Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(6):1057-1066. doi:10.7150/jca.23391 This issue Cite
Research Paper
A Meta-Analysis: Methylenetetrahydrofolate Reductase C677T Polymorphism in Gastric Cancer Patients Treated with 5-Fu Based Chemotherapy Predicts Serious Hematologic Toxicity but Not Prognosis
Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai, China.
Received 2017-10-18; Accepted 2018-1-29; Published 2018-2-28
Abstract
5-fluorouracil (5-Fu) metabolism associated enzyme, methylenetetrahydrofolate reductase (MTHFR)'s polymorphism C677T can affect enzyme activity and a series of studies have been performed to examine the association of this MTHFR polymorphism with the clinical outcomes of gastric cancer (GC) patients treated with 5-Fu based chemotherapies. However, the results are inconsistent and inconclusive. Therefore, a more comprehensive summary like meta-analysis on this topic is needed. We performed a systematic literature search of PubMed and Embase up to May 20, 2017. Researches exploring MTHFR polymorphisms C677T's relationship with the clinical outcomes (response rate, overall survival and toxicity) of GC patients treated with 5-Fu based chemotherapy were included. The association was measured by odds ratios (ORs) or hazard ratios (HRs) combined with their 95% confidence intervals (CIs) using random/fixed effects model according to the studies' heterogeneity. Subgroup, sensitivity and publication bias analyses were conducted. Thirteen studies were finally included in this meta-analysis. No significant association was found between response rate [TT/ (CC+CT) OR=1.31, 95% CI: 0.62-2.76] or overall survival [(CT+TT)/CC HR=1.05, 95% CI: 0.86-1.26; TT/(CT+CC) HR=1.48, 95% CI: 0.53-4.15] and MTHFR polymorphism C677T. However, GC patients with CC or CT genotype tended to experience less severe hematologic toxicity than those with TT genotype [(CC+CT)/TT OR=0.66, 95% CI: 0.48-0.91]. In conclusion, MTHFR C677T polymorphism predicts severe hematologic toxicity in GC patients receiving 5-Fu based chemotherapy, but not the efficiency.
Keywords: Gastric cancer, MTHFR C677T polymorphism, 5-Fu based chemotherapy, Meta-analysis
Introduction
Although early-stage and locally-advanced gastric cancer (GC) can be treated by surgery, the truth is that many patients have to face local recurrence or distant metastasis even after gastrectomy [1]. Besides that, approximately a quarter of patients have inoperable disease at diagnosis [2]. Thus, GC is one of the most common cases where multimodality therapy (MDT) is often applied. It is widely accepted that chemotherapy is an indispensable part of MDT, since both palliative chemotherapy for advanced disease and perioperative chemotherapy have been proved to improve survival and quality of life in patients with GC [3] and 5-fluorouracil (5-Fu) based regimens are most generally used in this area.
However, what cannot be ignored in clinical settings is that, even with the help of new agents such as capecitabine, S-1 which seem to be superior to older drugs, the expected survival for advanced GC is still poor. [4,5] Furthermore, chemotherapies may sometimes bring patients severe, unpredictable toxicity without any tumor response. Despite of what have been explored a lot in randomized control trials---the regimens' factors like dose, drug combinations, the inherited genetic variability probably also has something to do with the treatment outcomes, as dozens of recent researches infer. [6,7]
A growing body of evidence suggests that inter-individual variation in drug-metabolizing enzymes may affect anticancer drug efficacy by influencing related enzyme activities.[8] Methylenetetrahydrofolate Reductase (MTHFR) which has been found to be associated with 5-Fu metabolism recently is the key enzyme in metabolism of folic acid-homocysteine which catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methylenetetrahydrofolate.[9,10] Human's gene which expresses MTHFR locates on chromosome 1p, and several single nucleotide polymorphism (SNP) loci that associate with enzyme activity exist on that gene[11]. Among those SNP loci, polymorphisms in MTHFR C677T (rs1801133) is most widely investigated [9]. Although a series of studies have been performed to examine the association of MTHFR polymorphism C677T with the clinical outcomes of GC patients treated with 5-Fu based chemotherapies, the results were inconsistent and inconclusive. Therefore, we conducted a meta-analysis to evaluate the association of MTHFR polymorphism C677T with the prognosis and toxicity of GC patients treated with 5-Fu based chemotherapies.
Methods
Searching strategy
Studies focusing on MTHFR and gastric cancer were initially searched using PubMed, with the terms “Methylenetetrahydrofolate Reductase (NADPH2) / Methylenetetrahydrofolate Reductase (NADPH) / Methylene-THF Reductase (NADPH) / Methylenetetrahydrofolate Reductase / 5,10-Methylenetetrahydrofolate Reductase (NADPH) / Methylene Tetrahydrofolate Reductase / Tetrahydrofolate Reductase, Methylene / MTHFR” and “Stomach Neoplasms / Neoplasm, Stomach / Stomach Neoplasm / Neoplasms, Stomach / Gastric Neoplasms / Gastric Neoplasm / Neoplasm, Gastric / Neoplasms, Gastric / Cancer of Stomach / Stomach Cancers / Gastric Cancer / Cancer, Gastric / Cancers, Gastric / Gastric Cancers / Stomach Cancer / Cancer, Stomach / Cancers, Stomach / Cancer of the Stomach / Gastric Cancer, Familial Diffuse”. Another online search engine, Embase was searched with the terms “5,10 methylenetetrahydrofolate reductase (FADH2) / MTHFR / 5, 10 methylenetetrahydrofolate reductase (FADH2)” and “gastric cancer / stomach cancer”.
