Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(6):959-967. doi:10.7150/jca.22787 This issue Cite
Research Paper
Increased Paxillin expression in prostate cancer is associated with advanced pathological features, lymph node metastases and biochemical recurrence
1. Departments of Urology, The First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, China
2. Departments of Pathology, The First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, China
* Contributed equally
Received 2017-9-11; Accepted 2018-1-31; Published 2018-2-28
Abstract
Purpose Paxillin regulates cell-cell adhesion, and altered Paxillin expression has been associated with human carcinogenesis. This study analyzed the association between Paxillin expression in prostate cancer (PCa) tissues with clinicopathological features, lymph node metastasis and biochemical PCa recurrence.
Methods A total of 386 tissue specimens from PCa patients who received radical prostatectomy and 60 tissue specimens from benign prostatic hyperplasia (BPH) cases were collected to construct tissue microarrays, which were subsequently immunostained for Paxillin expression. Thirty positive lymph node tissue specimens and 10 healthy prostate tissue specimens were randomly selected for Paxillin immunostaining.
Results The association between Paxillin expression, lymph node metastasis and biochemical PCa recurrence was analyzed. Paxillin expression was significantly higher in PCa than both normal and BPH tissues (P<0.001) and was correlated with preoperative prostate-specific antigen level, Gleason score, clinical tumor stage, lymph node metastasis, positive surgical margin, extracapsular extension and seminal vesicle invasion (P<0.05 for all). Logistic regression analysis showed that Paxillin and Gleason score were independent risk factors for PCa lymph node metastasis (P<0.05). The receiver operating characteristic (ROC) curve indicated that Paxillin expression (AUC=0.723) more accurately predicted PCa lymph node metastasis than Gleason score (AUC=0.692). Kaplan-Meier curve analysis showed that increased Paxillin expression was associated with shortened biochemical-free survival (BFS) after radical prostatectomy (P<0.001).
Conclusion Paxillin was significantly upregulated in PCa compared with BPH and normal tissues and associated with lymph node metastasis and shortened BFS of PCa. Further study will investigate the underlying molecular mechanism and the role of Paxillin in PCa.
Keywords: paxillin, prostate cancer, lymph node metastasis, pathological feature, biochemical-free survival, prediction
Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed malignancies in men and is ranked the second most diagnosed male malignancy worldwide.[1] Although the majority of PCas progress slowly, it resulted in more than a quarter million cancer-related deaths globally in 2008[2]. The risk factors for PCa include older age, African ethnicity and a family history of the disease. Indeed, approximately 62% of all PCa cases in the United States are diagnosed in men aged 65 years and older.[2] Prostate cancer treatment includes surgery, radiation therapy, hormone therapy, chemotherapy and targeted therapy; treatment selection is dependent on staging and identifying histological subtypes.[3] Radical prostatectomy is one of the most effective PCa therapies; however, whether pelvic lymph node dissection (PLND) confers clinical advantage is contentious,[4] as most studies have indicated that PLND is only necessary for moderate- and high-risk PCa patients.[5-7] Like most other cancers, PCa lymph node metastasis can lead to mortality; thus, the development of biomarkers to predict tumor metastasis prior to surgery will aid oncologists in making optimal clinical decisions and treatment plans.[8, 9]
Tumor metastasis is a complex process in which cancer cells become capable of leaving the primary tumor site via the lymphatic system and/or the bloodstream and then establish a secondary tumor site in other parts of the body.[10] Paxillin is a scaffold protein composed of five N-terminal LD motifs, four C-terminal LIM domains and several SH3 domains, which allow Paxillin to form a large number of protein-protein interactions.[11] Paxillin regulates cell-cell adhesion by binding to a variety of adhesion molecules, such as integrins and actin to induce tumor cytoskeletal remodeling that leads to adhesion and migration.[12] In PCa, a previous study demonstrated that Paxillin could promote PCa cell migration and invasion, while another study showed that Paxillin was an important factor in mediating PCa bone metastasis.[13, 14]
Thus, this study sought to detect Paxillin expression in PCa versus normal and benign prostatic hyperplasia (BPH) tissue specimens and associate Paxillin expression with clinicopathological characteristics, lymph node metastasis and biochemical recurrence in PCa patients.
Materials and methods
Tissue specimen and data collection
The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Fujian Medical University (reference number: 2013085). All patients provided written informed consent. We collected 386 tissue specimens from PCa patients between January 2008 and January 2013 from the First Affiliated Hospital of Fujian Medical University. The patients were diagnosed with PCa according to EAU guidelines[3] and underwent radical prostatectomy plus PLND without any preoperative adjuvant endocrine therapy or radiotherapy (335 patients received standard PLND with a resection range including obturator formamen and external iliac vein peripheral lymph nodes, while 51 patients received extended PLND with peripheral lymph node fat tissue having an upper bound to the common iliac artery bifurcation, a lower bound to the femoral canal, both sides to the pelvic wall and a back bound to the obturator nerves, blood vessels and anterior internal vein).[15] We also collected 60 random BPH tissue specimens and 10 cases of healthy prostate tissue specimens from our hospital as controls. Among the samples, 30 positive lymph node cases were randomly selected for Paxillin immunohistochemical staining. Clinical data, including age, prostate-specific antigen (PSA), prostate volume, PSA density (PSAD), body mass index (BMI), clinical tumor (cT) stage, numbers of lymph node metastasis, positive surgical margin, extracapsular extension and seminal vesicle invasion were collected (Table 1).
