Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(5):792-796. doi:10.7150/jca.22656 This issue Cite
Research Paper
Serum expression level of IL-6 at the diagnosis time contributes to the long-term prognosis of SCLC patients
1. Department of General Surgery, Shanghai Pulmonary Hospital, School of Medicine, Tongji University
2. Department of Oncology, Shanghai Pulmonary Hospital, School of Medicine, Tongji University
3. Department of Thoracic Surgery, Shanghai Pulmonary Hospital, School of Medicine, Tongji University
4. Department of Respiratory Medicine, Shanghai Pulmonary Hospital, School of Medicine, Tongji University
5. Department of Bioengineering, University of Illinois at Chicago
6. Department of Pathology, Shanghai Pulmonary Hospital, School of Medicine, Tongji University
7. Department of Central Laboratory, Shanghai Pulmonary Hospital, School of Medicine, Tongji University
# Equal contribution
Received 2017-9-3; Accepted 2017-12-9; Published 2018-2-11
Abstract
Cytokines are vital mediators involved in tumor immunity. We aimed to explore whether the expression levels of IL-1β, TNF-α and IL-6 have impacts on prognosis of SCLC patients. In this study, we concluded 707 non-operable SCLC patients at stage III or IV into this study and analyzed the relationships between interleukins and OS/PFS by cox regression analysis and Kaplan-Meier analysis (log-rank test). As a result, under current standard chemotherapy, SCLC patients with higher IL-6 expression level had a shortened OS compared with those with normal level (HR: 0.381, 95%CI: 0.177-0.822, p=0.014). Furthermore, IL-6 expression level contributed mostly to patients without a smoking history. Non-smoking patients with a high IL-6 level showed a 6 months shortened OS than those with normal IL-6 level (10.50 vs 16.90 months, p=0.003 by Log-Rank test in Kaplan-Meier analysis). IL-6 had no obvious impacts on first-line PFS in these SCLC patients. To conclude, IL-6 acts as an independent factor of long-term prognosis of SCLC patients under current therapy.
Keywords: small cell lung cancer, cytokine, IL-6, prognosis, tumor immunity
Introduction
Small cell lung cancer is a subtype of lung cancer with extremely high malignance, which accounts for about 15%. Most patients are diagnosed at advanced stages with a starting subjective symptom caused by tumor metastases[1]. For advanced patients, the median OS is just 8 to 13 months, and the 5-year survival rate is about 5%[2]. For decades, a combination of etoposide and a platinum agent has been a cornerstone in SCLC treatment[3]. Although SCLC is sensitive to primary chemotherapy, the relapse rate is over 80% with a 4 to 5 months' median OS after the recurrence[2].
Immune system has been widely studied as a key procedure in tumorigenesis. Cancer is thought to develop from transformed cells, and immune system is supposed to identify the new pathogens and then clear the potential cancer-inducing cells[4, 5]. However, tumor cells succeed to escape from immune surveillance without immune activity[4, 6] via different ways, including immunological ignorance and immune escape. Expression immunosuppressive factors is a major strategy in tumor immune escape. The role of various cytokines has been demonstrated in immunosuppression. For example, TGF-β inhibits T cell proliferation by blocking IL-2 secretion[7] to suppress the immune system, and protects tumor cells from identified by immune cells[8]. Researchers also demonstrated that tumor progression can be reduced by TGF-β inhibition[9].
Interleukin is a main subtype of cytokines, and is essential in activating and regulating immune cells and mediating the activation, proliferation and differentiation of T cells as well as B cells. Interleukin contributes to tumor diseases in many aspects based on the multiple functions. For examples, IL-1 and IL-1β is involved in tumor progression, clinical outcome and risk of gastric cancer, breast cancer and non-small cell lung cancer [10-15]. IL-6 is reported to be involved in tumor progression of breast cancer[16] in molecular experiments[17] and clinical outcomes[18], and also in prostate cancer[19, 20].
The involvements of interleukin in lung cancer are also explored and reported. In terms of patient-based exploration and analysis, IL-6[21] was found to have an increased expression in NSCLC patients. A lot of interleukins, such as serum levels of IL-2 [22], IL-10[23], IL-8[24], IL-6 [25] were reported to have impacts on patients' prognosis of NSCLC. Only in few researches with small sample size, IL-8 was increased in SCLC patients[26] and IL-2 [27, 28] was reported to act as predictors of prognosis in SCLC.
