Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(19):3984-3991. doi:10.7150/jca.20971 This issue Cite
Research Paper
Post-Resection Exhaustion of Intra-Platelet Serotonin: Also an Indicator of Early Hepatocellular Carcinoma Recurrence?
1. Cardiovascular and Gastroenterological Surgery, Graduate School of Medical and Dental Sciences, Kagoshima University 890-8520, Kagoshima, Japan
2. Department of Laboratory and Vascular Medicine, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima 890-8520, Japan
3. Department of Surgery, Kirishima Medical Center, Kirishima 899-5112, Japan.
4. Department of Surgery, Kagoshima Medical Center, National Hospital Organization, Kagoshima 892-0853, Japan
5. Department of Epidemiology and Preventive Medicine, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima 890-8520, Japan
* These authors share joint senior authorship
Received 2017-5-11; Accepted 2017-9-20; Published 2017-10-23
Abstract
Purpose: Serotonin (5-hydroxytryptamine, 5-HT) is well known for its growth stimulatory effect on several types of carcinoma and tumor cells. Since a large portion of 5-HT is stored and transported by platelets, the aim of this study was to assess the influence of platelet-sequestered 5-HT on post-resection hepatocellular carcinoma (HCC) recurrence.
Methods: This pilot study was conducted in a cohort of forty patients diagnosed with HCC undergoing partial hepatectomy. 5-HT levels in serum, plasma and intra-platelet (IP) were monitored preoperatively and four weeks after liver resection. The patients were followed every three months after the surgery.
Results: Follow-up was standardized to a fixed length of time. Fifteen patients (37.5%) developed HCC recurrence during 18 months follow-up. Patients with recurrence had significantly reduced serum and IP 5-HT levels at four weeks of liver resection (P = 0.003 and P = 0.014 respectively). Accordingly, in the Cox regression hazard model, serum and IP 5-HT were able to independently predict the recurrence (hazard ratio = 0.1, 95% confidence interval = 0.01 - 0.75 and hazard ratio = 0.1, 95% confidence interval = 0.01 - 0.89 respectively). The optimal cut-off value of 42.77 ng/ml for serum [area under the curve (AUC): 0.78, P = 0.003] and 0.3117 ng per 106 platelets (AUC: 0.733, P = 0.015), on receiver operating characteristic (ROC) curve corresponded to maximum sensitivity and specificity of prediction. The disease free interval was significantly worse in patients with low serum and IP 5-HT (P = 0.001 and P = 0.029 respectively).
Conclusion: IP 5-HT monitored during early follow-up, after liver resection may represent a useful marker of early HCC recurrence.
Keywords: Hepatocellular Carcinoma, liver cirrhosis, liver resection, platelet, serotonin
Introduction
Beyond the confines of its role as a neurotransmitter, serotonin (5-hydroxytrypamine, 5-HT) has also been described for its mitogenic property in a wide range of normal and tumor cells. In this context, some studies have proposed associations between 5-HT or its receptors and different cancers[1-5].
Primarily synthesized by the enterochromaffin cells in the intestinal mucosa, the circulating 5-HT is largely taken up and reserved in circulating platelets[6]. This platelet-sequestered 5-HT (intra-platelet 5-HT, IP 5-HT) serves critical functions in different pathophysiological events. Its role as an inducer of liver regeneration after partial hepatectomy (PH) has already been acknowledged in molecular investigations as well as clinical trials[7, 8]. However, 5-HT also seems to have a negative effect in liver pathophysiology. In particular, it contributes to liver fibrosis[9], mediates oxidative stress in non-alcoholic steatotic hepatitis[10], and perpetuates viral hepatitis[11].
Resection is the recommended first line treatment for patients with single hepatocellular carcinoma (HCC) with well-preserved liver function[12]. Unfortunately, resection of very small tumors is also associated with higher recurrence rates[13]. Despite the relevance of disease recurrence in terms of overall survival, diagnostic tools have merely been described to predict HCC recurrence.
5-HT has already been identified as a marker for early detection of breast cancer recurrence [3]. Specific to HCC, 5-HT promoted tumor growth both in vivo and in vitro models[4, 5]. 5-HT has also been proposed as a diagnostic marker of HCC[14]. Concurrently, a diametrically opposite effect of 5-HT has been reported on its tumor inhibitory properties; a substantial exhaustion in the platelet uptake of 5-HT is evidently observed in clinical and translational studies[15, 16].