Construction of tissue microarray
Paraffin blocks from both PCa and BPH were retrieved from the Pathology Department and sectioned for hematoxylin and eosin (H&E) staining (4-µM-thick tissue sections) to confirm diagnosis and identify the representative areas for tissue microarray construction. A tissue microarray maker we designed was used to generate tissue microarrays using two 2 × 2 mm tissue cores of each case. In the end, we obtained tissue microarrays with 5 × 10 tissue cores of each for both PCa and BPH tissues and then sectioned 4-µm-thick tissue sections for Paxillin immunohistochemical staining. [16, 17]
Immunohistochemistry
Sections were de-waxed, rehydrated, and then antigen retrieval was performed using 0.1 M citric acid buffer (pH 5.0; Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China) in a high-pressure cooker. Following antigen retrieval, tissue microarrays were incubated with 3% H2O2 for 10 min to block endogenous peroxidase activity and subsequently with 20% normal goat serum at room temperature for 30 min. Next, the tissue microarray sections were incubated with a rabbit monoclonal anti-Paxillin antibody (Abcam, Cambridge, UK) at a dilution of 1:800 at 4°C overnight. The following day, the sections were briefly washed with phosphate buffered saline (PBS) three times and further incubated with secondary antibody (DAKO, Santa Clara, CA, USA) at 37°C for 30 min and subsequently with ChemMate™ EnVision™ Detection Kit (DAKO). The sections were subjected to color reaction with 3,3´-diaminobenzidine (DAB) solution, briefly counterstained with hematoxylin and mounted with a coverslip. Confirmed positive sections were used as positive controls, and PBS in place of primary antibody was used as a negative control. The immunostained tissue microarray sections were reviewed and scored under a light microscope (Olympus, Tokyo, Japan) by two blinded pathologists. Any discrepancies were resolved by re-reviewing the sections. Two semi-quantitative methods were conducted, and the total Paxillin immunostaining score included staining intensity and the proportion of positive cells. Staining intensity (I) was recorded as 0, absent; 1, weak; 2, moderate; 3, strong, while the proportion (P) of positive cells was recorded as 0, <5%); 1, 5%-25%; 2, 26%-50%; 3, 51%-75% and 4, >75%. A score for each histological grade (H-score) was determined as: H-score = Ʃ (I × P), as described previously. [18, 19] The final Paxillin expression score (E-score) was calculated using the value of the percentage positivity score multiplied by the staining intensity score, which ranged from 0 to 9. Paxillin expression level was defined as "-" (scores 0-1), "+" (scores 2-3), "++" (scores 4-5) and "+++" (scores ≥6).[20]
Statistical methods
SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Quantitative data were compared using independent samples t test, Mann-Whitney U test, Kruskal-Wallis test or ANOVA. Qualitative data were compared using independent sample chi-square test or Fisher exact test. Factors influencing lymph node metastasis predictors were analyzed using Logistic regression analysis. Receiver operating characteristic (ROC) curve was used to evaluate the predictive value of each indicator for PCa lymph node metastasis. Kaplan-Meier plots and the log-rank test were performed to assess the association of Paxillin expression with biochemical recurrence-free survival (BFS). P<0.05 was considered statistically significant.
Results
Differential Paxillin expression in PCa vs. normal and BPH tissue specimens
Clinical data of PCa and BPH patients were collected. The two groups were comparable with respect to age, BMI and prostate volume while PCa patients had significantly higher PSA level (Table 1). Immunohistochemical data showed that Paxillin protein was mainly expressed in the cytoplasm of positive cells, and Paxillin expression was significantly higher in PCa than normal and BPH tissues (Figure 1, Table 1). Normal prostate tissues were collected from patients aged 18 to 40 years who underwent radical cystectomy. Patients with prostatitis, BPH or PCa were excluded. Compared with Grade Group 1,[21] paxillin expression was enhanced in Grade Group 2 and Grade Group 3 (P<0.05). Compared with Grade Group 3, paxillin expression was enhanced in Grade Group 4 and Grade Group 5; however, there was no significant difference between paxillin expression in Grade Groups 3 and 2. There also was no significant difference between Grade Groups 4 and 5 with respect to the paxillin expression. Paxillin expression was also significantly higher in LN(+) PCa (Paxillin staining in primary prostate tissues from patients with lymph nodes metastasis) than in LN(-) PCa (Paxillin staining in primary prostate tissues from patients who did not show lymph node metastasis). Paxillin expression was also significantly higher in paired positive LN tissue than in paired PCa tissue (P<0.05 for all; Table 2 and Figure 2). However, there was no significant difference of Paxillin expression between normal and BPH tissue specimens (P>0.05) Table 2.
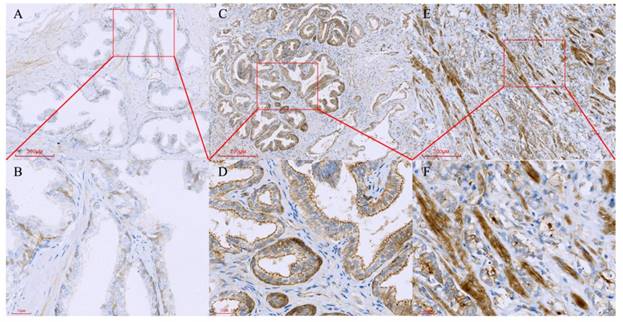
Tissue microarray containing normal prostate, benign prostate hyperplasia and prostate cancer tissues were immunostained with a monoclonal anti-Paxillin antibody. (A, B) Normal prostate, B is a high magnification of A; (C, D) BPH, D is a high magnification of C; (E, F) Prostate cancer, F is a high magnification of E.
