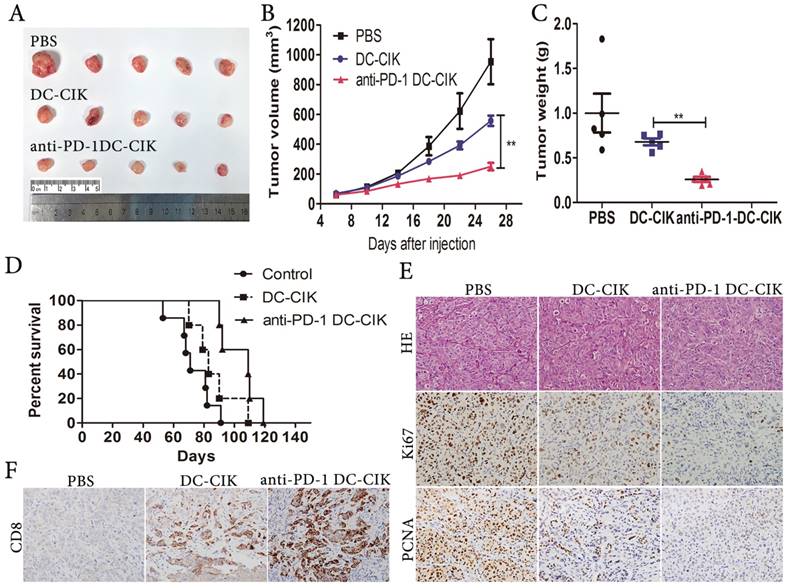
Anti-PD-1-treated DC-CIK cells suppressed HCC xenografts
To investigate if anti-PD-1-treated DC-CIK cells repressed HCC growth in vivo, we established subcutaneous tumor xenografts in nude mice. One-week later, all tumor-bearing animals were divided into 3 groups at random, to be infused every 2 days with anti-PD-1-treated DC-CIK cells, non-antibody-treated DC-CIK cells, or PBS. We observed considerable decreases in tumor weight and volume upon the administration of anti-PD-1-treated DC-CIK cells in comparison with the administration of non-antibody-treated DC-CIK cells (Fig. 4A-C). The median survival times of the PBS, DC-CIK, and anti-PD-1-treated DC-CIK cells groups were 71, 83, and 109 days respectively (Fig. 4D). Importantly, Kaplan-Meier analysis showed a significant increase in survival in the anti-PD-1-treated DC-CIK and control DC-CIK cell groups over the PBS group. Moreover, combination treatment prolonged survival to a meaningful extent over treatment with DC-CIK cells alone. Compared with the DC-CIK group, the anti-PD-1 treated DC-CIK group exhibited a dramatic reduction in Ki-67 and PCNA levels in tumor tissues, as assessed by immunohistochemistry (Fig. 4E). Owing to the high expression of CD8 on DC-CIK cells (Fig. 1B), we investigated the CD8 expression in the tumor tissues. Compared with the tumor tissues from the PBS and non-antibody-treated DC-CIK cell groups, the anti-PD-1-treated DC-CIK cells group tumors had greater CD8+ expression, as determined by immunohistochemical staining (Fig. 4F). These results suggest that the administration of anti-PD-1-treated DC-CIK cells is a potential therapeutic strategy in vivo.