As is mentioned above, the study of correlation of interleukin in NSCLC is far more than that in SCLC. Despite the reported meanings of interleukin in tumor progression and clinical prognosis of lung cancer, the role of interleukin in SCLC is still a blank due to the lack of supporting evidence from clinical data. Here, we analyzed the expression of three cytokines at the time of diagnosis, including TNF-α, IL-1β and IL-6, to explore whether these interleukins influence the clinical outcome of SCLC patients under current standard chemotherapy.
Materials and Methods
Ethical approval
This study was approved by the appropriate Ethics Committees of Shanghai Pulmonary Hospital and the approval number was 2015yxy55. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Study objects
We include 707 patients, who were diagnosed as SCLC pathologically from January 2012 to January 2016 in Shanghai Pulmonary Hospital, into this study. And all of these patients were at clinical stage III or stage IV without surgical resection history. All patients underwent chemotherapy with etoposide and cisplatin/carboplatin according to the SOC (standard of care) of SCLC.
ELISA assay And Cytokines reference range
All cytokines were performed using patients' peripheral blood by enzyme-linked immunosorbent assay (ELISA). The ELISA assay was performed following general steps with ELISA kit (SIEMENS, Siemens Healthcare Diagnostics Products Ltd.). The indexes were detected by IMMULITE 1000 Immunoassay System (SIEMENS, Immulite®1000).
All cytokines included into this study were inferred as the value at patients' first diagnosis. And the reference ranges of the selected cytokines, according to the given reference on the manual, were as follows: IL-1β: 0-5pg/ml, TNF-α: 0-8.1pg/ml, IL-6: 0-5.9pg/ml. The value of index was defined as higher one if was beyond the reference range.
Data collection
The basic clinical data were collected at the entry time, including patients' gender, age, clinical stages, smoking history and relative detection indexes. OS (overall survival) time was calculated from the first treating day to the death date caused by any reason. PFS (progression-free survival) time was calculated from the start date of treatment to the date of objective disease progression. If there's no death/disease progression record, the last follow-up date is used instead.
Statistical analysis
The statistical analysis was accomplished by SPSS software (version 20.0, SPSS Inc., Chicago, IL). The relationships between clinical features and major indicators and OS/PFS were evaluated by multivariate Cox regression analysis. Factor with a p level <0.05 was considered to have statistically significance. The cumulative death probability on significant index was assessed by Kaplan-Meier analysis (log-rank test).
Results
General information of studied cases
The general information of the 707 SCLC cases was listed in Supplementary Table 1. Simply, the study group was consisted of 89.1% female and 71.3% patients had a smoking history. All patients were at advanced clinical stage consisting of 49.4% stage III and 50.6% stage IV, and the average age was about 64 years old. 90.7% patients had the symptom of enlarged mediastinal lymph nodes. In terms of IHC neuroendocrine markers, the positive rate of CGA was 38.6%, 69.3% for SYN, and 81.8% for CD56. In terms of cytokines, 22.0% patients had a higher expression of IL-1β that beyond its normal range, 90.1% for TNF-α and 66.8% for IL-6 at the diagnosis time. All rates reported referred to valid percentages.
The associations of clinical features and interleukins with median OS in 707 SCLC cases by multivariate Cox regression analysis.