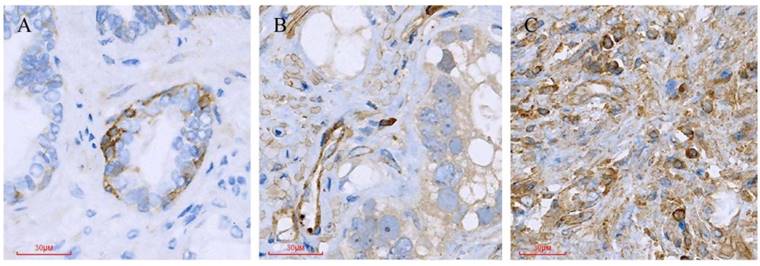
Different Paxillin protein expression levels in prostate cancer tissues. (A) Gleason pattern 2: + (1×1=1); (B) Gleason pattern 3: ++ (2×2=4); (C) Gleason pattern 5: +++ (3×3=9).
Clinicopathological Parameters of PCa and BPH patients
| Variables | PCa | BPH | P value |
|---|---|---|---|
| Number of cases | 386 | 60 | |
| Age(years) | |||
| Mean ± SD | 68.52±7.11 | 68.08±6.65 | 0.437 |
| Range | 46-79 | 47-82 | |
| BMI (kg/m2) | |||
| Mean ± SD | 25.77±4.42 | 26.30±3.99 | 0.352 |
| Range | 17.02-33.25 | 17.06-34.79 | |
| Prostate volume(ml) | |||
| Mean ± SD | 40.45±8.47 | 42.70±10.14 | 0.596 |
| Range | 20-59 | 35-100 | |
| PSA level(ng/ml) | |||
| Mean ± SD | 19.50±13.53 | 4.83±3.016 | <0.001 |
| Range | 2.03-62.27 | 0.54-15.05 | |
| Paxillin expression,n(%) | <0.001 | ||
| - | 89 | 58 | |
| + | 94 | 2 | |
| ++ | 102 | 0 | |
| +++ | 101 | 0 | |
| PSAD(ng/ml·cm3) | |||
| Mean ± SD | 0.52±0.44 | ||
| Range | 0.06-2.22 | ||
| Percentage of positive biopsiesa [n (%)] | |||
| <50% | 236(61.1) | ||
| ≥50% | 150(38.9) | ||
| cT stage [n (%)] | |||
| T1 | 60(15.5) | ||
| T2 | 259(67.1) | ||
| T3 | 67(17.4) | ||
| Gleason Score[n (%)] | |||
| <7 | 99(25.6) | ||
| 7 | 189(49.0) | ||
| >7 | 98(25.4) | ||
| Lymph node metastasis[n (%)] | |||
| Yes | 129(33.4) | ||
| No | 257(66.6) | ||
| Extracapsular extension[n (%)] | |||
| Yes | 67(17.4) | ||
| No | 319(82.6) | ||
| Seminal vesicle invasion[n (%)] | |||
| Yes | 21(5.4) | ||
| No | 365(94.6) | ||
| Positive surgical margin [n (%)] | |||
| Yes | 45(11.7) | ||
| No | 341(88.3) |
Differential Paxillin protein expression in paired positive lymph node tissue vs. paired PCa tissue specimens
Next, 30 cases of paired positive lymph node tissue specimens and paired PCa tissue specimens were randomly selected for Paxillin immunohistochemical staining. The data showed that Paxillin expression was significantly higher in paired positive lymph node tissue than paired PCa tissue specimens (Table 2).
Expression of Paxillin in Normal, BPH and Prostate Cancer tissue
| Specimen | n | H-score |
|---|---|---|
| Normal | 10 | 47.0±26.7 |
| BPH | 60 | 48.3±27.5 a |
| Grade Group+ 1 (GS≤6) | 99 | 82.9±41.3 b |
| Grade Group 2 (GS 3+4=7) | 109 | 145.5±52.7c |
| Grade Group 3 (GS 4+3=7) | 80 | 146.1±42.8d,e |
| Grade Group 4 (GS 4+4=8; 3+5=8; 5+3=8) | 61 | 162.1±44.2f |
| Grade Group 5 (GS 9-10) | 37 | 165.4±37.8g,h |
| LN(-) PCa # | 257 | 130.6±56.5 |
| LN(+) PCa * | 129 | 143.1±50.3i |
| Paired PCa Tissue | 30 | 140.7±44.9 |
| Paired positive LN Tissue | 30 | 164.0±42.9j |
Notes: The definition of H-score was defined as a score for each histological grade and was determined as: H-score = Ʃ (I × P), as described previously. Staining intensity (I) was recorded as 0, absent; 1, weak; 2, moderate; 3, strong, while the proportion of positive cells (P) was recorded as 0, <5%; 1, 5%-25%; 2, 26%-50%; 3, 51%-75% and 4, >75%[18, 19]. a Compared with normal prostate, P=0.890. b Compared with normal prostate, P=0.008. c Compared with Grade 1, P<0.001. d Compared with Grade Group1, P<0.001. e Compared with Grade Group 2, P=0.925, f compared with Grade Group 3, P=0.039. g Compared with Grade Group 3, P=0.032. h Compared with Grade Group 4, P=0.723. i Compared with LN(-) PCa, P=0.034. j Compared with Paired PCa Tissue, P=0.045. # Paxillin staining in primary prostate tissues from patients who did not show lymph node metastases. * Paxillin staining in primary prostate tissues from patients who had lymph node metastases. +The historical definition was applied to the New Grading System.[21]
Paxillin expression is associated with clinicopathological data
We then looked for associations between Paxillin protein expression and clinicopathological data of PCa patients. Higher Paxillin expression was associated with higher PSA levels, Gleason score, clinical tumor stage, lymph node metastasis, positive surgical margin, extracapsular extension and seminal vesicle invasion (P<0.05; Table 3), whereas Paxillin expression was not associated with patient age (P> 0.05; Table 3). Paxillin expression was more commonly observed in Gleason score >7 PCa tissue specimens.
