Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(3):584-593. doi:10.7150/jca.20940 This issue Cite
Research Paper
Role of Postoperative Concurrent Chemoradiotherapy for Esophageal Carcinoma: A meta-analysis of 2165 Patients
1. Department of Radiation Oncology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China;
2. Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA;
3. National Office for Cancer Prevention and Control, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China;
4. Department of VIP Medical Services, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Received 2017-5-9; Accepted 2017-8-31; Published 2018-1-1
Abstract
Purpose: This meta-analysis was aimed to evaluate the role of postoperative concurrent chemoradiotherapy (post-CCRT) for esophageal cancer patients after surgery.
Methods: We systematically searched PubMed, PMC, EMBASE, Cochrane Central Register of Controlled Trials, Chinese National Knowledge Infrastructure and Wanfang databases. Studies which compared CCRT with non-CCRT treatment for esophageal cancer patients after surgery were eligible. Outcomes of interest were odds ratios (OR) for overall survival (OS), local-regional recurrence rate, distant metastasis rate and adverse-event rate.
Results: Thirteen studies with 2165 patients were included in this meta-analysis. Post-CCRT significantly improved OS for esophageal cancer patients. Comparing the CCRT group with the non-CCRT one, the OR and 95% confidence interval (CI) for 1-year, 3-year and 5-year OS were 1.66 [1.30-2.11], 1.50 [1.24-1.81] and 1.54 [1.22-1.94], respectively. The local-regional recurrence rate was significantly reduced in the CCRT group (OR=0.58, 95% CI=0.46-0.72), but no significant difference was observed in the distant metastasis rate between the CCRT and non-CCRT groups (OR=0.94, 95% CI=0.68-1.30). Post-CCRT didn't increase the risk of pneumonitis, anastomotic stenosis or severe hematologic toxicities. Mild esophagitis in the CCRT group was increased but could be well tolerated.
Conclusions: This meta-analysis based on the largest-scale of published literature confirms that post-CCRT yields significant survival benefit and improves local-regional control with tolerable toxicity for patients with esophageal carcinoma.
Keywords: esophageal carcinoma, postoperative concurrent chemoradiotherapy, survival, recurrence, toxicity, meta-analysis
Introduction
Esophageal carcinoma is one of the most common malignancies and occupies the sixth leading cause of cancer-related death in the world [1]. About 49% of all new cases occurred in China [2]. Although neo-adjuvant chemoradiotherapy is recommended in resectable locally advanced esophageal carcinoma, what we have to be confronted with is that the initial treatment for majority of these patients trends to be surgery in China for various reasons. The effectiveness of surgery alone has been unsatisfactory, since the high relapse rate reaches up to 43.3%-50.0% [3-5]. Local-regional recurrence and distant metastasis remain to be the main causes of death after surgery. Therefore, postoperative multidisciplinary treatment including chemotherapy (CT) and radiotherapy (RT) has been vigorously implemented. Theoretically, postoperative concurrent chemoradiotherapy (post-CCRT) should be beneficial in improving the local-regional control as well as reducing the distant metastatic rate. But the results of the available clinical trials have not been consistent with each other. This meta-analysis aims to determine whether CCRT improves survival and decreases recurrence rates compared with non-CCRT strategies for patients who have underwent esophagectomy and lymphadenectomy.
Materials and Methods
Search strategy
We systematically performed a literature search of the following databases: PubMed, PMC, EMBASE, Cochrane Central Register of Controlled Trials, Wanfang and Chinese National Knowledge Infrastructure (CNKI). All trials published by July 15, 2017 were targeted. Computer retrieval was performed using the following retrieval language: (“thoracic esophageal cancer” OR “thoracic esophageal carcinoma”) AND (“postoperative” OR “adjuvant”) AND (“concurrent chemoradiotherapy” OR “concomitant chemoradiotherapy”). To ensure that no studies were missed, manual searches of reference lists were also performed.
Inclusion and exclusion criteria
Studies included in our analysis had to meet the following criteria: 1. clinical trials must compare post-CCRT with at least one of the following non-CCRT strategies: observation, postoperative CT (post-CT), postoperative RT (post-RT) or postoperative sequential chemoradiotherapy (post-SCRT) in the treatment of esophageal carcinoma after surgery; 2. data on survival, recurrence or toxicities had to be reported; 3. the language of publication was limited to English and Chinese with English abstract. All the randomized controlled trials (RCTs) and non-randomized controlled trials (NRCTs) were eligible. Articles for which the full text was not available were excluded.
Methodological quality assessment
The quality of RCTs was assessed using the Jadad scale, the scores of which range from 0 to 5, with higher scores indicating better reporting [6]. However, since blinding of patients and clinicians to interventions was not evaluated because it was considered impossible in these kinds of study, the highest score in our assessment should be 3 which was similar to one previous meta-analysis [7]. NRCTs was evaluated according to the MINORS in which the global ideal score is 24 for non-randomized comparative studies [8].
Data extraction
For each study the following data were extracted: first author, year of publication, the author's country, the tumor staging and histology, the treatment regimens, number of patients who received any regimen; the outcomes including 1-year survival, 3-year survival, 5-year survival, the local-regional recurrence rate, distant metastasis rate and incidence of toxicities. Data extraction was performed independently by two researchers.