Inclusion and exclusion criteria
Studies were included if they met the following criteria. First, objects of the study should have: (1) pathologically confirmed GC, (2) no concurrent uncontrolled medical illness, (3) treatment with 5-Fu-based chemotherapy, (4) measurable lesion if investigating response rate. Second, contents should illustrate clinical outcomes such as response rate, overall survival (OS) or toxicity involved with MTHFR polymorphism C677T. Meanwhile, Studies were excluded if they were: (1) in vitro studies; (2) not original research, such as review articles;(3) researches regarding MTHFR polymorphisms as risk factors for gastric cancer.
Data extraction and assessment
Data from each included study was extracted using Microsoft Excel spreadsheets, including information on authors, publishing years, patients' clinical stage and ethnicities, ways of presenting results, evaluation criteria of lesion, sample size, chemotherapeutic regimens and outcome indicators such as odds ratio (OR) for response rate and toxicity, hazard ratio (HR) for OS. Additionally, we evaluated the quality of the included studies using the Newcastle- Ottawa scale based on three aspects: selection, comparability, and exposure, with scores ranging from zero to nine. Studies with a score equal to or higher than five points were recognized to be high-quality ones [12], whereas studies with scores less than five points were regarded as low-quality ones which would be further excluded.
Statistical analysis
We analyzed the information which were discussed in included articles through Stata 12.0 (Stata Corporation, College Station, TX, USA). For SNP loci C677T, synthetic response rate, OS and toxicity were all calculable. Comparison of response rate and toxicity among patients with MTHFR polymorphism C677T were between genotype TT and CC+CT. Since some articles only presented data of CC and CT separately, we added the number of patients in these two groups up to form group CC+CT, then generated overall OR in meta-analysis through M-H model. Meanwhile, comparison of OS among patients with MTHFR polymorphism C677T took place between genotype CC and CT+TT, CC and TT, CC and CT or CT+CC and TT. Furthermore, we also combined the HRs together in articles which compared OS between genotype CC and TT, genotype CC and CT, separately, and then formed new result of comparison between genotype CC and CT+TT by synthesize the combined HRs and the already provided HRs together. All the HRs were combined through I-V model.
Flow diagram of study selection process
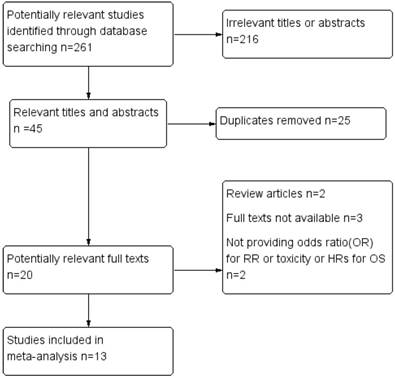
Heterogeneity between included studies would be considered significant if I2>25%. If heterogeneities were not present, the synthetic results would be illustrated using fixed effects model, if not, randomized effects model were used and moreover, in order to explain them, sensitivity analysis like dropping out one included article or subgroup analysis would be performed. Subgroup analysis based on patients' ethnicities, chemotherapy purpose or ways of presenting results would be conducted for further interpreting initial results. Publication bias were statistically tested by the Egger's test. All P values were two-sided and all CIs had a two-sided probability coverage of 95%.
Results
Included studies
Through applying the search strategy mentioned above, we found a total of two hundred and sixty-one studies in Pubmed and Embase. Two hundred and sixteen articles were removed due to irrelevant titles or abstracts, most of them were researches regarding MTHFR polymorphisms as risk factors for GC. After dropping out duplicates, twenty literatures were eligible for full text reviewing. Finally, seven case control studies and six cohort studies could be included in the meta-analysis [13-25], while the others were excluded because of following reasons: (1) review articles, (2) full texts not available, (3) neither providing ORs for response rate or toxicity nor providing HRs for OS. The process of study inclusion was listed in Figure 1. The sample size varied from 56 to 251 and the publication time was from 2004 to 2017. Most participants were Asian and European. The main characteristics of the thirteen included studies were listed in Table 2. All the eligible studies were of high quality owing to the fact that the NOS scores were higher than 5 points among the overall studies. Assessment of the quality of the eligible studies based on the NOS was listed in Table 1.