Increased prevalence of CD8+ T cell-associated genetic features in tumors is related to good prognosis in HCC patients
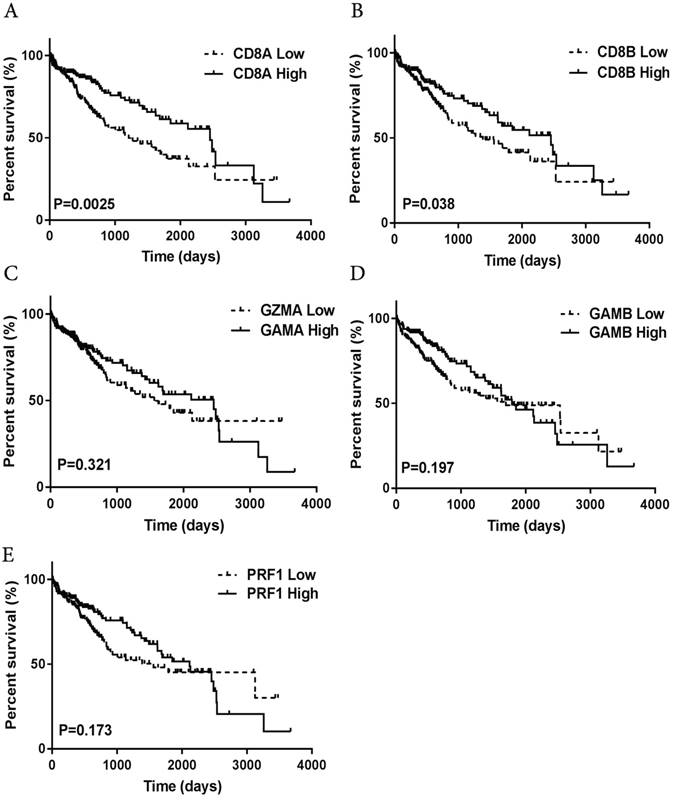
An immunostimulatory intratumoral immune context is associated with improved prognosis in cancer patients [21], so we next investigated the prognostic value of intratumoral T cells in HCC patients. We analyzed existing intratumoral lymphocyte subpopulation-specific mRNA signatures-CD8A, CD8B, granzyme A GZMA), granzyme B (GZMB) and perforin1 (PRF1) expression-in a large, standardized, cohort of 371 HCC patients that is publicly available through TCGA. We estimated the prognostic value of the differential expression of the genes on patient overall survival. High expression of the CD8A and CD8B genes was associated with significantly prolonged overall survival (Fig. 5A-B), whereas we found that high granzyme A, granzyme B, and perforin1 expression were associated with a slight, but not significant, survival benefit (Fig. 5C-E).
Pembrolizumab (PD-1 inhibitor) combined with DC-CIK cells attenuates the growth of HCC cells in vivo. (A) Representative picture of xenograft tumors formed. (B) Tumor volume was periodically measured for each mouse and growth curves were plotted. (C) Tumors were weighted. (D) Survival curves using Kaplan-Meier analysis. (E) Representative H&E as well as Ki67, and PCNA IHC staining of primary tumor tissues are shown. (F) Infiltration of DC-CIK cells in tumor tissues was shown by immunohistochemistry (CD8 staining).
Increased prevalence of CD8+ t cell-associated genetic signatures correlates with good prognosis in HCC patients. (A-E) TCGA HCC patient cohort (n=371)was stratified into “high-expression” or “low-expression” groups for genes associated with CD8A/B, granzyme A/B, perforin 1, followed by Kaplan-Meier plotting of patient's OS.
Discussion
Our study provides a novel approach of adoptive immunotherapy. Our findings suggest that blocking PD-1 on DC-CIK cells in vitro prior to infusion potentiated their anti-tumor killing capacity against liver cancer in vitro and in vivo.
CIK and DC-CIK cells represent the dominant adjuvant therapy in the field of HCC. In the present study, we showed the feasibility of the generation of CIK cells from PBMCs via culture with IFN-γ, anti-CD3 monoclonal antibody, and IL-2 for 2 to 3 weeks. Consistent with our findings, former studies have shown that CIK cells exhibit dual characteristics of NK and T cells [22, 23]. Several studies have reported the effects of CIK cells administered in combination with monoclonal antibodies targeting immune checkpoints molecules [24]. DCs are the foremost antigen-presenting cells that stimulate anti-cancer T cell immune responses. We employed whole tumor cell lysis to generate tumor antigens for DC maturation. This approach stimulates immunity against all tumor antigens, which induces a more complete cytotoxic reaction than stimulation with selected tumor antigens [25]. After co-culture of DC with CIK cells, the resulting cells have stronger proliferation activity than homologous CIK cells. At the same time, DC-CIK cells have a stronger cytotoxic activity, releasing a larger number of cytokines, and get better clinical benefit than CIK cells.
The PD1/PD-L1 pathway delivers inhibitory signals that negatively regulate the immune response. We found that a significant proportion of DC-CIK cells express PD-1. There is a high level of PD-L1 expression on liver cancer cell lines. PD1/PD-L1 axis is one of the mechanisms of tumor immune escape in liver cancer. PD1/PD-L1 antibodies are clinically used in many solid tumors and have unprecedented cure rates, making them one of the most promising methods for curing tumors. However, many patients have had to discontinue pembrolizumab therapy because of severe adverse effects [26]. We hypothesized that DC-CIK cells that express PD-1 are committed to cell death and lose the ability to kill the tumor cells. And that blocking PD-1/PD-L1 on DC-CIK cells would be sufficient to rescue their proliferation and survival without the adverse effects of pembrolizumab administration [27].