Statistical analyses
Meta-analysis was performed with the software of Review Manager Version 5.3 and STATA version 12. The statistical heterogeneity of each study was assessed by I2 statistic with planned cut-off for significance of I2 =50% [9]. If I2≤50% which indicated no significant heterogeneity existing between the included studies, a fixed-effects model was adopted; otherwise, a random-effects model was employed and sensitivity analysis was further carried out using the leave one-out approach if there were more than two studies. Pooled analysis was performed using the Mantel-Haenszel model and reported as odds ratio (OR) with 95% confidence intervals (CI). The statistical significance of the pooled OR was determined by the Z-test. In the analysis of overall survival, an OR greater than 1 indicated a higher survival rate in patients who received post-CCRT and the point estimate of the OR was considered significant at the P<0.05 level if the 95% CI did not include 1; while, in the analysis of recurrence or toxicities, an OR greater than 1 represented higher recurrence rate or risk of toxicities in patients who received post-CCRT and the point estimate of the OR was considered significant at the P<0.05 level if the 95% CI did not include 1.
The Begg's and Egger's test in STATA were used to assess the potential publication bias. P<0.05 was considered statistically significant and indicated possible publication bias. Further verification was carried out with the trim and fill method which was based on a formalization of the qualitative approach using the funnel plot [10]. The asymmetric part of the funnel plot was trimmed off and the true center of the funnel was estimated using the symmetric remainder, then the trimmed studies and their missing counterparts were replaced to produce a symmetrical funnel plot. The final estimates of the true mean and its variance were calculated based on the filled funnel plot. If the imputed values weren't markedly different from the original ones, it demonstrated that the publication bias had little effect on the pooled results indicating the strong reliability of the conclusion. While, when the values changed obviously after the trim and fill algorithm, we could not come to a safe conclusion because of the significant effect of the publication bias.
Results
Study characteristics
Overview of literature selection was showed in Figure 1. The characteristics details of all eligible studies were presented in Table 1. Thirteen studies published in 14 articles[11-24] were eligible since one of the included studies had reported the 3-year and 5-year outcomes respectively in two articles[13, 14]. The 13 studies consisted of three RCTs [12, 19, 23], one prospective non-randomized controlled study [18], one prospective historical controlled study [22] and eight retrospective control studies[11, 13-17, 20, 21, 24]. The total number of patients identified in these 13 trials was 2165, including 998 patients treated with post-CCRT and the remaining 1167 with non-CCRT treatment after surgery. Ten studies enrolled patients with squamous cell carcinoma (SCC) only[12-19, 22-24], while for the other three studies both SCC and adenocarcinoma (AC) were eligible [11, 20, 21]. The type of SCC comprised 94.6% of all cases and AC accounted for 5.4%. Tumor stage of the patients ranged from phase Ⅱ to phase Ⅳ. Eleven out of 13 studies were conducted in Asian countries, including eight in China, three in Japan. The remaining two studies were one in Canada and one in America.
Assessment of the studies' quality
The average Jadad score based on only three evaluation items (description of randomization, the right method of randomization and the dropouts and withdrawals) was 2.3 of 3 (Supplementary Table S1). The assessment details of the ten NRCTs was shown in Supplementary Table S2. The MINORS scores of all included NRCTs ranged between 16 and 22 with an average score of 18.2. Therefore, the overall methodological quality of included studies was relatively high.