MTHFR polymorphism C677T and 5-Fu based chemotherapy
Response rate
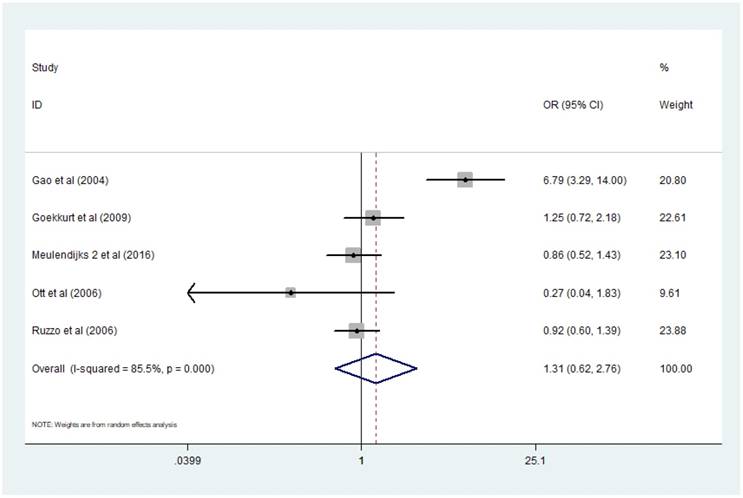
Five studies [13-17] presented data applicable for analyzing the association between MTHFR polymorphism C677T and response rate of GC patients treated with 5-Fu based chemotherapy. Here the definition of 'response' was complete remission (CR) or partial remission (PR) according to RECIST criterion. I2 value of heterogeneity test was more than 75% and a random-effect model was used. Combined analysis demonstrated that there was no significant difference of response rate between patients with genotype TT and patients with CC+CT [TT/ (CC+CT) OR=1.31, 95% CI: 0.62-2.76]. (Figure 2)
OS
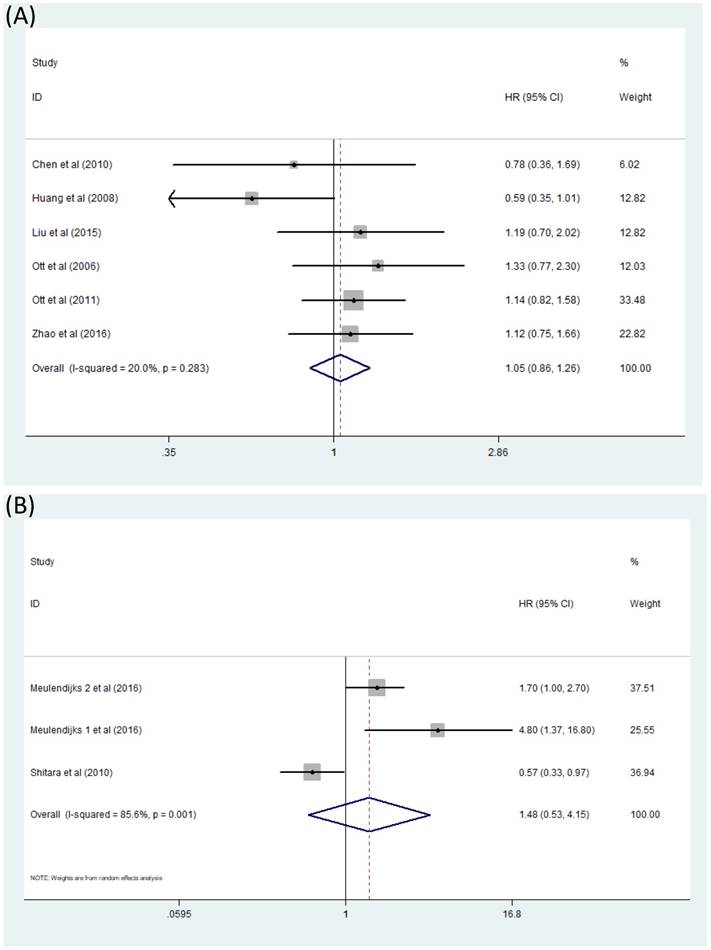
Comparison of OS between GC patients with genotype CT/TT and those with genotype CC when treated with 5-Fu based chemotherapy was exhibited directly in four articles [18-21]. Another three studies [16,20,22] presented results in a different way: OS of patients with genotype TT was compared with those with genotype CC and OS of patients with genotype CT was also compared against those with genotype CC. According to the statistical analysis procedure mentioned before, we processed these studies together to acquire a more comprehensive view of this question. The literatures included in this analysis contradicted, thus it almost seemed that OS of GC patients treated with 5-Fu based chemotherapy had nothing to do with MTHFR C677T polymorphism [(CT+TT)/CC HR=1.05, 95% CI: 0.86-1.26] (Figure 3A). This analysis was performed in a fixed effect model. Moreover, comparison between OS of GC patients with genotype CT+CC and OS of patients with genotype TT were also studied by some other researchers [15,23,24] but no relationship of significance could be found. [TT/(CT+CC) HR=1.48, 95% CI: 0.53-4.15] (Figure 3B).
Assessment of the quality of the eligible studies based on NOS.