An intricate defect in platelet functions has been described in patients with cirrhosis or underlying liver disease, including storage pool defect, defective signal transduction, glycoproteins dysfunction and impaired thromboxane synthesis[17-20]. Concomitantly, the presence of circulating exhausted platelets with depleted dense granules substances were found in patients with a heterogeneous group of malignant tumors[21, 22]. Recent advances in understanding platelet biology for its implications in molecular diagnostics in patients with different types of cancer have gained substantial attention in the oncology research.
Through this pilot project, we sought to investigate if IP 5-HT concentration represents a marker of HCC recurrence. Accordingly, we evaluated the feasibility of IP 5-HT for assessing early recurrent HCC from two perspectives: before and after resection of the primary tumor. We cross-examined the validity of IP 5-HT's association with HCC recurrence by evaluating some other platelet granule-released growth factors.
Patients and Methods
Prospective Study Cohorts
A total of forty patients with pathologically proven primary HCC who went on to have liver resection were enrolled in the study from May 2013 to June 2015. All the patients belonged to Child Pugh class A subgroup. Patient inclusion criteria were based on Clinical Practice Guidelines for Hepatocellular Carcinoma (Japanese Society of Hepato-Biliary-Pancreatic Surgery), 2013. This trial is registered in UMIN Clinical Trial Registry (UMIN000026380).
The institutional ethics committee (Kagoshima University # 24-155/ 26-77, Kirishima Medical Center # 2505 and Kagoshima Medical Center # 25-30) approved analyses of blood samples and patient data; all patients gave signed, informed consent. Study was conducted in accord with the ethical standards of the Committee on Human Experimentation of the institution in which the experiments were done or in accord with the ethical standards of the Helsinki Declaration of 1975.
Before liver resection, all patients underwent a thorough laboratory evaluation, including serum α-fetoprotein (AFP) and protein induced by vitamin K antagonist-II (PIVKA-II). Hepatic functional reserve was assessed by indocyanine green (ICG) clearance test, 99mTc-galactosyl human serum albumin (GSA) scintigraphy and Child-Pugh score. The diagnosis and staging of HCC were established with triple-phase computed tomography (CT) of the abdomen.
Follow up
Patients were followed with ultrasonography (USG) every 3 months after resection, and if USG showed any evidence of tumor, subsequent contrast-enhanced CT or magnetic resonance imaging (MRI) of the abdomen and non-contrast CT of the chest were performed. The diagnosis of intrahepatic recurrence was made on the basis of imaging alone if the tumor exhibited the typical enhancement characteristics.
Sample Preparation
Venous blood was collected preoperatively (PRE OP) and four weeks after surgery (POST OP). During this period, none of the patients received selective serotonin reuptake inhibitors (SSRIs). Complete blood count (CBC) was performed with an automated hematology analyzer, Sysmex XE-5000 (Sysmex Corporation, Kobe, Japan).
Serum and Plasma
Whole blood was collected in the serum separating tube and a citrate tube, containing 0.5 ml of sodium citrate (for plasma and platelet preparations), and an EDTA-2k tube (for cell count; Venoject II, Terumo Corp., Tokyo, Japan). Serum tube was incubated at room temperature for 30 minutes to allow clotting. Serum and plasma tubes were centrifuged at 1710 × g for 10 minutes. Only the top 75% of the resultant supernatant was carefully pipetted to avoid contamination.
Platelet Isolation
Venous blood in citrate tubes was centrifuged at 90 × g for 15 minutes. Again, only the top 75% of the resultant platelet rich plasma (PRP) was gently pipetted to avoid contamination. The PRP was centrifuged at 2810 × g to isolate platelets. The supernatant, platelet poor plasma (PPP), was collected precisely and decanted for the complete removal of the plasma from the pellets. Platelet pellets isolated from each 200 µl of PRP were suspended in 220 µl of lysis buffer (150 mM sodium chloride, 25 mM Tris-HCl pH 7.6, 1% Tergitol-type NP-40 and 0.1 % sodium dodecyl sulfate, 1 % sodium deoxycholate in distilled water to make 100 ml solution); after incubating for 20 minutes, the lysate solution was pipetted and vortexed until the pellets were completely dissolved in the solution. CBC was carried out in 3 preparations: whole blood, PRP and PPP.