Flow chart showing inclusion and exclusion of studies
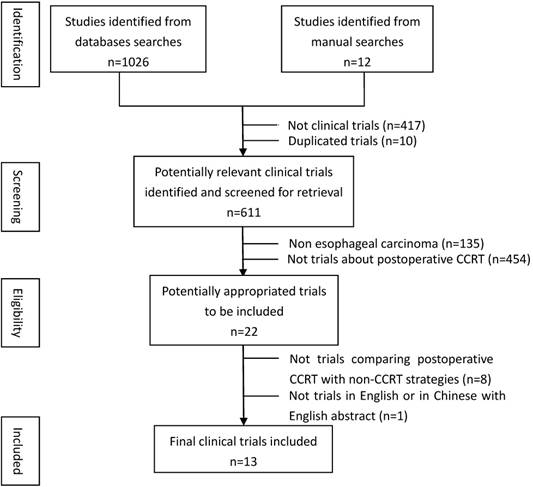
Characteristics of all clinical trials included in the meta-analysis
| Researcher and year | Country | Study type | Inclusion criterion | Histology | Radiotherapy | Chemotherapy | Treatment regimen | Patients number | Time after surgery |
|---|---|---|---|---|---|---|---|---|---|
| Saito 1993[22] | Japan | PHCS | Thoracic ESC; Ⅱ-Ⅳ | SCC | 40-50Gy 1.8-2.0Gy per day 5 days per week | CDDP 50mg/m2 D21 VDS 3mg/m2 D21 PLM 5mg D22-D26 Two cycles, interval of 4 weeks | S+CCRT S+RT | 35 26 | 1 month |
| Mukaida1998[20] | Japan | RCS | ESC | SCC and AC | 40-70Gy 1.8-2.0Gy per day 5 days per week | CDDP 50mg/m2 D1,D7 5-FU 500mg/m2 D3,D4,D5 VP-16 60mg/ m2 D3,D4,D5 Two cycles, interval of 4 weeks | S+CCRT S+RT S | 19 19 19 | 3-4 weeks |
| Bédard2001[11] | Canada | RCS | Thoracic ESC & EGJ cancer; T1-4,N1,M0 | SCC and AC | 50Gy 2.0Gy per day 5 days per week | CDDP 60mg/m2 D1 5-FU 200mg/m2 D1-D21 ±EPI 50mg/m2 D1-D21 First two cycles prior to radiation, then two concurrent with radiation, interval of 3 weeks | S+CT+CCRT S | 38 28 | NA |
| Rice2003[21] | USA | RCS | ESC; T3-4,N1,M1a | SCC and AC | 50.4-59.4Gy 1.8Gy per day 5days per week | CDDP 20mg/m2 D1-D4 5-FU 1000mg/m2 D1-D4 Two cycles, interval of 3 weeks | S+CCRT S | 31 52 | 3-10 weeks |
| Tachibana 2003[23] | Japan | RCT | Thoracic ESC; R0 resection | SCC | 45-50Gy 2.0Gy per day 5 days per week | CDDP 50mg/m2 D1,D5 5-FU 300mg/m2 D1-D35 | S+CCRT S+ CT | 22 23 | Within 2 months |
| Liu2005 [18] | China | PNRCS | Thoracic ESC; T3-4,N0-1,M0 | SCC | 55-60Gy 1.8Gy per day 5days per week | Concurrent: CDDP 30mg/m2 D1 for 6 weeks After completion of radiotherapy: CDDP 20mg/m2 D1-D5 5-FU 1000mg/m2 D1-D5 4 cycles, interval of 1 month | S+CCRT+CT S+RT | 30 30 | 2-3 weeks |
| Lv2010[19] | China | RCT | Thoracic ESC; Ⅱ-Ⅲ | SCC | Preoperative group:40Gy Postoperative group :50Gy 2.0Gy per day 5 days per week | CDDP 20mg/m2 D1-D3 TAX 135mg/m2 D1 2 cycles, interval of 3 weeks | CCRT+S S+CCRT S | 80 78 80 | 4-6 weeks |
| Cao 2010[12] | China | RCT | Thoracic ESC; Ⅱ-Ⅲ | SCC | 50Gy 2.0Gy per day 5 days per week | CDDP 20mg/m2 D1-D3 TAX 135mg/m2 D1 2 cycles, interval of 3 weeks | S+CCRT S | 74 77 | 4-6 weeks |
| Chen 2011[13], 2013[14] | China | RCS | Thoracic ESC; N1,M0 | SCC | 50Gy 2.0Gy per day 5 days per week | CDDP 80mg/m2 D1-D3 TAX 135mg/m2 D1 1-4 cycles, interval of 3 weeks | S+CCRT S+RT | 164 140 | 3-4 weeks |
| Wang2014[24] | China | RCS | ESC; R0 resection; ECE in metastatic lymph nodes | SCC | 45-54Gy 1.8-2.0Gy per day 5 days per week | CDDP 25mg/m2 D1-D3 5-FU 1000mg/m2 D1-D4 2 cycles, interval of 4 weeks | S+CCRT S | 43 47 | 3-6 weeks |
| Hsu2014[16] | China | RCS | ESC | SCC | 45-50.4Gy 1.8-2.0Gy per day 5 days per week | CDDP 80mg/m2 D1 5-FU 600mg/m2 D1-D4 LV 90mg/m2 D1-D4 2 cycles | S+CCRT S | 104 186 | NA |
| Hwang 2016[17] | China | RCS | ESC | SCC | NA | NA | S+CCRT S | 147 147 | 6 weeks |
| Hsu 2017[15] | China | RCS | ESC; R0 resection | SCC | NA | NA | S+CCRT S | 213 213 | 6 weeks |
Abbreviations: PHCS: prospective historical controlled study; ESC: esophageal carcinoma; SCC: squamous cell carcinoma; CDDP: cisplatin; VDS: vindesine; PLM: pepleomycin; S: surgery; CCRT: concurrent chemoradiotherapy; RT: radiotherapy; RCS: retrospective controlled study; AC: adenocarcinoma; 5-FU:5-fluorouracil; VP-16: etoposide; EPI: epirubicin; CT: chemotherapy; NA: not recorded or available; RCT: randomized controlled trial; PNRCS: prospective nonrandomized controlled study; TAX: paclitaxel; ECE: extracapsular extention; LV: leucovorin.