| Case control study | Selection | Comparability 5 | Exposure | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Definition 1 | Representative-ness 2 | Selection 3 | Definition 4 | Ascertainment 6 | Method 7 | Rate 8 | |||
| Huang et al (2008) | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Liu et al (2015) | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 5 |
| Ott et al (2006) | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Ott et al (2011) | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Roberto et al (2017) | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Shitara et al (2010) | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 5 |
| Zhao et al (2016) | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 5 |
| Cohort study | Selection | Comparability 5 | Outcome | Total | |||||
| Representative-ness 9 | Selection 10 | Ascertainment 6 | Demonstration 11 | Assessment 12 | Duration 13 | Adequacy 14 | |||
| Chen et al (2010) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Gao et al (2004) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Goekkurt et al (2009) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Meulendijks 1 et al 15 (2016) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Meulendijks 2 et al 16 (2016) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Ruzzo et al (2006) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
1Adequate definition of cases (0, 1); 2Consecutive or obviously representative series of cases (0, 1); 3Selection of controls: Community controls (0, 1); 4Definition of controls: No history of disease (0, 1); 5Study controls for the most important factor or any additional factor (0, 1, 2); 6Secure record (0, 1); 7Same method of ascertainment for cases and controls (0, 1); 8Same non-response rate for both groups (0, 1);9Truly or somewhat representative of the exposed cohort (0, 1); 10Selection of the non-exposed cohort (0, 1);11Demonstration that outcome of interest was not present at start of study (0, 1); 12Assessment of outcome (0, 1); 13Follow-up long enough for outcomes to occur (0, 1); 14Adequacy of follow up of cohorts (0, 1); 15Meulendijks 1 et al represents article published by this author in the journal Cancer; 16Meulendijks 2 et al represents article published by this author in the journal Pharmacogenomics.
Characteristics of studies included in the meta-analysis
| Study(reference) | Patients | Ways of Presenting Results | Ethnicity(Country) | Evaluationcriteria | Samplesize | Chemotherapeutic regimens | Outcomes |
|---|---|---|---|---|---|---|---|
| Chen et al (2010) | patients with unresectable advancedgastric carcinoma | 677T CT/TT vs CC | Mixed (TaiwanArgentina, South Korea andMexico) | NR | 65 | pemetrexed + cisplatin | OS |
| Gao et al (2004) | Patients with advanced GC | 677T TT vs CC/CT | Asian(China) | RECIST | 75 | 5-Fu et al. | RR and toxicity |
| Goekkurt et al (2009) | Patients with metastatic gastroesophageal adenocarcinoma | 677T TT vs CC/CT | European(Germany) | RECIST | 134 | fluorouracil+leucovorin+oxaliplatin or fluorouracil+leucovorin+ cisplatin | RR |
| Huang et al (2008) | Patients with GC after curative surgery | 677T CT/TT vs CC | Asian(China) | NR | 116 | 5-Fu/CF/oxaliplatin 5-Fu/CF/taxanes 5-Fc/CF/oxaliplatin/other regimens | OS |
| Liu et al (2015) | Patients with metastatic GC | 677T CT/TT vs CC | Asian(China) | RECIST | 108 | EOF: epirubicin+ oxaliplatin+ 5-Fu | OS |
| Meulendijks1 et al (2016) | Patients with advanced HER2(-) GC | 677T TT vs CC/CT | European( Netherlands ) | NR | 56 | B-DOC, bevacizumab, docetaxel, oxaliplatin, capecitabine; followed by Maintenance with Capecitabine | OS and toxicity |
| Meulendijks2 et al (2016) | Patients with advanced GC | 677T TT vs CC/CT | European( Netherlands ) | NR | 185 | B-DOC, bevacizumab, docetaxel, oxaliplatin, capecitabine;B-DOCT, bevacizumab, docetaxel, oxaliplatin, capecitabine, trastuzumab;DOC, docetaxel, oxaliplatin, capecitabine; ECC, epirubicin, cisplatin, capecitabine; | OS, RR and toxicity |
| Ott et al (2006) | Neoadjuvant chemotherapy forpatients with locally advanced GC | 677T TT vs CC CT vs CC | European(Germany) | Others | 135 | 5-Fu+cisplatin | RR and OS |
| Ott et al (2011) | Neoadjuvant chemotherapy forpatients with locally advanced GC | 677T TT vs CC CT vs CC | European(Germany) | Others | 144 | 5-Fu+leucovorin + cisplatin | OS |
| Roberto et al (2017) | adjuvant chemotherapy forpatients with early stage GC | 677T TT vs CC/CT | European(Italy) | NR | 142 | capecitabine | toxicity |
| Ruzzo et al (2006) | Patients with advanced GC | 677T TT vs CC/CT | European(Italy) | Others | 175 | fluorouracil/cisplatin | RR |
| Shitara et al (2010) | Patients with inoperable GC | 677T TT vs CC/CT | Asian(Japan) | NR | 132 | 5-Fu et al. | OS and toxicity |
| Zhao et al (2016) | stage II-III patients | 677T CT/TT vs CC TT vs CC CT vs CC | Asian(China) | NR | 251 | 5-Fu et al. | OS |
GC: gastric cancer; Others: evaluation criteria which were described in original papers; NR: not reporting; RR: response rate; OS: overall survival; Meulendijks 1 et al represents article published by this author in the journal Cancer; Meulendijks 2 et al represents article published by this author in the journal Pharmacogenomics.
Forest plot of comparison: response rate of patients with MTHFR C677T polymorphism TT vs CC+CT. Meulendijks 2 et al represents article published by this author in the journal Pharmacogenomics.