Quantification of Cytokines
Serum, plasma and platelet extracts were analyzed together by commercially available enzyme-linked immunosorbent assay (ELISA) tests for human serotonin (Enzo LifeSciences Inc., Farmingdale, NY, US) and, Platelet derived growth factor- BB (PDGF-BB), Epidermal growth factor (EGF) and Angiopoetin-1 (Ang -1) (Quantikine; R&D Systems, Minneapolis, MN, USA) according to the manufacturer's guidelines.
Calculation of IP 5-HT
We have established different methods to calculate the intra-platelet growth factors[23, 24]. Platelet content of 5-HT per 106 platelets was calculated using our previous equation[23]:
V1 × C1 / V2 × C2
V1 = Volume of lysate solution
C1 = 5-HT concentration in platelet lysate solution
V2 = Volume of PRP
C2 = Platelet count in PRP
Briefly, 220 μl of lysis buffer was added to the platelets isolated from each 200 μl of PRP. The concentration was adjusted to the platelet count obtained from the PRP.
Statistical Analysis
Statistical analyses were conducted with SPSS software (version 21; SPSS, Inc., Chicago, IL) and Graph Pad Prism (version 6.0d for Mac OS X, USA, GraphPad Software, San Diego California, USA), and were based on nonparametric tests (Mann-Whitney's U test, Wilcoxon's signed rank test, and Spearman's correlation). The Fisher's exact test was used to evaluate frequencies between categorical variables. Patients were divided in lower and higher postoperative serum and IP 5-HT group, and platelet counts based on the median value obtained from the non-recurrent group; on this basis, Cox's proportional hazards regression model was used for the univariable (UVA) and multivariable analyses (MVA) to determine the variables independently associated to recurrence. Because of the collinearity between serum and IP 5-HT, MVA was separately run for these two variables. Receiver operating characteristic (ROC) analysis was performed to assess the specificity and sensitivity of IP 5-HT levels to predict recurrence. Two-tailed P values of less than 0.05 were considered statistically significant. IP 5-HT was expressed per 106 platelets.
Results
During the study period, forty patients underwent liver resection for HCC. Since this study aimed to identify the early recurrence, we adopted 18 months follow-up providing a uniform exposure to the cohort. During this period, fifteen patients (37.5%) developed recurrence, and the characteristics of patients stratified according to the recurrence status are shown in Table 1. Of all the variables, only gender and preoperative INR were significantly different between the groups.
Diminution in Serum and IP 5-HT Concentration after Liver Resection
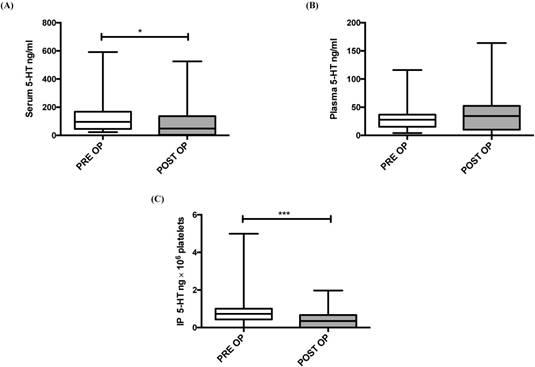
To investigate the impact of tumor resection on IP 5-HT concentrations, we examined the 5-HT concentrations just prior to the surgery and after four weeks of surgery. A significant decrease in serum (P = 0.013) and IP 5-HT (P < 0.001) was observed four weeks after liver resection (Figure 1, A and C). No significant difference was observed between pre and postoperative plasma 5-HT baseline values (P = 0. 253; Figure 1B).
Postoperative Serum or IP 5-HT can Independently Predict Early HCC Recurrence
In Table 2, we stratified the 5-HT concentrations and platelet counts based on recurrence. As shown, the postoperative 5-HT concentration was significantly lower in serum and IP of patients with recurrence (P = 0.003 and P = 0.014). Similarly, patients with recurrence had a significantly lower postoperative platelet count (P = 0.018). There was no difference in postoperative AFP concentration between the groups; however, we observed a weak positive correlation between the serum AFP and IP 5-HT concentrations (Figure S1).