Effects of post-CCRT on survival
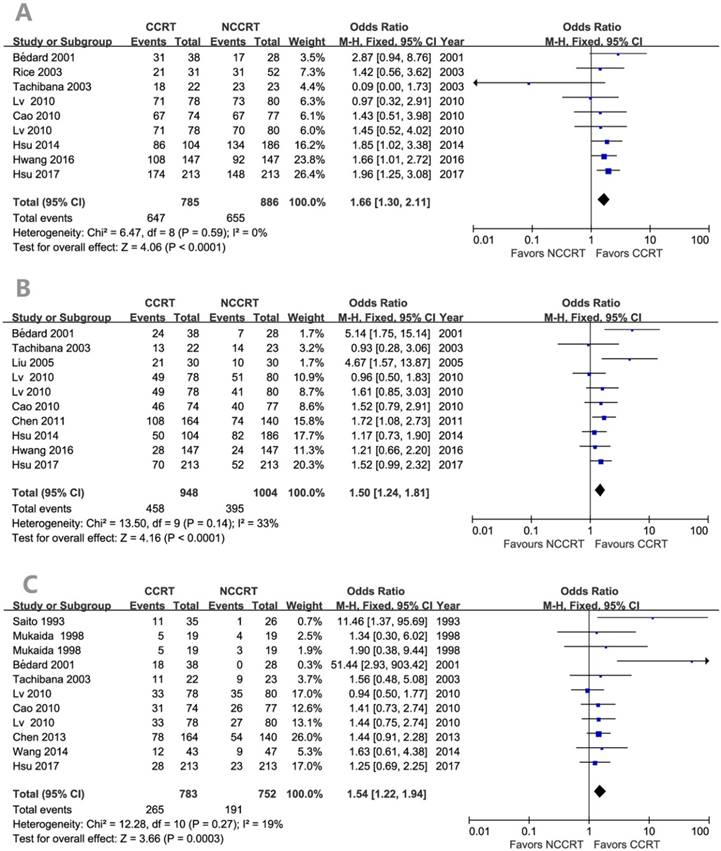
Figure 2 showed the effect of post-CCRT on the survival rate compared with the non-CCRT treatment after surgery. There was statistically significant benefit on overall survival in the post-CCRT group. No significant heterogeneity was detected among the included studies, so fixed effects-model was adopted for analysis. The values of OR for CCRT comparing with non-CCRT were 1.66 (95% CI=1.30-2.11, P<0.0001; Figure 2A) for 1-year survival, 1.50 (95% CI=1.24-1.81, P<0.0001; Figure 2B) for 3-year survival, and 1.54 (95% CI=1.22-1.94, P=0.0003; Figure 2C) for 5-year survival. There was no publication bias for the pooled estimates of 1-year and 3-year survival. Publication bias was detected in the 5-year survival result since the P values for the Begg's and Egger's test were both less than 0.05. However, after the trim and fill algorithm had been performed, the pooled analysis incorporating the hypothetical studies continued to show a statistically significant survival benefit of post-CCRT (OR=1.313, 95% CI=1.047-1.646, P=0.018).
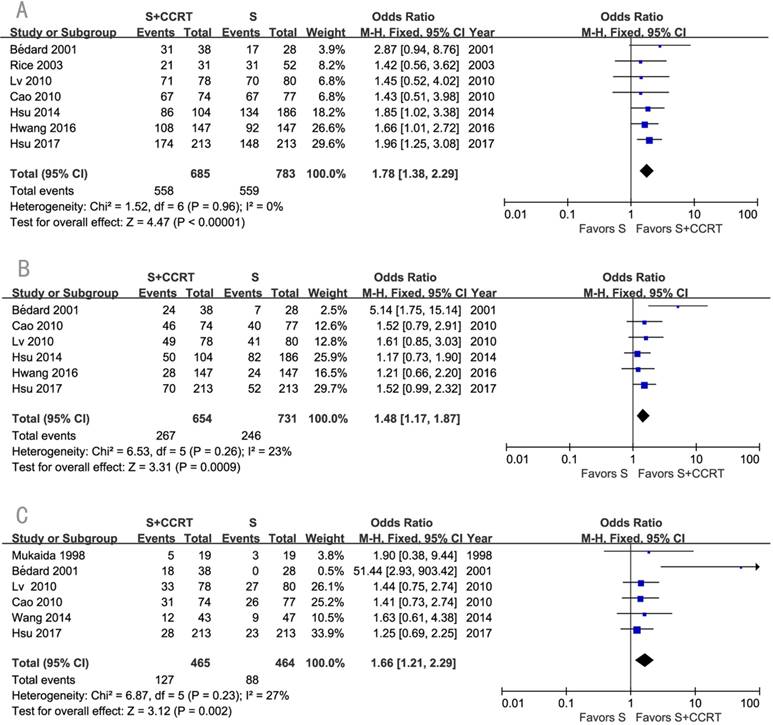
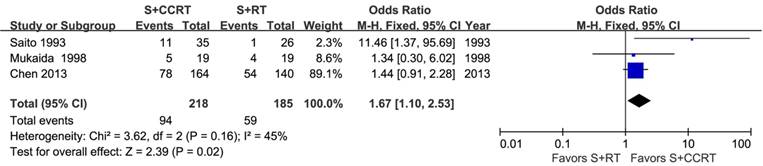
The survival benefits were also observed in the comparisons of post-CCRT with surgery alone (SA) or with post-RT. As shown in Figure 3, the values of OR for post-CCRT comparing with SA were 1.78 (95% CI=1.38-2.29, P<0.00001; Figure 3A) for 1-year survival, 1.48 (95% CI=1.17-1.87, P=0.0009; Figure 3B) for 3-year survival, and 1.66 (95% CI=1.21-2.29, P=0.002; Figure 3C) for 5-year survival. Figure 4 demonstrated that the OR of 5-year survival for post-CCRT comparing with post-RT was 1.67 (95% CI=1.10-2.53, P=0.02; Figure 4). Because there were no study reporting the 1-year survival and only two studies reporting the 3-year survival in the treatment of post-CCRT versus post-RT, we didn't do pooled analysis of them; since no study compared post-CCRT versus post-SCRT, we could not perform the pooled analysis either. Only one study compared post-CCRT with post-CT which showed non-significant survival benefit of post-CCRT probably because the patients number was too small [23].