Increasing evidence suggests that immune inhibition is critical for tumor development and treatment tolerance. Researchers have investigated factors that influence the efficacy of DC-CIK cells and the exhaustion of T cells, and agents that can optimize the tumor microenvironment to stimulate immune responses, including those that target immune checkpoints molecules like PD-1, have been administered to CIK cells [28, 29]. A retrospective study in hepatocellular carcinoma patients revealed that those with >5% PD-L1 expression in their tumor tissues had prolonged overall survival and recurrence-free survival in comparison with those with <5% PD-L1, upon treatment with CIK cell immunotherapy. Here, for the first time, we present additional evidence that using an immune checkpoint inhibitor to block the PD-1/PD-L1 axis could enhance the tumor killing capacity of DC-CIK cells. We conclude that adoptive transfer of DC-CIK cells pretreated with PD-1 blockade prolongs survival time to a greater extent than DC-CIK cells in HCC nude mouse model.
Time-lapse imaging indicated that adequate numbers of active DC-CIK cells are essential to kill tumor cells. The killing efficiencies of anti-PD-1-treated DC-CIK cells were higher than those of their respective controls. Importantly, DC-CIK cells pretreated with anti-PD-1 were able to escape anergy and effectively migrate into tumor sites. Our analysis of TCGA HCC patient data showed that molecular signatures with relatively high CD8A and CD8B gene expression are associated with good prognosis.
In our study, we found that pembrolizumab enhanced the expansion of DC-CIK cells. Furthermore, due to their enhanced tumor cell lysis, DC-CIK cells treated with anti-PD-1 significantly reduced tumor cell migration. Additionally, blockade increased the production of perforin1, granzyme B and IFN-γ by DC-CIK cells, indicating that perforin1, IFN-γ, and granzyme B play crucial roles in anti-HCC cytotoxic activity.
Our study demonstrated the efficacy of an immunotherapy that enhances the anti-tumor activity of DC-CIK cells using only a small quantity of PD-1 inhibitor, thereby avoiding the high cost and severe adverse reactions typically associated with pembrolizumab. We identified a prospective modality for the treatment of hepatocellular carcinoma. Our results encourage further study into the efficacy of patient-derived DC-CIK cells combined with anti-PD-1 antibody for treatment of hepatocellular carcinoma, especially as the expression of PD-1 on DC-CIK cells differs among individuals. Although it was beyond the scope of our study to investigate the specific pathways related to the anti-tumor activity of DC-CIK cells, it is critical to identify these pathways in future studies, in order to determine the biomarkers for tumor patients who are susceptible to the therapy. In the future, we will conduct clinical trials of DC-CIK cells combined with a PD-1 inhibitor or with other treatment methods, like RFA and TACE, as immunotherapy is most effective when combined with traditional therapeutic approaches.
Abbreviations
PD1: programmed cell death-1; CIK: cytokine-induced killer; DC: dendritic cell; DC-CIK: DC-stimulated CIK; HCC: hepatocellular carcinoma; TACE: transarterial chemoembolization; RFA: radiofrequency ablation; PBMC: peripheral blood mononuclear cell; LDH: lactate dehydrogenase; ELISA: enzyme-linked immunosorbent assay; TCGA: the Cancer Genome Atlas; PD-L1: programmed cell death ligand 1; FBS: fetal bovine serum; OD: optical density; PBS: phosphate-buffered saline; CFSE: carboxyfluorescein succinimidyl ester; GZMA: granzyme A; GZMB: granzyme B; PRF1: perforin1; OS: overall survival; SD: standard deviation.
Supplementary Material
Supplementary figures, table, and video legends.
Supplementary video 1.
Supplementary video 2.