Toxicity
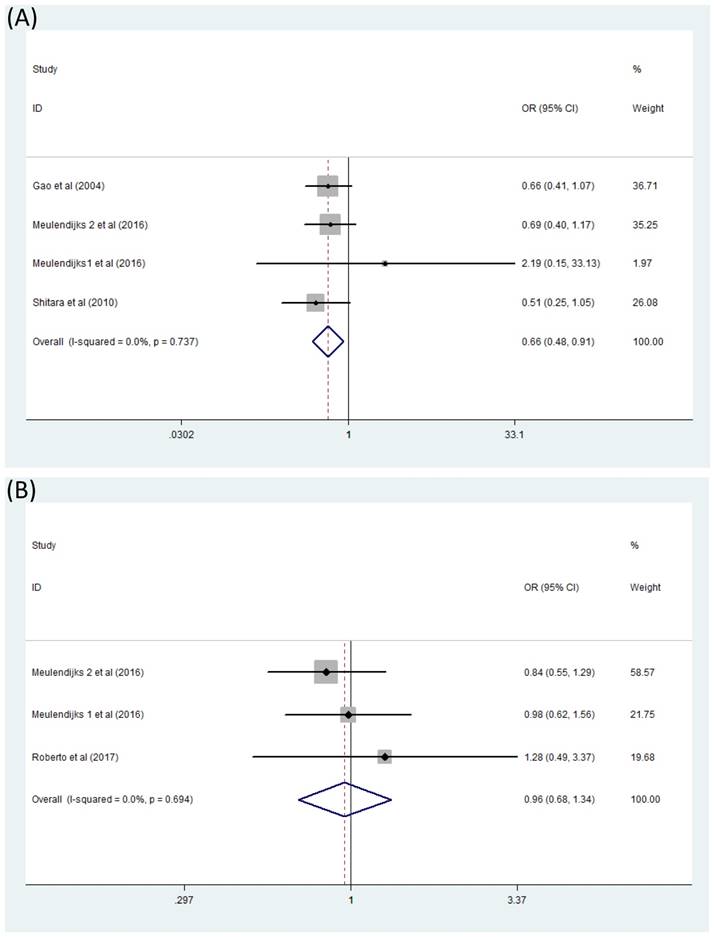
Difficulties occurred when we analyzed the association between MTHFR polymorphism C677T and toxicity of 5-Fu based chemotherapy because of very few reported literatures. Only two forest plots concerned about serious hematologic toxicity and serious global toxicity were finally generated. Here serious toxicity indicated adverse effects range from G3 to G4 and global toxicity were mainly composed of hematologic toxicity, gastrointestinal toxicity and hand-foot syndrome. Combined analysis of four studies [13,15,23,24] containing data on serious hematologic toxicity were finished by fixed effects model (I2=0) and exhibited that significant difference appeared in the comparison between patients with genotype TT and patients with CC+CT [(CC+CT)/TT OR=0.66, 95% CI: 0.48-0.91].(Figure 4A) However, this was not the same situation for serious global toxicity[15,23,25] which did not show any association with MTHFR polymorphism C677T through analyzing with fixed effects model(I2=0) [(CC+CT)/TT OR=0.96, 95% CI: 0.68-1.34] (Figure 4B).
Sensitivity and subgroup analyses
We performed sensitivity analysis by omitting one study at a time and recalculating the pooled OR and then figured out the source of the obvious heterogeneity in the combined analysis exploring the relationship of response rate and MTHFR C677T polymorphism in GC patients receiving 5-Fu based chemotherapy. The results of sensitivity analysis showed that heterogeneity came from Gao's study, but there was still no difference of significance between the response rate of patients in the two groups comparing with each other even after dropping out Gao's study [13] and applying fixed effects model [TT/(CC+CT) OR=0.87, 95% CI: 0.54-1.39].
Subgroup analyses were conducted for those studies that examine the association of OS with MTHFR C677T polymorphism in GC patients treated with 5-Fu based chemotherapy. We stratified the researches by the ways they presenting results first. In studies [18-21] directly comparing OS between GC patients with genotype CT+TT and those with genotype CC, only insignificant disadvantage of genotype CC over genotype CT+TT was discovered [(CT+TT)/CC HR=0.94, 95% CI: 0.73-1.22]. Other three studies [16,20,22] offered us results in another way: patients with genotype CC seem to own shorter OS than those with genotype TT. No significance existed as the HR of group TT versus CC was 0.85, 95% CI: [0.60, 1.20], and no significant results could be concluded when comparing OS of GC patients with genotype CT against genotype CC [CT/ CC HR=1.10, 95% CI: 0.77-1.57], either. Stratifying by patients' ethnicities, the pooled HRs of GC patients with genotype CT+TT versus those with genotype CC were 0.92 (95%CI: 0.66-1.28) for studies conducted in Asian [18-21] and 1.19 (95%CI: 0.90-1.58) in European [16,22]. With stratified analysis that was based on the chemotherapy purpose, the pooled HRs of genotype CT+TT against CC were 1.03 (95%CI: 0.76-1.38) in the subgroup 'perioperative' [16,20,21,22] and 1.04 (95%CI: 0.67-1.61) in subgroup 'palliative' [18,19]. The results of subgroup analyses are listed in Table 3.
Forest plot of comparison: OS of patients with MTHFR C677T polymorphism. (A) CT+TT vs CC. (B) TT vs CT+CC. Meulendijks 1 et al represents article published by this author in the journal Cancer; Meulendijks 2 et al represents article published by this author in the journal Pharmacogenomics.