Association of Paxillin Expression with Clinicopathological Features of Prostate Cancer.
| Variables | N | Paxillin expression | P-value | |||
|---|---|---|---|---|---|---|
| - | + | ++ | +++ | |||
| Total, n (%) | 386 | 89 | 94 | 102 | 101 | |
| Age (yrs) | ||||||
| <70 | 189(49.0) | 41(46.1) | 50(53.2) | 49(48.0) | 49(48.5) | 0.960 |
| ≧70 | 197(51.0) | 48(53.9) | 44(46.8) | 53(52.0) | 52(51.5) | |
| PSA Level (ng/ml) | ||||||
| <10 | 62(16.1) | 17(19.1) | 25(26.6) | 11(10.8) | 9(8.9) | 0.014 |
| 10-20 | 252(65.3) | 52(58.4) | 56(59.6) | 72(70.6) | 72(71.3) | |
| >20 | 72(18.7) | 20(22.5) | 13(13.8) | 19(18.6) | 20(19.8) | |
| Gleason Score | ||||||
| <7 | 99(25.6) | 48(53.9) | 32(34.0) | 15(14.7) | 4(4.0) | <0.001 |
| 7 | 189(49.0) | 26(29.2) | 43(45.7) | 52(51.0) | 68(67.3) | |
| >7 | 98(25.4) | 15(16.9) | 19(20.2) | 35(34.3) | 29(28.7) | |
| cT stage | ||||||
| T1 | 60(15.5) | 38(42.7) | 11(11.7) | 6(5.9) | 5(5.0) | <0.001 |
| T2 | 259(67.1) | 41(46.1) | 78(83.0) | 80(78.4) | 60(59.4) | |
| T3 | 67(17.4) | 10(11.2) | 5(5.3) | 16(15.7) | 36(35.6) | |
| Lymph node metastasis | ||||||
| Yes | 129(33.4) | 7(7.9) | 24(25.5) | 41(40.2) | 57(56.4) | <0.001 |
| No | 257(66.6) | 82(92.1) | 70(74.5) | 61(59.8) | 44(43.6) | |
| Extracapsular extension | ||||||
| Yes | 67(17.4) | 10(11.2) | 5(5.3) | 16(15.7) | 36(35.6) | <0.001 |
| No | 319(82.6) | 79(88.6) | 89(94.7) | 86(84.3) | 65(64.4) | |
| Seminal vesicle invasion | ||||||
| Yes | 21(5.4) | 0(0.0) | 3(3.2) | 10(9.8) | 8(7.9) | 0.004 |
| No | 365(94.6) | 89(100.0) | 91(96.8) | 92(90.2) | 93(92.1) | |
| Positive surgical margin | ||||||
| Yes | 45(11.7) | 9(10.1) | 4(4.3) | 11(10.8) | 21(20.8) | 0.006 |
| No | 341(88.3) | 80(89.9) | 90(95.7) | 91(89.2) | 80(79.2) | |
Association of clinicopathological factors and Paxillin expression with pelvic lymph node metastasis in PCa
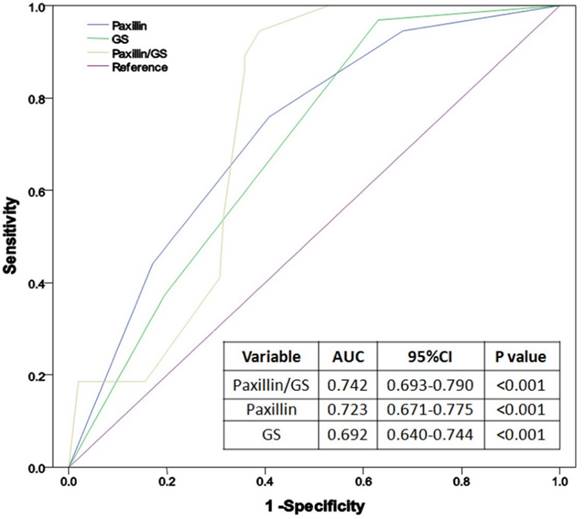
We then performed subgroup analysis to associate clinicopathological factors and Paxillin expression with pelvic lymph node metastasis in PCa. The results showed that PCa metastasis to pelvic lymph nodes was associated with Paxillin expression, higher PSA level, Gleason score, clinical tumor stage, positive surgery margin, extracapsular extension and seminal vesicle invasion (all P<0.05; Table 4), but not with age, patient BMI, prostate volume, PSAD or the percentage of positive prostate needle biopsies (Table 4). Multivariate logistic regression analysis showed that Gleason score and Paxillin expression were independent risk factors for PCa lymph node metastasis (P< 0.001, Table 5). ROC curve analysis showed that the area under the curve (AUC) of Paxillin expression (AUC=0.723) was much higher than Gleason score (AUC=0.692), while their combination could further enhance the accuracy in predicting PCa lymph node metastasis (AUC=0.742, Figure 3).