Clinical and Pathological Data at the Time of Resection of Primary Tumor
| Variable Demographical and Clinical Data (N=40) | Recurrent Cases (n = 15) | Non-Recurrent Cases (n = 25) | P -Value |
|---|---|---|---|
| Age, Median (Min., Max.) | 71 (55, 83) | 74 (51, 80) | 0.726 |
| Sex, n (%) | 0.002 | ||
| Male | 9 (60%) | 21 (84%) | |
| Female | 6 (40%) | 4 (16%) | |
| Etiology, n (%) | 0.254 | ||
| HBV | 4 (26.66%) | 8 (32%) | |
| HCV | 7 (46.77%) | 5 (20%) | |
| None | 4 (26.66%) | 12 (48%) | |
| Fibrosis, n (%) | 0.083 | ||
| 0 | 0 (0%) | 4 (16%) | |
| 1-2 | 6 (40%) | 12 (48%) | |
| 3-4 | 9 (60%) | 9 (36%) | |
| Total bilirubin, mg/dl | 0.725 | ||
| Median (Min., Max) | 0.8 (0.6, 1.5) | 0.7 (0.5, 2.5) | |
| ≥ 1.0 | 1.3 (1, 1.5) | 1.2 (1.1, 2.5) | |
| INR | 0.026 | ||
| Median (Min., Max) | 1.09 (0.92, 2.43) | 1.02 (0.89, 1.29) | |
| ≥ 1.0 | 1.09 (1, 2.43) | 1.04 (1, 1.29) | |
| AFP, ng/ml | 0.791 | ||
| Median (SD) | 24.30 (2072) | 15.7 (2344) | |
| ≥ 20 | 102 (2703) | 914.8 (3671) | |
| Extent of resection, n (%) | 0.502 | ||
| Minor hepatectomy | 11 (73.33%) | 15 (60%) | |
| Major hepatectomy | 4 (26.67%) | 10 (40%) | |
| Tumor stage, n (%) | 0.543 | ||
| I-II | 11 (73.33%) | 17 (68%) | |
| III-IV | 4 (26.67%) | 8 (32%) | |
| Tumor size, n (%) | 0.730 | ||
| < 5 cm | 11 (73.33%) | 18 (72%) | |
| ≥ 5 cm | 4 (26.67%) | 7 (28%) | |
| Histological grade, n (%) | 0.686 | ||
| Well to moderate | 13 (86.67%) | 19 (76%) | |
| Poor | 2 (13.33%) | 6 (24%) | |
Min.: Minimum, Max.: Maximum, n/N: numbers, HBV: Hepatitis B Virus, HCV: Hepatitis C Virus, INR: International Normalised Ratio
5-HT Concentrations and Platelet Count on the Basis of Recurrence. Association Between Cytokine of Interest (Serum, Plasma and IP 5-HT), POST OP Platelet Count and AFP, and HCC Recurrence by Mann-Whitney Test
| Variables | Recurrent Cases | Non-Recurrent Cases | P- Value |
|---|---|---|---|
| PRE OP Serum 5-HT, ng/ml | 0.100 | ||
| Median (Min., Max.) | 56.78 (24.3, 591.9) | 109.4 (22.8, 389.2) | |
| PRE OP Plasma 5-HT, ng/ml | 0.470 | ||
| Median (Min., Max.) | 25.68 (4, 72) | 29.40 (5.1, 116.1) | |
| PRE OP IP 5-HT, ng | 0.630 | ||
| Median (Min., Max.) | 0.62 (0, 3) | 0.79 (0, 5) | |
| PRE OP Platelet count, ×103/μl | 0.121 | ||
| Median (Min., Max.) | 134 (61, 255) | 156 (69, 387) | |
| POST OP Serum 5-HT, ng/ml | 0.003 | ||
| Median (Min., Max.) | 6.06 (0, 204) | 91.14 (0, 525) | |
| POST OP Plasma 5HT, ng/ml | 0.065 | ||
| Median (Min., Max.) | 20.40 (0, 67.7) | 40.9 (0, 164) | |
| POST OP IP 5-HT, ng | 0.014 | ||
| Median (Min., Max.) | 0.2 (0, 1.4) | 0.50 (0, 2) | |
| POST OP Platelet count, × 103/μl | 0.018 | ||
| Median (Min., Max.) | 13.60 (12, 29) | 17.30 (10, 29) | |
| POST OP AFP, ng/ml | 0.548 | ||
| Median (Min., Max) | 4.5 (1.4, 844) | 3.7 (1.3, 382) |
Min.: Minimum, Max.: Maximum, PRE OP: Preoperative, POST OP: Postoperative, IP: Intra-Platelet, 5-HT: 5-hydroxytryptamine, IP 5-HT expressed per 106 platelets
Univariable and Multivariable Analyses by Cox Proportional Hazard Model