| Variables | n(N=707) | mOS(95%CI) | HR(95%CI) | p |
|---|---|---|---|---|
| GENDER | ||||
| male | 630 | 9.20(7.98-10.41) | 0.847(0.237-3.026) | 0.799 |
| female | 77 | 11.93(8.79-15.07) | ||
| AGE | ||||
| ≤64 | 366 | 10.50(8.69-12.30) | 0.673(0.389-1.165) | 0.157 |
| >64 | 341 | 8.76(6.91-10.61) | ||
| CLINICAL STAGE | ||||
| III | 349 | 13.06(10.94-15.19) | 0.562(0.316-0.998) | 0.049* |
| IV | 358 | 7.33(5.81-8.48) | ||
| ECOG PS | ||||
| 0-1 | 512 | 10.53(9.14-11.92) | 0.656(0.258-1.668) | 0.376 |
| 2-3 | 33 | 5.13(3.45-6.80) | ||
| SMOKING HISTORY | ||||
| yes | 468 | 9.36(7.91-10.81) | 1.939(0.812-4.628) | 0.136 |
| no | 188 | 11.93(9.15-14.71) | ||
| TUMOR LOCATION | ||||
| central | 618 | 9.46(8.29-10.63) | 2.493(0.972-6.399) | 0.057 |
| peripheral | 87 | 9.36(3.60-15.12) | ||
| MEDIASTINAL LYMPH NODES | ||||
| enlarged | 641 | 9.40(8.22-10.57) | 1.050(0.699-1.578) | 0.815 |
| normal | 44 | 10.93(2.97-18.89) | ||
| CGA | ||||
| positive | 263 | 6.70(4.98-8.42) | 1.463(0.808-2.648) | 0.209 |
| negative | 418 | 11.36(7.61-15.12) | ||
| SYN | ||||
| positive | 487 | 8.80(7.81-9.78) | 1.257(0.664-2.380) | 0.483 |
| negative | 216 | 13.36(9.98-16.74) | ||
| CD56 | ||||
| positive | 378 | 12.56(9.49-15.63) | 0.697(0.324-1.503) | 0.357 |
| negative | 84 | 16.90(4.20-29.60) | ||
| IL-1β | ||||
| normal | 327 | 10.86(9.00-12.73) | 0.894(0.652-1.228) | 0.490 |
| higher | 92 | 8.56(4.85-12.27) | ||
| TNF-α | ||||
| normal | 238 | 9.20(7.17-11.22) | 2.041(0.574-7.261) | 0.271 |
| higher | 112 | 8.50(6.40-10.59) | ||
| IL-6 | ||||
| normal | 135 | 13.06(8.24-17.88) | 0.381(0.177-0.822) | 0.014* |
| higher | 272 | 10.00(8.18-11.81) |
Note: * refers to p<0.05.
IL-6 was an independent prognostic factor of OS in 707 non-operated advanced SCLC
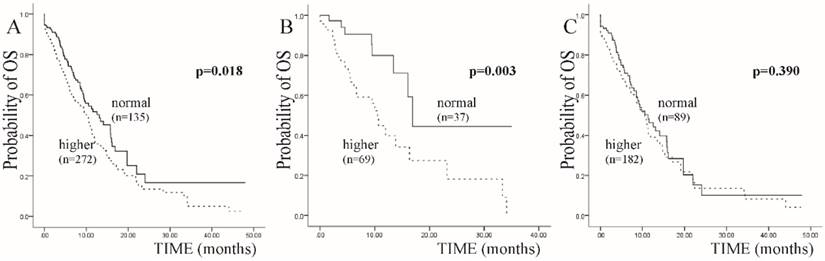
We conducted the survival analysis in these 707 SCLC cases by multivariate Cox regression analysis of OS. All of the clinical features, including the three interleukins, were included into the analysis. As a result, clinical stage and the expression of IL-6 were independent prognostic factors with a p value <0.05 (Table 1). Focusing on IL-6, patients with a higher expression level, which means the expression level was beyond the normal range, experienced a 3 months shortened OS compared with those with a normal expression level at the first diagnosis (HR: 0.381, 95%CI: 0.177-0.822, p=0.014) (Table 1 and Figure 1A).
IL-6 mainly influenced the prognosis of non-smoking SCLC patients
In further exploration, the expression level of IL-6 at the diagnosis time mainly influenced the OS of non-smoking SCLC patient in this study. To be detailed, in all patients with IL-6 examination, 106 patients had no smoking history. In these 106 patients, those who had a higher IL-6 expression level showed a 6 months shortened OS than those with a normal IL-6 level at the diagnosis time (10.50 vs 16.90 months, p=0.003 by Log-Rank test in Kaplan-Meier analysis). And in another subgroup consisting of smoking patients, there was no obvious difference between median OS of higher and normal IL-6 expression level (10.53 vs 11.30 months, p=0.390) (Figure 1B and 1C).