Association of Clinicopathological Features with Lymph Node Metastasis of Prostate Cancer.
| Variables | N | Lymph node metastasis | p value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Total, n (%) | 386 | 257(66.6) | 129(33.4) | |
| Age (yrs.) | ||||
| <70 | 189(49.0) | 124(48.2) | 65(50.4) | 0.692 |
| ≥70 | 197(51.0) | 133(51.8) | 64(49.6) | |
| BMI | ||||
| <25 | 196(50.8) | 134(52.1) | 62(48.1) | 0.450 |
| ≥25 | 190(49.2) | 123(47.9) | 67(51.9) | |
| Prostate volume(cm3) | ||||
| ≤35 | 124(32.1) | 81(31.5) | 43(33.3) | 0.719 |
| >35 | 262(67.9) | 176(68.5) | 86(66.7) | |
| Percentage of positive biopsies | ||||
| <50% | 236(61.1) | 158(61.5) | 78(60.5) | 0.847 |
| ≥50% | 150(38.9) | 99(38.5) | 51(39.5) | |
| PSA Level (ng/ml) | ||||
| <10 | 62(16.1) | 51(19.8) | 11(8.5) | 0.001 |
| 10-20 | 252(65.3) | 169(65.8) | 83(64.3) | |
| >20 | 72(18.7) | 37(14.4) | 35(27.1) | |
| PSAD (ng/ml·cm3) | ||||
| <0.15 | 24(6.2) | 18(7.0) | 6(4.7) | 0.367 |
| ≥0.15 | 362(93.8) | 239(93.0) | 123(95.3) | |
| Gleason Score | ||||
| <7 | 99(25.6) | 95(37.0) | 4(3.1) | <0.001 |
| 7 | 189(49.0) | 112(43.6) | 77(59.7) | |
| >7 | 98(25.4) | 50(19.5) | 48(37.2) | |
| cT stage | ||||
| T1 | 60(15.5) | 54(21.0) | 6(4.7) | <0.001 |
| T2 | 259(67.1) | 169(65.8) | 90(69.8) | |
| T3 | 67(17.4) | 34(13.2) | 33(25.6) | |
| Extracapsular extension | ||||
| Yes | 67(17.4) | 34(13.2) | 33(25.6) | <0.001 |
| No | 319(82.6) | 223(86.8) | 96(74.4) | |
| Seminal vesicle invasion | ||||
| Yes | 21(5.4) | 9(3.5) | 12(9.3) | 0.018 |
| No | 365(94.6) | 248(96.5) | 117(90.7) | |
| Positive surgical margin | ||||
| Yes | 45(11.7) | 20(7.8) | 25(19.4) | 0.001 |
| No | 341(88.3) | 237(92.2) | 104(80.6) | |
| Paxillin expression | ||||
| - | 89(23.1) | 82(31.9) | 7(5.4) | <0.001 |
| + | 94(24.4) | 70(27.2) | 24(18.6) | |
| ++ | 102(26.4) | 61(23.7) | 41(31.8) | |
| +++ | 101(26.2) | 44(17.1) | 57(44.2) | |
Multivariable Analysis of Clinicopathological Features for Association with Lymph Node Metastasis of Prostate Cancer.
| Variables | OR(95% CI) | P value |
|---|---|---|
| Multivariable analysis | ||
| PSA Level(<10 vs.10-20 vs. >20) | 1.629(0.932-2.848) | 0.087 |
| Gleason Score(<7 vs. 7 vs. >7) | 2.255(1.449-3.508) | <0.001 |
| cT stage (T1 vs. T2 vs. T3) | 1.062(0.359-3.139) | 0.913 |
| Extracapsular extension | 0.596(0.157-2.259) | 0.447 |
| Seminal vesicle invasion | 1.008(0.271-3.752) | 0.990 |
| Positive surgical margin | 1.302(0.472-3.597) | 0.610 |
| Paxillin (- / + / ++ / +++) | 2.108(1.638-2.713) | <0.001 |
Receiver operating characteristic (ROC) curves of Paxillin, Gleason score and their combination in the diagnosis of prostate cancer lymph node metastasis.
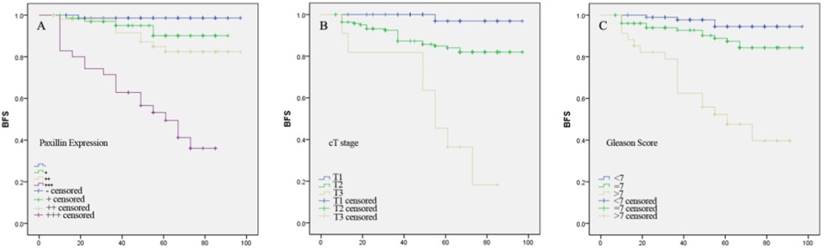
Kaplan-Meier curve analyses of biochemical recurrence-free survival of prostate cancer patients stratified by (A) Paxillin expression, (B) tumor cT stage and (C) Gleason score.