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | P- Value | HR | 95 % CI | P- Value | |
| Sex (M/F) | 1.03 | 0.33-3.33 | 0.95 | |||
| Fibrosis Grade (0-2/ 3-4) | 0.50 | 0.18-1.41 | 0.19 | |||
| PRE OP INR (<1/ ≥ 1) | 0.17 | 0.02-1.29 | 0.09 | |||
| POST OP Serum 5-HT | 0.09 | 0.01-0.69 | 0.02 | 0.1 | 0.01-0.75 | 0.03 |
| POST OP IP 5-HT | 0.10 | 0.01-0.78 | 0.02 | 0.11 | 0.01-0.89 | 0.03 |
| POST OP Platelet count | 0.36 | 0.11-1.13 | 0.08 | 0.41 | 0.13-1.32 | 0.13 |
M: Male, F: Female, PRE OP: Preoperative, POST OP: Postoperative, IP: Intra-Platelet, 5-HT: 5- hydroxytryptamine, HR: Hazard ratio
Since we observed a significant association of postoperative serum and IP 5-HT concentrations with HCC recurrence, it was of interest to investigate, if postoperative serum or IP 5-HT level could independently predict early HCC recurrence. Therefore, we performed univariable and multivariable analyses using the Cox proportional hazard model (Table 3). To specifically test for independence of serum and IP 5-HT from platelets, platelet count was always included as a covariate in MVA. Strikingly, postoperative serum and IP 5-HT were able to predict early recurrence independently (hazard ratio = 0.1, confidence interval = 0.01 - 0.75 and hazard ratio = 0.1, confidence interval = 0.01 - 0.89 respectively).
5-HT is stored in the dense-granules of platelet; to examine if a similar phenomenon occurs with the alpha-granules secreted growth factors, we analyzed platelet derived growth factor- BB (PDGF-BB), epidermal growth factor (EGF) and Angiopoetin-1, in serum, which were previously studied for their role in liver pathophysiology. We observed a similar association between HCC recurrence and serum PDGF-BB, Angiopoetin-1 and EGF (Table S1); moreover, PDGF-BB also yielded a statistically significant result. Furthermore, we found, a moderately weak, yet positive correlation between serum 5-HT and PDGF-BB concentrations in both pre and postoperative samples (r = 0.35, P = 0.02 and r = 0.32, P = 0.04 respectively; Figure S2).
Determination of Cut-off Serum and IP 5-HT Concentration to Predict Early HCC Recurrence
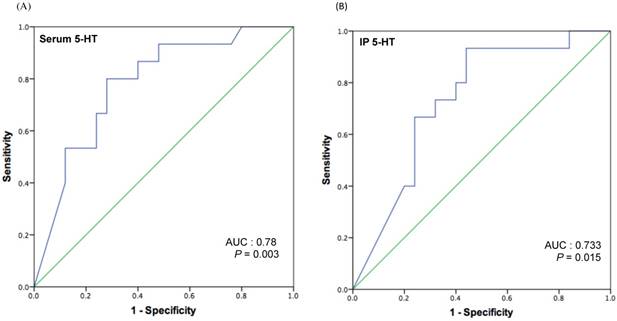
Because we had observed a significantly lower postoperative serum and IP 5-HT concentrations in patients with recurrence, we aimed to further characterize the potential of postoperative 5-HT values to predict early recurrence. Receiver operating characteristic (ROC) curves for serum and IP 5-HT was plotted (Figure 2, A and B), revealing significant predictive values of serum [area under curve (AUC) = 0.78; P = 0.003] and IP 5-HT (AUC = 0.733; P = 0.015). With this ROC plot, a cut-off level of 42.77 ng/ml of serum and 0.311 ng (per 106 platelets) of IP 5-HT was chosen to identify patients likely to develop recurrence with a specificity of 72% for serum and 68% IP, and sensitivity of 80% or 66.7% for serum and IP respectively. This holds the positive predictive value (PPV) of 63.16% and negative predictive value (NPV) of 85.71 % for serum 5-HT, and PPV of 56 % and NPV of 77.8% for IP 5-HT.