Forest plots of odds ratio (OR) of overall survival in comparison of postoperative CCRT arm to NCCRT arm. (A) 1-year overall survival; Publication bias: Begg's test, P = 0.118; Egger's test, P = 0.048. (B) 3-year overall survival; Publication bias: Begg's test, P = 0.858; Egger's test, P = 0.239. (C) 5-year overall survival; Publication bias: Begg's test, P = 0.020; Egger's test, P = 0.014. CI: confidence interval; CCRT: concurrent chemoradiotherapy; NCCRT: non-concurrent chemoradiotherapy
Forest plots of odds ratio (OR) of overall survival in comparison of S+CCRT arm to S arm. (A) 1-year overall survival; (B) 3-year overall survival; (C) 5-year overall survival. CI: confidence interval; S+CCRT: surgery plus concurrent chemoradiotherapy; S: surgery alone
Forest plot of odds ratio (OR) of 5-year overall survival in comparison of S+CCRT arm to S+RT arm. CI: confidence interval; S+CCRT: surgery plus concurrent chemoradiotherapy; S+RT: surgery plus radiotherapy
Two studies performed respectively by Bédard et al. [11] and Liu et al. [18] added consolidation CT in the CCRT group but not in the control group. In order to rule out the impact of consolidation CT, further analysis was carried out which excluded these two studies. The analysis showed that comparing post-CCRT group with postoperative non-CCRT one, the OR values were 1.61 (95% CI=1.26-2.07, P=0.0002) for 1-year survival, 1.38 (95% CI=1.13-1.68, P=0.002) for 3-year survival, and 1.41 (95% CI=1.11-1.78, P=0.004) for 5-year survival (Table 2).
Meta-analysis outcomes of postoperative CCRT versus non-CCRT for included studies except two in which consolidation chemotherapy were performed.
| Outcomes | Included studies | Sample size | Analysis model | Test for overall effect | I2 value for heterogeneity | P value for Begg's | P value for Egger's | ||
|---|---|---|---|---|---|---|---|---|---|
| CCRT | NCCRT | OR (95% CI) | P | ||||||
| 1-year survival | 7 | 669 | 858 | F | 1.61 (1.26-2.07) | 0.0002 | 0% | 0.009 | 0.002 |
| 3-year survival | 7 | 802 | 946 | F | 1.38 (1.13-1.68) | 0.0020 | 0% | 0.386 | 0.222 |
| 5-year survival | 8 | 648 | 724 | F | 1.41 (1.11-1.78) | 0.0040 | 0% | 0.107 | 0.080 |
Abbreviations: CCRT: concurrent chemoradiotherapy; NCCRT: non-concurrent chemoradiotherapy; F: fixed-effects model; OR: odds ratio; CI: confidence interval.
Meta-analysis outcomes of postoperative CCRT versus non-CCRT for SCC
| Outcomes | Included studies | Sample size | Analysis model | Test for overall effect | I2 value for heterogeneity | P value for Begg's | P value for Egger's | ||
|---|---|---|---|---|---|---|---|---|---|
| CCRT | NCCRT | OR (95% CI) | P | ||||||
| 1-year survival | 6 | 638 | 806 | F | 1.63 (1.26,2.11) | 0.0002 | 0% | 0.007 | 0.005 |
| 3-year survival | 8 | 832 | 976 | F | 1.44 (1.18,1.74) | 0.0003 | 3% | 0.917 | 0.675 |
| 5-year survival | 7 | 629 | 686 | F | 1.40 (1.10,1.78) | 0.0060 | 0% | 0.174 | 0.074 |
Abbreviations: CCRT: concurrent chemoradiotherapy; NCCRT: non-concurrent chemoradiotherapy, F: fixed-effects model, OR: odds ratio, CI: confidence interval.
When we focused on the studies which only included patients with SCC, OR values for post-CCRT group versus postoperative non-CCRT group were 1.63 (95% CI=1.26-2.11, P=0.0002) for 1-year survival, 1.44 (95% CI=1.18-1.74, P=0.0003) for 3-year survival, and 1.40 (95% CI=1.10-1.78, P=0.006) for 5-year survival (Table 3). No publication bias was detected for the pooled estimates of 3-year and 5-year survival. And further trim and fill analysis certified that the publication bias in the analysis of 1-year survival had little influence on the pooled estimates (imputed OR=1.666, 95% CI=1.282-2.165, P=0.000).