Publication bias
No statistically significance of publication bias were detected by Egger's test in the combined analysis trying to figure out MTHFR C677T polymorphism's relationship with response rate [P=0.839] or OS [P=0.430] in GC patients receiving 5-Fu based chemotherapy.
Forest plot of comparison: Toxicity of patients with MTHFR C677T polymorphism (CC+CT vs TT). (A) serious hematologic toxicity, (B) serious global toxicity. Meulendijks 1 et al represents article published by this author in the journal Cancer; Meulendijks 2 et al represents article published by this author in the journal Pharmacogenomics.
Association between OS and MTHFR C677T polymorphism in GC patients treated with 5-Fu based chemotherapy stratified by ways of presenting results, patients' ethnicities or chemotherapy purpose.
| Subgroups | Number of Studies | Test of heterogeneity | Test of Association | ||||
|---|---|---|---|---|---|---|---|
| p | I2 | HR | 95%CI | Z | p | ||
| Ways of presenting results | |||||||
| CT/TT: CC | 4 | 0.21 | 34% | 0.92 | [0.66,1.28] | 0.49 | 0.62 |
| TT: CC | 3 | 0.78 | 0% | 0.85 | [0.60,1.20] | 0.92 | 0.36 |
| CT:CC | 3 | 0.19 | 40% | 1.10 | [0.77,1.57] | 0.51 | 0.61 |
| Ethnicities | |||||||
| Asian | 4 | 0.21 | 34% | 0.92 | [0.66,1.28] | 0.49 | 0.62 |
| European | 2 | 0.64 | 0% | 1.19 | [0.90,1.58] | 1.20 | 0.23 |
| Chemotherapy Purpose | |||||||
| Palliative | 2 | 0.38 | 0% | 1.04 | [0.67,1.61] | 0.17 | 0.86 |
| Perioperative | 4 | 0.14 | 45% | 1.03 | [0.76,1.38] | 0.17 | 0.86 |
Discussion
MTHFR is among those drug-metabolizing enzymes whose inter-individual variation may affect anticancer drug efficacy. Polymorphism MTHFR C677T is most widely investigated as they may lead to change in related enzyme activities, thus influencing 5-Fu metabolism. A series of researches have been conducted to explore the relation between this MTHFR polymorphism and the clinical outcomes of GC patients treated with 5-Fu based chemotherapies, but the results still remain controversial and uncertain. To our knowledge, though one meta-analysis with ambiguous conclusion on this question written by Wang et al [2] has already came out in 2012, quite a few new articles focusing on this issue have been published since that time and an updated systematic review may be needed to provide a more comprehensive view. Moreover, here we may also discuss what has not been investigated thoroughly due to limited resources in that article, MTHFR polymorphisms' relationship with the toxicity on GC patients who have received 5-Fu based chemotherapy, with more available data.
Well defined search strategy and strict inclusion and exclusion criteria increase the strength of this meta-analysis. In addition, we process these raw statistics as what is described in 'Methods' so that results of more universality can be generated. Performing subgroup analysis is another way to figuring out heterogeneity and promote the reliability of our review. However, some deficiencies still exist in our study. Firstly, near half of the included studies are retrospective, which may decrease our conclusion's ability to convince the causal relationship. Secondly, potential selection bias is introduced because of different studying objects inclusion criteria in included studies. For example, Roberto et al. [25] include patients with early stage GC but Goekkurt et al [14] include patients with metastatic gastroesophageal adenocarcinoma. Thirdly, although all chemotherapies investigated here are 5-Fu based chemotherapies, the regimen in each single study is quite different from one another and this factor is not taken into consideration when performing meta-analysis. Fourthly, identifying all relevant literatures is impossible for us even with great efforts. Moreover, the number of included studies in each comparison is rather small which may decrease the significance of evaluation publication bias.
Nowadays, the 5-Fu, either itself or other refined versions such as S-1, is considered as standard agent in GC chemotherapy, in combination with other agents like platinum or not.[27] Theoretically, after uptake by cancer cells, 5-Fu metabolizes to fluorodeoxyuridine monophosphate (FdUMP), thus inhibits thymidylate synthase (TS) from catalyzing deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) and finally destroys the steady state of DNA metabolism in cancer cells.[28] Here in this process, MTHFR also plays an important role as FdUMP exerts inhibition on TS through forming trimer with TS and 5,10-methylenetetrahydrofolate, which is the substrate of MTHFR.[26] What can be deduced is that decreased MTHFR activity leads to more substrate accumulation and more trimers forming, therefore confers a more effective TS inhibition and increased 5-Fu efficacy.
MTHFR C677T single-nucleotide polymorphism is found to be associated with reduced enzymatic activity and the mutant genotype TT may result in up to 70% reduction in MTHFR activity [11]. However, increasing published reports during recent years does not change the conclusion made by Wang et al [2] in 2012 significantly or indicate increased 5-Fu efficacy with decreased MTHFR activity. Our meta-analysis reveals no correlation of significance between MTHFR C677T polymorphism and response rate of GC patients accepting 5-Fu based chemotherapy, no matter before [TT/ (CC+CT) OR=1.31, 95% CI: 0.62-2.72] or after [TT/ (CC+CT) OR=0.87, 95% CI: 0.54-1.39] the sensitivity analysis and it turns out that OS of GC patients treated with 5-Fu chemotherapy has nothing to do with MTHFR C677T polymorphism [(CT+TT)/CC HR=1.05, 95% CI: 0.86-1.26; TT/(CT+CC) HR=1.48, 95% CI: 0.53-4.15]. What's more, the subgroup analysis conducted among researches exploring OS's relation with MTHFR C677T polymorphism in GC patients receiving 5-Fu based chemotherapy reveals no differences of significance in subgroups when stratified by ways of presenting results, patients' ethnicities or chemotherapy purpose.