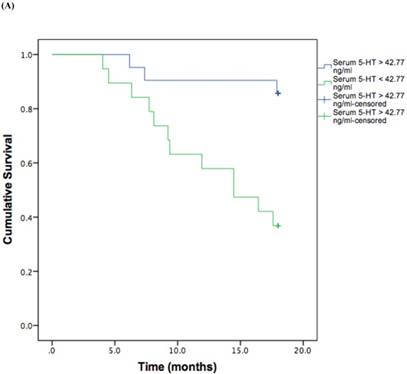
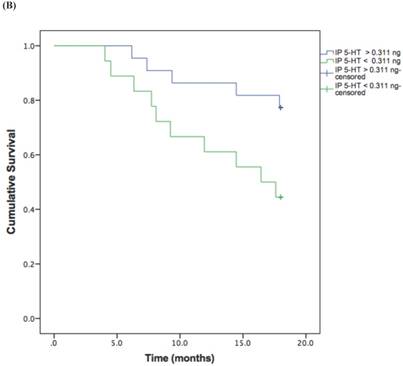
Based on the cut-off values obtained from the ROC curve, patients were divided in high and low 5-HT in serum and IP. A log rank test was run to determine the differences in disease free interval (DFI) distribution between groups with high and low 5-HT (Figure 3, A and B).
The DFI patterns differed significantly between the patients with high and low 5-HT; patients with lower serum {χ2 (2) = 10.118, P = 0.001} or IP {χ2 (2) = 4.729, P = 0.029} 5-HT displayed a significantly shorter DFI.
Discussion
Through this study, we introduce post-resection IP 5-HT as one of the indicators of early HCC recurrence. In this study, we monitored IP 5-HT in two distinct events: with the primary tumor and after resection of the tumor. The concentrations of postoperative 5-HT in serum or platelets, four weeks after liver resection, predicted the early HCC recurrence; the peculiar finding that stems from this study is the explicit low concentrations of postoperative 5-HT in all three-blood preparations from patients with recurrence, which is further supported by the results obtained from other platelet-sequestered growth factors.
5-HT concentration before (PRE OP) and 4 weeks after liver resection (POST OP) in: serum (A), plasma (B) and IP (C). IP 5-HT concentration was expressed per 106 platelets. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
ROC curve analysis for serum (A) and IP 5-HT (B) levels to determine a cut-off value to predict early HCC recurrence after liver resection.
Kaplan-Meier disease-free interval curves according to serum 5-HT (A) (42.77 ng/ml; P = 0.001, log-rank test) or IP 5-HT (B) (0.311 ng × 106 platelets; P = 0.029 log-rank test).
An emerging body of evidence suggests both deleterious and beneficial actions of platelet on liver function. In our previous study, we have demonstrated how IP VEGF-A shifts its kinetics from cancer to liver regeneration[23]. Accordingly, there are fairly contradictory reports on platelet 5-HT's involvement in liver diseases[25]. For instance, IP 5-HT is known to exert both pro-regenerative and pro-fibrotic effect in the liver[26]. These signals are, however, dependent on the cellular and cytokine microenvironment specific to the stage and type of liver injury[27].
Previous studies have demonstrated elevated 5-HT concentrations in patients with cancer[1, 14, 28]. Our finding is apparently in contrast with most of the previous studies, showing low postoperative (IP) 5-HT in patients with early HCC recurrence. This discrepancy could be attributed to different inclusion criteria, different neoplastic conditions and existence of liver disease. The degree of liver fibrosis doesn't only affect the platelet count but also account for intrinsic platelet hypofunction[19, 20, 29]. Furthermore, storage pool defect has been observed in patients with cirrhosis, shrinking the effect of granules release on activation. The 'cirrhotic platelets' have decreased platelet factor 4 (PF4) and P-selectin in the alpha granules, and reduced ATP and 5-HT in the dense bodies[17, 18, 20]. Patients with chronic liver injury (cirrhosis) tend to develop early HCC recurrence after liver resection[30]. Likewise, we observed a positive, provisionally significant, association between fibrosis grade and early recurrence in our cohort. Moreover, a similar tendency obtained with alpha-granule secreted growth factors and a positive correlation obtained between PDGF-BB and 5-HT prompt us to speculate the existence of platelet storage granule defects in patients with early recurrence. Thus, these results also must take into account the dynamic changes in platelet function augmented by stressful events, like post liver resection, in patients with advanced fibrosis.