Association between clinicopathological factors and Paxillin expression with PCa biochemical recurrence
Univariate Cox proportional hazards analysis showed that higher pre-operative PSA level, higher Gleason score, advanced cT stage, extracapsular extension, seminal vesicle invasion, positive surgical margin and higher Paxillin expression were associated with PCa biochemical recurrence after radical prostatectomy. Multivariate Cox proportional hazards analysis further revealed that higher Gleason score, advanced cT stage and higher Paxillin expression were all independent predictors for PCa biochemical recurrence after radical prostatectomy (P<0.05; Table 6).
Kaplan-Meier curve analysis showed that higher Paxillin expression, advanced cT stage and higher Gleason score were associated with shortened biochemical recurrence-free survival patients after radical prostatectomy (P<0.001 for all; Figure 4).
Univariate and multivariate Cox proportional hazards analyses of prostate cancer biochemical recurrence-free survival.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (yrs.; < 70 vs. ≥ 70) | 1.268 (0.639-2.514) | NS | ||
| BMI (≤ 25 vs. > 25) | 1.702 (0.865-3.352) | NS | ||
| Prostate Volume (≤ 35 vs. > 35) | 0.876 (0.433-1.771) | NS | ||
| Percentage of positive biopsies (< 50% vs. ≥ 50%) | 0.485 (0.226-1.039) | NS | ||
| PSA level (< 10 vs. ≥ 10) | 2.117 (1.137-3.943) | 0.018 | 0.799 (0.212-3.006) | NS |
| PSAD (< 0.15 vs. ≥ 0.15) | 2.540 (0.347-18.575) | NS | ||
| Gleason score (<7 vs. 7 vs. >7) | 4.220 (2.518-0.073) | <0.001 | 2.484 (1.253-4.925) | 0.009 |
| cT stage (T1 vs. T2 vs. T3) | 5.512 (2.814-10.794) | <0.001 | 4.324 (1.210-15.451) | 0.024 |
| Extracapsular extension | 3.402 (1.315-8.803) | 0.012 | 0.156 (0.021-1.145) | NS |
| Seminal vesicle invasion | 5.577 (1.333-23.330) | 0.019 | 9.050 (0.689-118.873) | NS |
| Positive surgical margin | 5.701 (2.005-16.205) | 0.001 | 1.183 (0.151-9.262) | NS |
| Paxillin (- / + / ++ / +++) | 3.430 (2.264-5.195) | <0.001 | 3.322 (1.987-5.551) | <0.001 |
NS, not significant.
Discussion
Prostate cancer is the most common malignancy in men and a major cause of cancer-associated death.[1] In some moderate/high-risk cases, there is no disease progression for a long period of time, whereas lymph node and bone metastasis may occur in certain low-risk cases at early stages.[7, 22] To date, the application of PLND to all PCa patients remains controversial, and previous studies have suggested that PLND may only help clinicians accurately evaluate tumor staging and improve patient prognosis to a certain extent.[23, 24] However, other studies have concluded that PLND does not affect the prognosis of PCa patients with postoperatively confirmed pTxN0 stages, even if the patients had a relatively high PSA level, pathological grade, or capsule invasion before surgery.[25, 26] Moreover, PLND may also result in complications, such as deep vein thrombosis of the lower limbs, lymphedema and pelvic infection, in particular in patients treated with extensive PLND.[27] Thus, some surgeons suggest that low-risk PCa does not require PLND and that standard or extended PLND should only be performed in moderate- and high-risk cases.[28, 29] In our study, 335 patients received standard PLND, with a resection range including obturator formamen and external iliac vein peripheral lymph nodes, while 51 patients received extended PLND with peripheral lymph node fat tissue having an upper bound to the common iliac artery bifurcation, a lower bound to the femoral canal, both sides to the pelvic wall and a back bound to the obturator nerves, blood vessels and anterior internal vein.[15] Currently, there is a lack of a standardized protocol or specific tumor markers to evaluate or predict the risk of PCa metastasis to pelvic lymph nodes.
Paxillin is a multifunctional and multi-domain focal adhesion adapter protein that serves an important scaffolding role at focal adhesions, recruiting structural and signaling molecules that are involved in cell motility and migration. As a major participant in the regulation of cell movement, Paxillin plays distinct roles in cancer development and metastasis. [30] Sen et al.[13] demonstrated that Paxillin promoted PCa cell migration and invasion, while Bokobza et al. showed that Paxillin was an important factor that mediates PCa bone metastasis. [14]
Our study evaluated Paxillin expression in 386 PCa, 60 BPH and 10 normal prostate tissue specimens for associations between lymph node metastasis and other clinicopathological data. Paxillin was highly expressed in PCa tissues compared with BPH and normal prostate tissues. Paxillin expression was associated with higher PSA levels, Gleason score, tumor staging, lymph node metastasis, positive surgical margin, extracapsular extension and seminal vesicle invasion. We also found that Paxillin expression was significantly higher in LN(+) PCa than LN(-) PCa, which indicated that Paxillin may play an important role in PCa lymph node metastasis.