Toxicity of certain chemotherapies is always a great concern in clinical situation, no matter for doctors or for patients. However, few researches investigating the relation between MTHFR C677T polymorphism and toxicity of 5-Fu based chemotherapy in GC patients are available in 2012 and Wang et al just find three studies [14,24,29] which are applicable for analyzing the association in between. Two of them find no results of significance[14,24]; Another one reports that the mutant genotype TT is associated with higher frequency of non-hematologic toxicity (nausea/vomiting).[29] Five years later, however, we seem to discover an intriguing result through analyzing the slowly increasing researches of this aspect: GC patients with CC or CT genotype tend to experience less severe hematologic toxicity than those with TT genotype when treated with 5-Fu based chemotherapy[(CC+CT)/TT OR=0.66, 95% CI: 0.48-0.91]. Actually, it is a phenomenon which is in accord with the theoretically deduction discussed above to some extent. The mutant genotype TT may result in up to 70% reduction in MTHFR activity thus leads to a more effective TS inhibition and increased 5-Fu efficacy. Meanwhile, from another aspect, for cytotoxic agents like 5-Fu, increased efficacy probably means increased toxicity and this is just the case for the mutant genotype TT as it seems to indicate higher frequency of severe hematologic toxicity in GC patients treated with 5-Fu based chemotherapy. Moreover, a possible deduction can be made here: The high possibility of severe hematologic toxicity brought by 5-Fu based chemotherapy in GC patients with genotype TT probably forces these patients to reduce dose or stop primary plan and switch to other ones ahead of time, thus makes genotype TT's effect of increasing 5-Fu efficacy and influencing clinical outcomes not very obvious and this is just what we see from the ambiguous results when comparing response rate and OS among GC patients with different genotypes. When it comes to serious global toxicity, no significant disadvantages of genotype TT are shown over another two genotypes [(CC+CT)/TT OR=0.96, 95% CI: 0.68-1.34].
In addition to the chemotherapy purpose, patients' races and elevated toxicity in patients with certain genotypes, those unclear results in regard to the relation between MTHFR polymorphism and prognosis of GC patients treated with 5-Fu based chemotherapy may be ascribed to two other reasons such as: (1) Multiple genes like ERCC1,GSTs rather than a single gene play certain roles in the complicated mechanisms that determines the prognosis of GC patients treated with 5-Fu based chemotherapies[2] and other involved genes are not taken into consideration in this meta-analysis.(2) Chemotherapy which usually combines several drugs rather than only one drug is delivered to patients, thus drug interaction may also change the effect of MTHFR polymorphism.
Conclusions and Implications
In conclusion, we newly found that the MTHFR C677T polymorphism TT may indicated higher probability of severe hematologic toxicity in GC patients treated with 5-Fu based chemotherapy. Meanwhile, just like previous researches, no relations between MTHFR C677T polymorphism and the efficacy of 5-Fu based chemotherapy in GC patients were revealed in this meta-analysis. Thus, we probably should pay close attention to GC patients' genotyping results of MTHFR C677T before their chemotherapy starts and patient's blood routine if a GC patient with mutant genotype TT is going to receive 5-Fu based chemotherapy. However, these results should be treated with caution because of potential bias and confounding factors. As a consequence, more better-designed high relevant large clinical trials are an urgent need in the future.