Acknowledgements
This work was supported by National Nature Science Fund of China (No. 81572797), Natural Science Fund of Guangdong Province (No. 2015A030313261, No. 2016A030311015 and No. 2018A030313730), and the Science and Technology Program of Guangzhou, China (No. 201604020009).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E. et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. The Lancet Oncology. 2009;10:1111-8
2. Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2017;389:56-66
3. Kean LS, Turka LA, Blazar BR. Advances in targeting co-inhibitory and co-stimulatory pathways in transplantation settings: the Yin to the Yang of cancer immunotherapy. Immunological reviews. 2017;276:192-212
4. Deng X, Nakamura Y. Cancer Precision Medicine: From Cancer Screening to Drug Selection and Personalized Immunotherapy. Trends Pharmacol Sci. 2017;38:15-24
5. Barreira da Silva R, Laird ME, Yatim N, Fiette L, Ingersoll MA, Albert ML. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat Immunol. 2015;16:850-8
6. Takayama T, Sekine T, Kondo Y, Kakizoe T, Makuuchi M. Adjuvant adoptive immunotherapy against hepatocellular carcinoma. Hepatology (Baltimore, Md). 1998;28:1436-7
7. Fearon D. Combination immunotherapy for cancer. The Journal of experimental medicine. 2016;213:1115
8. Wang Y, Xu Z, Zhou F, Sun Y, Chen J, Li L. et al. The combination of dendritic cells-cytotoxic T lymphocytes/cytokine-induced killer (DC-CTL/CIK) therapy exerts immune and clinical responses in patients with malignant tumors. Experimental hematology & oncology. 2015;4:32
9. Xia Zhao C-YJ, Guo-Qiang Liu, Dao-Xin Ma, Hui-Fang Ding, Min Xu, Jian Xing. Immunomodulatory effect of DC/CIK combined with chemotherapy in multiple myeloma and the clinical efficacy. Int J Clin Exp Pathol. 2015;8:13146-55
10. Schmidt-Wolf GD, Negrin RS, Schmidt-Wolf IG. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Annals of hematology. 1997;74:51-6
11. Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J. et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res. 2014;20:4262-73
12. Park HJ, Kusnadi A, Lee EJ, Kim WW, Cho BC, Lee IJ. et al. Tumor-infiltrating regulatory T cells delineated by upregulation of PD-1 and inhibitory receptors. Cell Immunol. 2012;278:76-83
13. Abate-Daga D, Hanada K, Davis JL, Yang JC, Rosenberg SA, Morgan RA. Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes. Blood. 2013;122:1399-410
14. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027-34
15. Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature medicine. 1999;5:1365-9
16. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature medicine. 2002;8:793-800
17. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443-54
18. Zhao M, Luo R, Liu Y, Gao L, Fu Z, Fu Q. et al. miR-3188 regulates nasopharyngeal carcinoma proliferation and chemosensitivity through a FOXO1-modulated positive feedback loop with mTOR-p-PI3K/AKT-c-JUN. Nature communications. 2016;7:11309
19. Ren X, Yang X, Cheng B, Chen X, Zhang T, He Q. et al. HOPX hypermethylation promotes metastasis via activating SNAIL transcription in nasopharyngeal carcinoma. Nature communications. 2017;8:14053
20. Sharma P, Allison JP. The future of immune checkpoint therapy. Science (New York, NY). 2015;348:56-61
21. Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609-17
22. Cappuzzello E, Sommaggio R, Zanovello P, Rosato A. Cytokines for the induction of antitumor effectors: The paradigm of Cytokine-Induced Killer (CIK) cells. Cytokine & growth factor reviews. 2017;36:99-105
23. Rong XX, Wei F, Lin XL, Qin YJ, Chen L, Wang HY. et al. Recognition and killing of cancer stem-like cell population in hepatocellular carcinoma cells by cytokine-induced killer cells via NKG2d-ligands recognition. Oncoimmunology. 2016;5:e1086060
24. Hui Z, Zhang X, Ren B, Li R, Ren X. Rapid Response of Advanced Squamous Non-Small Cell Lung Cancer with Thrombocytopenia after First-Line Treatment with Pembrolizumab Plus Autologous Cytokine-Induced Killer Cells. Front Immunol. 2015;6:633
25. Yufeng D, Guocheng Z, Dongliang X, Rong F, Yuhong C, Ruying L. et al. Whole-tumor-antigen-pulsed dendritic cells elicit cytotoxic T-cell response against pediatric nasopharyngeal carcinoma in vitro. Medical oncology. 2009;26:78-85
26. Cousin S, Italiano A. Molecular Pathways: Immune Checkpoint Antibodies and their Toxicities. Clin Cancer Res. 2016;22:4550-5
27. Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105:15016-21
28. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917-27
29. Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935-45
Author contact
![]() Corresponding authors: E-mail addresses: liaimin2005com (A. Li) and luorc02163.com (R.Luo)
Corresponding authors: E-mail addresses: liaimin2005com (A. Li) and luorc02163.com (R.Luo)

 Global reach, higher impact
Global reach, higher impact