Increased cell proliferation and motility could promote tumor growth and distant metastasis. Cell migration includes actin phosphorylation and cytoskeletal remodeling.[31, 32] Paxillin is a focal adhesion protein that mediates interactions between integrins and extracellular matrix and can also bind to a variety of cell-cell adhesion molecules, such as integrin and actin, to induce cytoskeleton remodeling; thus, increased Paxillin expression could result in increased cell adhesion and migration.[33] Mackinnon et al.[11] showed that Paxillin overexpression occurs during the earliest stages of lung cancer development, and that the most common Paxillin mutation (A127T) showed increased proliferation and invasive tumor growth, suggesting an important role for Paxillin in lung cancer development. In PCa, Paxillin expression promoted tumor cell migration and invasion in vitro and associated with PCa bone metastasis ex vivo.[13, 14] Our current data further confirmed these studies.
The complete molecular mechanisms underlying Paxillin, a focal-adhesion-associated tyrosine-phosphorylated protein, activity have remained elusive. In 1998, Turner et al.[34] demonstrated that Paxillin becomes tyrosine phosphorylated in response to multiple stimuli, including cell adhesion. However, it was shown that Low-molecular-weight protein tyrosine phosphatase (LMWPTP) overexpression, which correlates with earlier PCa recurrence and reduced patient survival, results in reduced Paxillin phosphorylation in PCa cells.[35] Jackson et al. claimed that focal adhesion dynamics were influenced by the serine and tyrosine phosphorylation state of Paxillin; stating effects on motility and adherence were accompanied by increased serine and decreased tyrosine phosphorylation.[36] In conclusion, the role of Paxillin phosphorylation and the role of total Paxillin protein levels both need to be further studied.
Clinically, PCa mainly metastasizes to the pelvic lymph nodes, contributing to an unfavorable prognosis for PCa patients.[37] Previous studies have shown that Paxillin expression was associated with lymph node metastasis in lung and colon cancer.[11, 38] In this study, we demonstrated that Paxillin expression and other clinicopathological factors, such as raised PSA level, Gleason score, tumor grade, positive surgery margin, extracapsular extension and seminal vesicle invasion, were associated with PCa metastasis to the pelvic lymph nodes. Thus, we speculate that PCa metastasis is a multi-factoral-induced phenomenon, and further studies are needed to elucidate the underlying molecular mechanisms that can be targeted to effectively control disease progression and provide a means to evaluate and predict PCa metastasis.
This study had some limitations. For example, we could have analyzed the number of tumor-positive lymph nodes versus total numbers of lymph nodes in this study, which may have led to a more accurate determination of the association between Paxillin expression and lymph node metastasis. We did not carry this out, as the surgeons who performed PLND might not have had consistent techniques, leading to inconsistencies in the extent of lymph node dissection. Moreover, some patients received a standard PLND, while others received extended PLND. In addition, this study was retrospective and not well controlled. In conclusion, our data demonstrated that upregulated Paxillin expression was associated with PCa malignant behaviors, lymph node metastasis and shortened biochemical-free survival.
Acknowledgements
This study was supported by Natural Science Foundation of Fujian Province, China (Grant No. 2015J01393 and 2017J01197) and the Funds for Creative Program of Health and Family Planning Commission of Fujian Province, China (Grant No. 2016-CX-27).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the First Affiliated Hospital of Fujian Medical University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Attard G, Parker C, Eeles RA, Schröder F, Tomlins SA, Tannock I. et al. Prostate cancer. Lancet. 2015;387:70-82
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90
3. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T. et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124-37
4. Bogdanovic J, Sekulic V. Re: Firas Abdollah, Giorgio Gandaglia, Nazareno Suardi, et al. More Extensive Pelvic Lymph Node Dissection Improves Survival in Patients with Node-positive Prostate Cancer. Eur Urol. 2015;67:212-9
5. Thurairaja R, Studer UE, Burkhard FC. Indications, extent, and benefits of pelvic lymph node dissection for patients with bladder and prostate cancer. Oncologist. 2009;14:40-51
6. Ordon M, Nam RK. Lymph node assessment and lymphadenectomy in prostate cancer. J Surg Oncol. 2009;99:215-24
7. Mitsuzuka K, Koie T, Narita S, Kaiho Y, Yoneyama T, Kawamura S. et al. Is pelvic lymph node dissection required at radical prostatectomy for low-risk prostate cancer? Int J Urol. 2013;20:1092-6
8. Denkçeken T, Canpolat M, Baykara M, Başsorgun İ, Aktaşsamur A. Diagnosis of pelvic lymph node metastasis in prostate cancer using single optical fiber probe. Int J Biol Macromol. 2015;90:63-7
9. Briganti A, Abdollah F, Nini A, Suardi N, Gallina A, Capitanio U. et al. Performance characteristics of computed tomography in detecting lymph node metastases in contemporary patients with prostate cancer treated with extended pelvic lymph node dissection. Eur Urol. 2012;61:1132-8
10. Klein CA. Cancer. The metastasis cascade. Science. 2008;321:1785-7