Effects of post-CCRT on recurrence
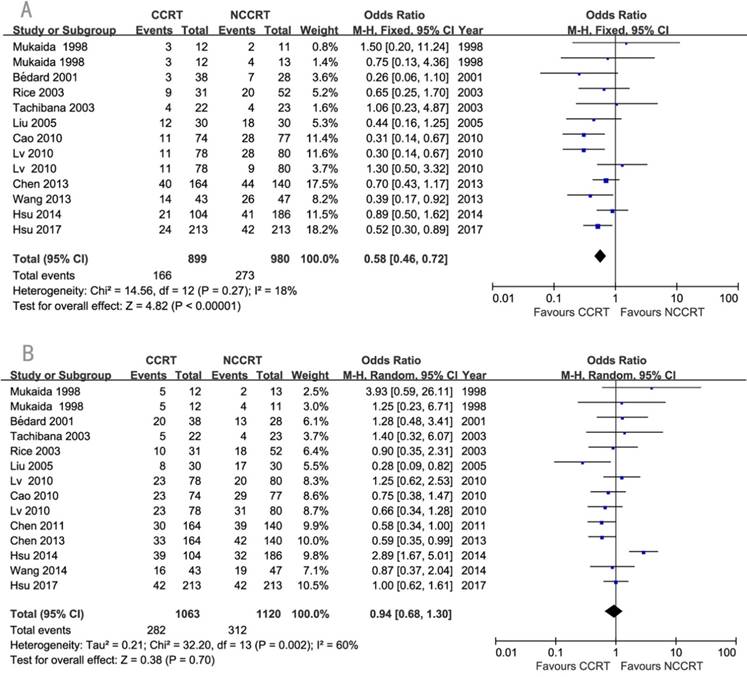
The detailed information about recurrence was available in eleven studies. Local-regional recurrence rate was significantly lower in the CCRT group compared with non-CCRT group (OR=0.58, 95% CI=0.46-0.72, P<0.00001; Figure 5A). Since there was heterogeneity regarding the distant metastasis among the eleven studies (I2=60%), a random-effects model of analysis was used. There was no significant difference in the comparison of distant metastasis rate between the two groups (OR=0.94, 95% CI=0.68-1.30 P=0.70; Figure 5B). Sensitivity analysis was further performed with each study removed in turn. The pooled estimates of distant metastasis remained non-significant after each study was removed except the removal of Hsu 2014 which changed it to significant, indicating this study might influenced the pooled estimate. No publication biases for the estimates of recurrences were detected.
Toxicity of post-CCRT
In the group of post-CCRT, the most common treatment-related mild toxicities (grade 1-2) were hypohemoglobinemia (25.7%-94.7%), leucocytopenia (33.3%-76.2%), thrombocytopenia (0-13.3%), nausea/vomiting (19.5%-86.7%), esophagitis (37.2%-66.7%) and stomatitis (17.1%-22.7%). The most prevalent severe complications (grade 3-4) were leucocytopenia (0-36.8%), hypohemoglobinemia (0-16.7%), thrombocytopenia (0-10.5%), nausea/vomiting (0-18.4%), and stomatitis (0-5.3%). Almost no studies reported grade 3 or worse late toxicities except for one in which 6.7% patients developed grade 3 lung toxicity, and 1 patient (0.6%) suffered grade 5 lung toxicity [14]. Toxicity comparison results were available in three studies which compared post-CCRT with post-CT or post-RT [13, 18, 23]. The pooled analysis results revealed that post-CCRT didn't increase the risk of grade 3-4 anemia (OR=1.26, 95% CI=0.34-4.73, P=0.73) and thrombocytopenia (OR=0.84, 95% CI=0.25-2.82, P=0.77) compared with post-CT or post-RT. Compared with post-RT, post-CCRT increased the risk of esophagitis (OR=1.71, 95% CI=1.09-2.66, P=0.02) but not pneumonitis (OR=0.89, 95% CI=0.55-1.44, P=0.63) or anastomotic stenosis (OR=0.54, 95% CI=0.18-1.59, P=0.26)(Supplementary Table S3). On the whole, with supportive care and symptomatic treatment, most of the patients could tolerate toxic reactions caused by post-CCRT.
Discussion
Esophageal carcinoma is an aggressive malignancy with a poor prognosis. In East Asia, the majority of patients are esophageal SCC and receive surgery as the initial treatment, especially in China. But the most optimal postoperative treatment remains unclear. The only one previously published meta-analysis conducted by Zheng [25] indicated that patients with esophageal cancer after surgery could gain survival benefit from post-CCRT. But this meta-analysis with relatively small sample sizes only included two RCTs plus five NRCTs with a total of 523 patients. What's more, the methodological quality of all included studies hadn't been assessed in detail. Comparatively speaking, one more RCT and five more NRCTs up to July 15, 2017 were added in our meta-analysis, and the methodological quality was assessed to make sure that all studies included were of relatively high quality. This guaranteed the result's reliability of our study. Our meta-analysis based on the 2165 esophageal cancer patients confirmed that post-CCRT can significantly improve the overall survival as well as the local-regional control as compared with non-CCRT strategies after surgery. What's more, subgroup analyses showed that patients receiving post-CCRT had greater survival benefits than those treated with post-RT or SA. Because there was only one study comparing post-CCRT with post-CT and no study comparing post-CCRT with post-SCRT, we could not do pooled analysis about these groups. Taken together, the efficacy superiority of post-CCRT indicated that radiotherapy and concurrent chemotherapy could achieve good synergistic effect, which was also confirmed in the preoperative CCRT [26-28] and definitive CCRT [29, 30] for patients with esophageal carcinoma. There were two studies involving consolidation CT in the CCRT group but not in the control group. In order to rule out the impact of consolidation CT, further analysis was performed which excluded these two studies and the result still favored CCRT group in the comparison of long-term survival. This further confirmed the value of post-CCRT. In our study, post-CCRT significantly improved local-regional control but had no significant influence on the distant metastasis indicating that the survival improvement was mainly attributed to the better local-regional control in the CCRT group. This was in accordance with the preoperative CCRT and definitive CCRT in the treatment of esophageal carcinoma. What's more, meta-analysis of head and neck cancer also confirmed that post-CCRT significantly improved survival and local-regional control but not the distant control [31].