IL-6 has no obvious impacts on first-line PFS in non-operated advanced SCLC
We also conducted the analysis on IL-6 expression level and first-line PFS to explore the correlations. After multivariate Cox regression analysis of all relating factors, only clinical stage acted as independent factors of first-line PFS with a p value <0.05. There was no obvious difference between normal and higher IL-6 groups (p=0.157) (Table 2).
Discussion
In this study, we found that the plasma IL-6 expression level at diagnosis time had impacts on long-term prognosis of advanced SCLC patients. As is known, IL-6 is a vital cytokine with multiple functions, and the most significant one is the role of STAT3 signaling activator. Through STAT3 signaling pathway, IL-6 plays a positive role in MDSC generation[29].
MDSC (myeloid-derived suppressor cell) was firstly described in cancer patients and murine models decades ago, which includes mature myeloid cells and immature myelo-monocytic precursors. It is demonstrated that MDSC can reduce the proliferation of antigen-specific CD8+ T cells, increase the apoptosis and dysfunction of T cells, so that lead to immunosuppression. In terms of tumor diseases, the number of MDSCs increases significantly in many kinds of tumors, such as NSCLC and breast cancer. Furthermore, the production of MDSC can impede effective anti-tumor immunity and reduce the effect of tumor treatment.
Kaplan-Meier curves for SCLC patients with IL-6 expression level at diagnosis time. A: In all included SCLC patients. B: In subgroup of non-smoking SCLC patients. C: In subgroup of smoking SCLC patients.
The associations of clinical features and interleukins with median PFS in 707 SCLC cases by multivariate Cox regression analysis.
| Variables | mPFS(95%CI) | HR(95%CI) | p |
|---|---|---|---|
| GENDER | |||
| male | 8.60(7.50-9.69) | 1.445(0.255-8.186) | 0.677 |
| female | 8.50(3.67-13.32) | ||
| AGE | |||
| ≤64 | 8.30(7.12-9.48) | 2.716(0.989-7.460) | 0.053 |
| >64 | 8.90(7.12-10.67) | ||
| CLINICAL STAGE | |||
| III | 10.13(8.65-11.61) | 0.330(0.123-0.889) | 0.028* |
| IV | 6.36(4.99-7.74) | ||
| ECOG PS | |||
| 0-1 | 8.50(7.20-9.79) | 0.097(0.008-1.145) | 0.064 |
| 2-3 | ND | ||
| SMOKING HISTORY | |||
| yes | 8.46(7.44-9.48) | 1.061(0.313-3.592) | 0.924 |
| no | 8.20(5.87-10.52) | ||
| TUMOR LOCATION | |||
| central | 8.46(7.24-9.68) | 1.654(0.468-5.845) | 0.435 |
| peripheral | 10.76(6.40-15.13) | ||
| MEDIASTINAL LYMPH NODES | |||
| enlarged | 8.60(7.27-9.92) | 0.630(0.172-2.308) | 0.486 |
| normal | 8.30(5.52-11.07) | ||
| CGA | |||
| positive | 8.96(7.06-10.86) | 2.501(0.834-7.497) | 0.102 |
| negative | 8.16(6.96-9.36) | ||
| SYN | |||
| positive | 8.73(7.08-10.38) | 2.501(0.834-7.497) | 0.102 |
| negative | 8.30(6.49-10.10) | ||
| CD56 | |||
| positive | 8.46(7.72-9.20) | 0.470(0.143-1.546) | 0.214 |
| negative | 6.36(2.84-9.89) | ||
| IL-1β | |||
| normal | 8.96(7.43-10.50) | 0.991(0.593-1.659) | 0.974 |
| higher | 8.73(6.54-10.91) | ||
| TNF-α | |||
| normal | 7.00(3.34-10.65) | 2.339(0.326-16.790) | 0.398 |
| higher | 9.40(8.21-10.59) | ||
| IL-6 | |||
| normal | 8.73(6.69-10.76) | 1.955(0.772-4.954) | 0.157 |
| higher | 8.90(7.68-10.11) |
Note: * refers to p<0.05.