Acknowledgements
This research was supported by grants from Science and Technology Committee of Shanghai (No.15411961900).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Macdonald JS. Treatment of localized gastric cancer. Semin Oncol. 2004;31:566-73
2. Wang Z. et al. Polymorphisms in ERCC1, GSTs, TS and MTHFR predict clinical outcomes of gastric cancer patients treated with platinum/5-Fu-based chemotherapy: a systematic review. BMC Gastroenterol. 2012;12:137-50
3. Wagner AD. et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903-9
4. Lee JJ. et al. A multicenter phase II study of S-1 plus paclitaxel as first-line therapy for patients with advanced or recurrent unresectable gastric cancer. Cancer Chemother Pharmacol. 2009;63:1083-90
5. Lee JL. et al. A randomized multicenter phase II trial of capecitabine versus S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer. 2008;99:584-90
6. Sadee W. et al. Pharmacogenetics/genomics and personalized medicine. Hum Mol Genet. 2005;14:207-14
7. Yamayoshi Y. et al. Cancer pharmacogenomics: international trends. Int J Clin Oncol. 2005;10:5-13
8. Ichikawa W. Prediction of clinical outcome of fluoropyrimidine-based chemotherapy for gastric cancer patients, in terms of the 5-fluorouracil metabolic pathway. Gastric Cancer. 2006;9:145-155
9. Zhao Y. et al. MTHFR C677T polymorphism is associated with tumor response to preoperative chemoradiotherapy: a result based on previous reports. Med Sci Monit. 2015;21:3068-76
10. Joerger M. et al. Germline TYMS genotype is highly predictive in patients with metastatic gastrointestinal malignancies receiving capecitabine-based chemotherapy. Cancer Chemother Pharmacol. 2015;75:763-72
11. Ueland PM. et al. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci. 2001;22:195-201
12. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-5
13. Gao CM. et al. Polymorphism of methylenetetrahydrofolate reductase and sensitivity of stomach cancer to fluoropyrimidine-based chemotherapy. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:1054-8
14. Goekkurt E. et al. Pharmacogenetic analyses of a phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil and leucovorin plus either oxaliplatin or cisplatin: a study of the arbeitsgemeinschaft internistische onkologie. J Clin Oncol. 2009;27:2863-73
15. Meulendijks D. et al. Pharmacogenetic variants associated with outcome in patients with advanced gastric cancer treated with fluoropyrimidine and platinum-based triplet combinations: a pooled analysis of three prospective studies. Pharmacogenomics J. 2016 [Epub ahead of print]
16. Ott K. et al. The thymidylate synthase tandem repeat promoter polymorphism: a predictor for tumor-related survival in neoadjuvant treated locally advanced gastric cancer. Int J Cancer. 2006;119(12):2885-94
17. Ruzzo A. et al. Pharmacogenetic profiling and clinical outcome of patients with advanced gastric cancer treated with palliative chemotherapy. J Clin Oncol. 2006;24(12):1883-91
18. Liu R. et al. Influences of ERCC1, ERCC2, XRCC1, GSTP1, GSTT1, and MTHFR polymorphisms on clinical outcomes in gastric cancer patients treated with EOF chemotherapy. Tumour Biol. 2016;37:1753-62
19. Chen JS. et al. A phase I/II and pharmacogenomic study of pemetrexed and cisplatin in patients with unresectable, advanced gastric carcinoma. Anticancer Drugs. 2010;21:777-84
20. Zhao T. et al. The effects of genomic polymorphisms in one-carbon metabolism pathways on survival of gastric cancer patients received fluorouracil-based adjuvant therapy. Sci Rep. 2016;6:28019-30
21. Huang ZH. et al. The polymorphisms of TS and MTHFR predict survival of gastric cancer patients treated with fluorouracil-based adjuvant chemotherapy in Chinese population. Cancer Chemother Pharmacol. 2009;63:911-8
22. Ott K. et al. DNA repair gene and MTHFR gene polymorphisms as prognostic markers in locally advanced adenocarcinoma of the esophagus or stomach treated with cisplatin and 5-fluorouracil-based neoadjuvant chemotherapy. Ann Surg Oncol. 2011;18:2688-98
23. Meulendijks D. et al. Bevacizumab combined with docetaxel, oxaliplatin, and capecitabine, followed by maintenance with capecitabine and bevacizumab, as first-line treatment of patients with advanced HER2-negative gastric cancer: a multicenter phase 2 study. Cancer. 2016;122:1434-43
24. Shitara K. et al. Folate intake along with genetic polymorphisms in methylenetetrahydrofolate reductase and thymidylate synthase in patients with advanced gastric cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:1311-9
25. Roberto M. et al. Evaluation of 5-fluorouracil degradation rate and Pharmacogenetic profiling to predict toxicity following adjuvant Capecitabine. Eur J Clin Pharmacol. 2017;73:157-64
26. Blank. S, et al. A retrospective comparative exploratory study on two methylentetrahydrofolate reductase (MTHFR) polymorphisms in esophagogastric cancer: the A1298C MTHFR polymorphism is an independent prognostic factor only in neoadjuvantly treated gastric cancer patients. BMC Cancer. 2014;14:58-73
27. Wohrer SS. et al. Palliative chemotherapy for advanced gastric cancer. Ann Oncol. 2004;15:1585-95
28. Ishida Y. et al. Association of thymidylate synthase gene polymorphism with its mRNA and protein expression and with prognosis in gastric cancer. Anticancer Res. 2002;22:2805-10
29. Lu JW. et al. Relationship of methylenetetrahydrofolate reductase C677T polymorphism and chemosensitivity to 5-fluorouracil in gastric carcinoma. Chinese J Cancer. 2004;23:958-62
Author contact
![]() Corresponding author: Yuehong Cui, MD PhD, Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai, China, 180# Fenglin Road, Xuhui District, Shanghai 200032, PR China. Tel: 86-21-6404199; Fax: 86-21-6404199; E-mail: cui.yuehongsh.cn or Tianshu Liu, MD PhD, Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai, China, 180# Fenglin Road, Xuhui District, Shanghai 200032, PR China. Tel:86-21-64041990; Fax:86-21-64041990; E-mail: liutianshu1969com
Corresponding author: Yuehong Cui, MD PhD, Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai, China, 180# Fenglin Road, Xuhui District, Shanghai 200032, PR China. Tel: 86-21-6404199; Fax: 86-21-6404199; E-mail: cui.yuehongsh.cn or Tianshu Liu, MD PhD, Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai, China, 180# Fenglin Road, Xuhui District, Shanghai 200032, PR China. Tel:86-21-64041990; Fax:86-21-64041990; E-mail: liutianshu1969com

 Global reach, higher impact
Global reach, higher impact