Forest plots of odds ratio (OR) of recurrence in comparison of postoperative CCRT arm to NCCRT arm. (A) local-regional recurrence; Publication bias: Begg's test, P=0.583; Egger's test, P=0.998. (B) distant metastasis; Publication bias: Begg's test, P=0.189; Egger's test, P=0.753. CI: confidence interval; CCRT: concurrent chemoradiotherapy; NCCRT: non-concurrent chemoradiotherapy
This meta-analysis is highly representative for including all the studies concerning about post-CCRT in the treatment of esophageal carcinoma all over the world. However, of the 13 studies included in this meta-analysis 8 were conducted in China and 3 in Japan. Therefore, our meta-analysis is of greater value in the guidance of treatment for esophageal cancer patients after surgery in East Asia, especially in China. SCC is the predominant histopathological type of esophageal cancer in East Asia, which is different from that of western countries. Since there are differences of pathogenetic mechanism and pathobiological behavior between AC and SCC, the optimized treatment modality may also be different. In our study, with SCC accounting for approximately 95% of all cases, subgroup analysis was performed with trials only including patients with SCC and the pooled result confirmed that post-CCRT could significantly improve the prognosis for these patients. Subgroup analysis with AC was not carried out for the lack of eligible study which enrolled patients with AC exclusively.
In order to improve therapeutic efficacy in the treatment of operable esophageal carcinoma, chemoradiotherapy is generally combined with surgery either preoperatively or postoperatively, especially for the locally advanced disease. Therefore, adverse effects of chemoradiotherapy and the tolerance of the patients should be of great concern, particularly for those who suffered from malnutrition caused by esophagus disease and the further strike of the surgery. CCRT could significantly improve the treatment efficacy, but frequently at the expense of increased toxicity. Actually, studies included in our meta-analysis showed that common toxicities related to post-CCRT were hypohemoglobinemia, leucocytopenia, thrombocytopenia, nausea/vomiting, esophagitis and stomatitis. Most of them were mild graded 1-2. Post-CCRT didn't significantly increase the risk of pneumonitis, anastomotic stenosis and severe hematologic toxicity compared with post-CT or post-RT. Though esophagitis was significantly increased in the CCRT group, it all graded no more than 2 and could be well tolerated after symptomatic and supportive treatment. What's more, meta-analyses studying about preoperative CCRT in the treatment of resectable esophageal carcinoma confirmed that CCRT was associated with no increased risk of postoperative morbidity or perioperative mortality [7, 32]. To sum up, it was safe, feasible and effective for the application of CCRT both in the preoperative and postoperative treatment for esophagus cancer patients.
Although our meta-analysis confirmed significant improvement in both loco-regional control and overall survival with post-CCRT in the treatment of esophageal carcinoma, there are still some issues need further clarification. First of all, we didn't mention how to identify and select the appropriate population most likely to benefit from post-CCRT. Large-scale retrospective studies and prospective trials are warranted in order to find out optimal target population. Furthermore, studies included in our meta-analysis revealed that even after post-CCRT, the distant metastasis rate remains high ranging from 18.3% to 52.6%. Whether the consolidation or induction chemotherapy is needed deserves further exploration. What's more, though post-CCRT could provide survival benefit for esophageal cancer patients, the studies conducted with SEER database indicated that preoperative CCRT (or RT) provides superior survival compared with post-CCRT (or RT)[33, 34]. A network meta-analysis published online recently also confirmed that preoperative CCRT followed by surgery should be the most effective strategy in improving survival of resectable esophageal cancer [35]. So we may need to promote preoperative CCRT to maximize the treatment efficacy for esophageal carcinoma. Finally, the optimal radiation dose, target volume, and the most suitable concurrent chemotherapy regimen were not involved in our study. The future trials should be aimed to identify the optimum regimen of post-CCRT.
Conclusions
In conclusion, this meta-analysis including the largest-scale of relatively high quality trials confirms the value of post-CCRT in the treatment of esophageal carcinoma, especially in SCC. Post-CCRT brings about significant survival benefit and improves local-regional control without increased risk of severe toxicities compared with non-CCRT treatment. CCRT merits consideration as a preferred treatment for patients with esophageal carcinoma after surgery. Prospective large scale phase III randomized clinical trial is warranted in the future.
Supplementary Material
Supplementary tables.
Abbreviations
CCRT: concurrent chemoradiotherapy; OR: odds ratios; OS: overall survival; CI: confidence interval; CT: chemotherapy; RT: radiotherapy; SCRT: sequential chemoradiotherapy; RCTs: randomized controlled trials; NRCTs: non-randomized controlled trials; SCC: squamous cell carcinoma; AC: adenocarcinoma; SA: surgery alone.
Acknowledgements
The authors thank Professor Feng Sun for the suggestions and guidance in the statistical analysis. This work was supported by grants from National Natural Science Foundation of China (No. 81372418).