As is reported previously, IL6/STAT3 signaling pathway takes a part in tumor invasion by promoting the gathering and activity of MDSC, including in prostate cancer[30] and breast cancer[31]. Moreover, tumor cells may secrete IL-6 to gather and activate MDSC, and also induce MDSC on self-secretion of IL-6, which induces a positive feedback between tumors and MDSC[32]. Hence, the tumor becomes more aggressive and has a rapid progression. And we can infer that the high expression level of IL-6 also contribute to the malignance the SCLC and lead to the worse clinical outcome by promoting MDSC. What's more, recent studies have shown that some certain anti-tumor regimens, including gemcitabine and sunitinib malate, may eliminate or reverse the production of MDSCs and then improve the therapy efficacy of tumor treatment. And from that, we can get new ideals about future researches on tumor treatment, including SCLC therapy strategy.
We have also taken note of the deficiency of this study. Firstly, the interleukins examined were far too little as compared to the big interleukins family. Secondly, the number of studied SCLC cases was relatively large, but the follow-up time was not long enough, especially for the cases diagnosed in 2015 and 2016. Thirdly, the conclusion would be more convincing and conclusive if validation test or experiment was conducted.
To conclude, we firstly analyzed the relationship between three cytokines, including TNF-α, IL-1β, IL-6, and prognosis of non-operable SCLC patients based on a relatively large sample size. Patients with higher IL-6 expression level showed a shortened OS than those with normal level under current standard SCLC therapy. The impacts were more obvious in patients without smoking history, and in details, higher IL-6 level at diagnosis time led to a 6 months shortened OS. IL-6 was an independent factor of long-term prognosis in SCLC patients.
Supplementary Material
Supplementary table 1.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.81572269, No. 81672279), Science and Technology Commission of Shanghai Municipality (No.14411966400, No.17ZR1423500, No.16ZR1428900, No.15ZR1434500), and Shanghai Health Bureau Foundation (No. 201440397).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Klautke G, Sauer R, Fietkau R. Combined treatment modality in small cell lung cancer: the impact of radiotherapy on survival. Strahlentherapie und Onkologie. 2008;184:61-6
2. Puglisi M, Dolly S, Faria A, Myerson JS, Popat S, O'Brien MER. Treatment options for small cell lung cancer |[ndash]| do we have more choice|[quest]. British Journal of Cancer. 2010;102:629-38
3. Sundstrøm S, Bremnes RM, Kaasa S, Aasebø U, Hatlevoll R, Dahle R. et al. Cisplatin and Etoposide Regimen Is Superior to Cyclophosphamide, Epirubicin, and Vincristine Regimen in Small-Cell Lung Cancer: Results From a Randomized Phase III Trial With 5 Years' Follow-Up. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 2001;37:4665
4. Sogn JA. Tumor immunology: the glass is half full. Immunity. 1999;9:757-63
5. Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nature Immunology. 2001;2:293-9
6. Chen L. Immunological ignorance of silent antigens as an explanation of tumor evasion. Immunology Today. 1972;19:27-30
7. Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455-71
8. Kim S, Buchlis G, Fridlender ZG, Sun J, Kapoor V, Guanjun. et al. Systemic Blockade of Transforming Growth Factor-β (TGF-β) Signaling Augments the Efficacy of Immunogene Therapy. Cancer Research. 2008;68:10247
9. Gaspar NJ, Li L, Kapoun AM, Medicherla S, Reddy M, Li G. et al. Inhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasiveness. Molecular Pharmacology. 2007;72:152-61
10. El-Omar EM, Carrington M, Chow WH, Mccoll KE, Bream JH, Young HA. et al. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412:99 -
11. Zeng ZR, Hu PJ, Hu S, Pang RP, Chen MH, Ng M. et al. Association of interleukin 1B gene polymorphism and gastric cancers in high and low prevalence regions in China. Gastroenterology. 2003;124:1684-9
12. Zienolddiny S, Ryberg D, Maggini V, Skaug V, Canzian F, Haugen A. Polymorphisms of the interleukin-1 β gene are associated with increased risk of non-small cell lung cancer. International Journal of Cancer Journal International Du Cancer. 2004;109:353
13. Camargo MC, Mera R, Correa P, Peek RM, Fontham ETH, Goodman KJ. et al. Interleukin-1β and Interleukin-1 Receptor Antagonist Gene Polymorphisms and Gastric Cancer: A Meta-analysis. Cancer Epidemiology Biomarkers & Prevention. 2006;15:1674-87