11. Mackinnon AC, Tretiakova M, Henderson L, Mehta RG, Yan BC, Joseph L. et al. Paxillin expression and amplification in early lung lesions of high-risk patients, lung adenocarcinoma and metastatic disease. J Clin Pathol. 2011;64:16-24
12. Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE. et al. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901-15
13. Sen A, De Castro I, Defranco DB, Deng FM, Melamed J, Kapur P. et al. Paxillin mediates extranuclear and intranuclear signaling in prostate cancer proliferation. J Clin Invest. 2012;122:2469-81
14. Bokobza SM, Ye L, Kynaston HG, Jiang WG. Growth and differentiation factor-9 promotes adhesive and motile capacity of prostate cancer cells by up-regulating FAK and Paxillin via Smad dependent pathway. Oncol Rep. 2010;24:1653-9
15. Heidenreich A, Ohlmann CH, Polyakov S. Anatomical extent of pelvic lymphadenectomy in patients undergoing radical prostatectomy. Eur Urol. 2007;52:29-37
16. Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med. 2005;103:89-101
17. Richani K, Romero R, Kim YM, Cushenberry E, Soto E, Han YM. et al. Tissue microarray: an effective high-throughput method to study the placenta for clinical and research purposes. J Matern Fetal Neonatal Med. 2006;19:509-15
18. Sakamoto S, McCann RO, Dhir R, Kyprianou N. Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res. 2010;70:1885-95
19. Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT. et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419-25
20. Ma HQ, Liang XT, Zhao JJ, Wang H, Sun JC, Chen YB. et al. Decreased expression of Neurensin-2 correlates with poor prognosis in hepatocellular carcinoma. World J Gastroenterol. 2009;15:4844-8
21. Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol. 2016;40:244-52
22. Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G. et al. Aggressive variants of castration-resistant prostate cancer. Clinical Cancer Research An Official Journal of the American Association for Cancer Research. 2014;20:2846-50
23. Abdollah F, Gandaglia G, Suardi N, Capitanio U, Salonia A, Nini A. et al. More extensive pelvic lymph node dissection improves survival in patients with node-positive prostate cancer. Eur Urol. 2015;67:212-9
24. Moschini M, Briganti A, Murphy CR, Bianchi M, Gandaglia G, Montorsi F. et al. Outcomes for Patients with Clinical Lymphadenopathy Treated with Radical Prostatectomy. Eur Urol. 2016;69:193-6
25. DiMarco DS, Zincke H, Sebo TJ, Slezak J, Bergstralh EJ, Blute ML. The extent of lymphadenectomy for pTXNO prostate cancer does not affect prostate cancer outcome in the prostate specific antigen era. J Urol. 2005;173:1121-5
26. Murphy AM, Berkman DS, Desai M, Benson MC, Mckiernan JM, Badani KK. The number of negative pelvic lymph nodes removed does not affect the risk of biochemical failure after radical prostatectomy. Bju International. 2010;105:176-9
27. Suardi N, Larcher A, Haese A, Ficarra V, Govorov A, Buffi NM. et al. Indication for and extension of pelvic lymph node dissection during robot-assisted radical prostatectomy: an analysis of five European institutions. Eur Urol. 2014;66:635-43
28. Schiavina R, Manferrari F, Garofalo M, Bertaccini A, Vagnoni V, Guidi M. et al. The extent of pelvic lymph node dissection correlates with the biochemical recurrence rate in patients with intermediate- and high-risk prostate cancer. BJU Int. 2011;108:1262-8
29. Ji J, Yuan H, Wang L, Hou J. Is the impact of the extent of lymphadenectomy in radical prostatectomy related to the disease risk? A single center prospective study. J Surg Res. 2012;178:779-84
30. Lopez-Colome AM, Lee-Rivera I, Benavides-Hidalgo R, Lopez E. Paxillin: a crossroad in pathological cell migration. J Hematol Oncol. 2017;10:50
31. Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445-57
32. O'Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. 2008;409:635-49
33. Stiegler AL, Draheim KM, Li X, Chayen NE, Calderwood DA, Boggon TJ. Structural basis for paxillin binding and focal adhesion targeting of beta-parvin. J Biol Chem. 2012;287:32566-77
34. Turner CE. Paxillin. Int J Biochem Cell Biol. 1998;30:955-9
35. Ruela-de-Sousa RR, Hoekstra E, Hoogland AM, Queiroz KC, Peppelenbosch MP, Stubbs AP. et al. Low-Molecular-Weight Protein Tyrosine Phosphatase Predicts Prostate Cancer Outcome by Increasing the Metastatic Potential. Eur Urol. 2016;69:710-9
36. Jackson JL, Young MR. Protein phosphatase-2A modulates the serine and tyrosine phosphorylation of paxillin in Lewis lung carcinoma tumor variants. Clin Exp Metastasis. 2002;19:409-15
37. Verhagen PC, Schröder FH, Collette L, Bangma CH. Does local treatment of the prostate in advanced and/or lymph node metastatic disease improve efficacy of androgen-deprivation therapy? A systematic review. Eur Urol. 2010;58:261-9
38. Du C, Wang X, Zhang J, Liu X, Zhu J, Liu Y. Paxillin is positively correlated with the clinicopathological factors of colorectal cancer, and knockdown of Paxillin improves sensitivity to cetuximab in colorectal cancer cells. Oncol Rep. 2016;35:409-17
Author contact
![]() Corresponding authors: Ning Xu (drxunedu.cn) and Xue-Yi Xue (xuexueyiedu.cn), Department of Urology, The First Affiliated Hospital of Fujian Medical University. No. 20 Chazhong Road, Fuzhou 350005, China. Tel/Fax: +86-059187981687
Corresponding authors: Ning Xu (drxunedu.cn) and Xue-Yi Xue (xuexueyiedu.cn), Department of Urology, The First Affiliated Hospital of Fujian Medical University. No. 20 Chazhong Road, Fuzhou 350005, China. Tel/Fax: +86-059187981687

 Global reach, higher impact
Global reach, higher impact