The primary data were presented in oral presentation at ASTRO's 58th Annual Meeting in Boston, MA, September 25-28, 2016.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel R, Ferlay J. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
2. Stewart BW, Wild CP. World Cancer Report 2014. International Agency for Research on Cancer: IARC Press. 2014
3. Chen G, Wang Z, Liu X-y, Liu F-y. Recurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor-Lewis esophagectomy. World J Surg. 2007;31:1108-15
4. Liu Q, Cai XW, Wu B, Zhu ZF, Chen HQ, Fu XL. Patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma: implications for the clinical target volume design of postoperative radiotherapy. PLoS One. 2014;9:e97225
5. Nakagawa S, Kanda T, Kosugi S-i, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198:205-11
6. Jadad AR, Moore RA, Carroll D. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12
7. Kumagai K, Rouvelas I, Tsai JA. et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg. 2014;101:321-38
8. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-6
9. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-60
10. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-63
11. Bedard EL, Inculet RI, Malthaner RA, Brecevic E, Vincent M, Dar R. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer. 2001;91:2423-30
12. Cao X, Lv J, Zhu B, An H. A prospective comparison between surgery alone and postoperative chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Chin J Oncol. 2010;32:452-5
13. Chen J, Pan J, Li J, Liu J. Clinical study of postoperative chemoradiotherapy of thoracic esophageal squamous cell carcinoma with positive lymph nodes. Chin J Radiat Oncol. 2011;20:287-90
14. Chen J, Pan J, Liu J. et al. Postoperative radiation therapy with or without concurrent chemotherapy for node-positive thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;86:671-7
15. Hsu PK, Chen HS, Huang CS. et al. Patterns of recurrence after oesophagectomy and postoperative chemoradiotherapy versus surgery alone for oesophageal squamous cell carcinoma. Br J Surg. 2017;104:90-7
16. Hsu P-K, Huang C-S, Wang B-Y, Wu Y-C, Hsu W-H. Survival benefits of postoperative chemoradiation for lymph node-positive esophageal squamous cell carcinoma. Ann Thorac Surg. 2014;97:1734-41
17. Hwang JY, Chen HS, Hsu PK. et al. A Propensity-matched Analysis Comparing Survival After Esophagectomy Followed by Adjuvant Chemoradiation to Surgery Alone for Esophageal Squamous Cell Carcinoma. Ann Surg. 2016;264:100-6
18. Liu H-C, Hung S-K, Huang C-J. et al. Esophagectomy for locally advanced esophageal cancer, followed by chemoradiotherapy and adjuvant chemotherapy. World J Gastroenterol. 2005;11:5367
19. Lv J, Cao X-F, Zhu B, Ji L, Tao L, Wang D-D. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol. 2010;16:1649
20. Mukaida H, Hirai T, Yamashita Y. et al. Clinical evaluation of adjuvant chemoradiotherapy with CDDP, 5-Fu, and VP-16 for advanced esophageal cancer. Jpn J Thorac Cardiovasc Surg. 1998;46:11-7
21. Rice TW, Adelstein DJ, Chidel MA. et al. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg. 2003;126:1590-6
22. Saito T, Shigemitsu Y, Kinoshita T. et al. Cisplatin, vindesine, pepleomycin and concurrent radiation therapy following esophagectomy with lymph adenectomy for patients with an esophageal carcinoma. Oncology. 1993;50:293-7
23. Tachibana M, Yoshimura H, Kinugasa S. et al. Postoperative chemotherapy vs. chemoradiotherapy for thoracic esophageal cancer: a prospective randomized clinical trial. Eur J Surg Oncol. 2003;29:580-7
24. Wang Z, Luan Z, Zhang W. et al. Postoperative chemoradiotherapy improves survival in esophageal squamous cell cancer with extracapsular lymph node extension. Neoplasma. 2013;61:732-8
25. Zheng B, Zheng W, Zhu Y, Lin XY, Xu BH, Chen C. Role of adjuvant chemoradiotherapy in treatment of resectable esophageal carcinoma: a meta-analysis. Chin Med J (Engl). 2013;126:1178-82
26. Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538-43
27. Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226-34
28. Jin HL, Zhu H, Ling TS, Zhang HJ, Shi RH. Neoadjuvant chemoradiotherapy for resectable esophageal carcinoma: a meta-analysis. World J Gastroenterol. 2009;15:5983-91
29. Cooper JS, Guo MD, Herskovic A. et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-7
30. Zhu LL, Yuan L, Wang H. et al. A Meta-Analysis of Concurrent Chemoradiotherapy for Advanced Esophageal Cancer. PLoS One. 2015;10:e0128616
31. Winquist E, Oliver T, Gilbert R. Postoperative chemoradiotherapy for advanced squamous cell carcinoma of the head and neck: a systematic review with meta-analysis. Head Neck. 2007;29:38-46
32. Sjoquist KM, Burmeister BH, Smithers BM. et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681-92
33. Hong JC, Murphy JD, Wang SJ, Koong AC, Chang DT. Chemoradiotherapy before and after surgery for locally advanced esophageal cancer: a SEER-Medicare analysis. Ann Surg Oncol. 2013;20:3999-4007
34. Wojcieszynski AP, Berman AT, Wan F. et al. The impact of radiation therapy sequencing on survival and cardiopulmonary mortality in the combined modality treatment of patients with esophageal cancer. Cancer. 2013;119:1976-84
35. Pasquali S, Yim G, Vohra RS. et al. Survival After Neoadjuvant and Adjuvant Treatments Compared to Surgery Alone for Resectable Esophageal Carcinoma: A Network Meta-analysis. Ann Surg. 2016
Author contact
![]() Corresponding author: Zhouguang Hui, Department of Radiation Oncology & Department of VIP Medical Services, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 17 Panjiayuan Nanli, Chaoyang District, Beijing 100021, China. E-mail: drhuizgcom; Tel: (010)87787656
Corresponding author: Zhouguang Hui, Department of Radiation Oncology & Department of VIP Medical Services, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 17 Panjiayuan Nanli, Chaoyang District, Beijing 100021, China. E-mail: drhuizgcom; Tel: (010)87787656

 Global reach, higher impact
Global reach, higher impact