14. Liu J, Zhai X, Jin G, Hu Z, Wang S, Wang X. et al. Functional variants in the promoter of interleukin-1beta are associated with an increased risk of breast cancer: a case-control analysis in a Chinese population. International Journal of Cancer. 2006;118:2554-8
15. Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman SH. et al. The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. International Journal of Oncology. 2003;23:269
16. Knüpfer H, Preiß R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Research and Treatment. 2007;102:129-35
17. Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N. et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940-7
18. Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-α-positive human breast cancer. Faseb Journal Official Publication of the Federation of American Societies for Experimental Biology. 2007;21:3763-70
19. Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine & Growth Factor Reviews. 2001;12:33-40
20. Culig Z, Steiner H, Bartsch G, Hobisch A. Interleukin-6 regulation of prostate cancer cell growth. Journal of Cellular Biochemistry. 2005;95:497
21. Carpagnano GE, Resta O, Foschinobarbaro MP, Gramiccioni E, Carpagnano F. Interleukin-6 is increased in breath condensate of patients with non-small cell lung cancer. International Journal of Biological Markers. 2002;17:141-5
22. Orditura M, Romano C, De VF, Galizia G, Lieto E, Infusino S. et al. Behaviour of interleukin-2 serum levels in advanced non-small-cell lung cancer patients: relationship with response to therapy and survival. Cancer Immunology, Immunotherapy. 2000;49:530-6
23. Vita FD, Orditura M, Galizia G, Romano C, Roscigno A, Lieto E. et al. Serum Interleukin-10 Levels as a Prognostic Factor in Advanced Non-small Cell Lung Cancer Patients. Chest. 2000;117:365-73
24. Orditura M, De VF, Catalano G, Infusino S, Lieto E, Martinelli E. et al. Elevated serum levels of interleukin-8 in advanced non-small cell lung cancer patients: relationship with prognosis. Journal of Interferon & Cytokine Research. 2002;22:1129-35
25. Songür N, Kuru B, Kalkan F, Ozdilekcan C, Cakmak H, Hizel N. Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori. 2004;90:196-200
26. Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Serum vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) levels in small cell lung cancer. Cancer Investigation. 2006;24:492-6
27. Fischer JR, Schindel M, Bülzebruck H, Lahm H, Krammer PH, Drings P. Long-term survival in small cell lung cancer patients is correlated with high interleukin-2 secretion at diagnosis. Journal of Cancer Research and Clinical Oncology. 2000;126:730-3
28. Fischer JR, Schindel M, Bülzebruck H, Lahm H, Krammer PH, Drings P. Decrease of Interleukin-2 secretion is a new independent prognostic factor associated with poor survival in patients with small-cell lung cancer. Annals of Oncology. 1997;8:457
29. Lechner MG, Liebertz DJEpstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. Journal of Immunology. 2010;185:2273
30. Wu CT, Hsieh CC, Lin CC, Chen WC, Hong JH, Chen MF. Significance of IL-6 in the transition of hormone-resistant prostate cancer and the induction of myeloid-derived suppressor cells. Journal of Molecular Medicine. 2013;91:649-50
31. Oh K, Lee OY, Shon SY, Nam O, Ryu PM, Seo MW. et al. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Research. 2013;15:R79
32. Beury DW, Parker KH, Nyandjo M, Sinha P, Carter KA, Ostrandrosenberg S. Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors. Journal of Leukocyte Biology. 2014;96:1109-18
Author contact
![]() Corresponding author: Li-Ping Zhang, Department of Pathology, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, No. 507, Zhengmin Road, Shanghai, 200433, P.R. China. Tel: +86 021 65115006; Email: lipingzhang2002com and Bo Su, Department of Central Laboratory, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, No. 507, Zhengmin Road, Shanghai, 200433, P.R. China. Tel: +86 021 65115006; Email: su_bo_scom
Corresponding author: Li-Ping Zhang, Department of Pathology, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, No. 507, Zhengmin Road, Shanghai, 200433, P.R. China. Tel: +86 021 65115006; Email: lipingzhang2002com and Bo Su, Department of Central Laboratory, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, No. 507, Zhengmin Road, Shanghai, 200433, P.R. China. Tel: +86 021 65115006; Email: su_bo_scom

 Global reach, higher impact
Global reach, higher impact