Dysfunction of serotonergic systems with gastrointestinal microbiota dysbiosis resulting in an impaired uptake of serotonin by platelets from gut has been implicated in different conditions[31, 32]. It is now known that serotonergic mechanisms are altered in patients with underlying liver diseases. A decrease in the number of platelet membrane acceptors for 5-HT occurs in some neoplastic diseases[33]. Likewise, it is worth taking into consideration the mechanism, which could also interpret the functional significance of depleted IP 5-HT in patients with early HCC recurrence.
In a recent study, Shehta et al. has reported that the recurrence rate increases at lower platelet counts, and decreases at higher platelet counts after liver resection in cirrhotic patients[34]. In consonance with these findings, we also observed an increase recurrence rate in patients with post-resection exhaustion of platelet count and IP 5-HT. In addition to IP 5-HT exhaustion, some other platelet-sequestered growth factors also demonstrated a similar tendency in patients with early HCC recurrence. Taken together, another plausible speculation that cannot be fully excluded is that the phenomenon associated with recurrence might influence both the qualitative and quantitative properties of the platelets, or the reverse phenomenon may provoke the recurrence.
An increment in the concentration of 5-HT is known to exhibit a pro-mitogenic effect in cancers[4, 5, 35], while some studies describe a significant reduction in the kinetics of serotonin uptake by platelets in some cancers[15, 16]. Intriguingly, there are several reports, which found, even if not unequivocally, that using 5-HT or serotonergic agonists can be implemented in tumor growth inhibition[16, 36, 37]. From these diverging opinions, it makes sense to argue that based on the tumor type, associated events, the dose and the receptors involved, 5-HT might hinder the process of tumor growth; a great deal of revised evidence will be needed before we can know how this mechanism actually ensues.
This pilot study has some limitations that need to be pointed out. Main among these is the relatively small sample size. Furthermore, our study doesn't focus on the mechanistic evidence. Despite identical results in all three-blood preparations, we couldn't achieve statistically significant results with plasma preparations, and it could be attributed to our suboptimal plasma preparation technique; a slight deviation from the absolute technique described earlier by Starlinger et. al.[38]. However, we have shown a similar phenomenon with other platelet-alpha granules sequestered growth factors that strengthen the findings obtained from this small cohort. From another perspective, evaluation of 5-HT concentrations after resection of the tumor might represent a different spectrum of pathomechanisms to cancer recurrence.
Taken together, depleted IP 5-HT during early follow-up, after liver resection, was found to be a useful marker for prediction of early HCC recurrence. Since 5-HT is readily accessible in serum and reflects the platelet 5-HT, it may represent a potential biomarker to identify patients that require monitoring and consideration for recurrence. Nevertheless, the predictive potential of post-resection exhausted IP 5-HT in HCC recurrence needs to be further validated in large-scale studies. Moreover, the presence of 5-HT depleted platelets in patients with early HCC recurrence also emphasizes the need to delineate the precise molecular basis of platelet's pleiotropic actions and its functional consequences in the process of carcinogenesis.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This work was supported by the program of Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science, JSPS KAKENHI Grant number: 16F16420. This study was supported also in part by the Grant-in-Aid for Scientific Research (B: 16H05229), Japan.
We extend our sincere thanks to Dr. Hiroyuki Yamamoto for his expert opinion on statistical issues. We thank Dr. Hizuru Kumemura and Dr. Keisuke Yano for helping in data collections. We are indebted to Dr. Rohit K. Chaudhary (AIIMS), Dr. Benn Gooch (Oxford, UK), Miss Anna Löwe, (Tübingen, Germany) and Miss Nobue Uto (Kagoshima University) for their suggestions and kind assistance. We also thank Joint research laboratory, Kagoshima University Graduate School of Medical and Dental sciences, for allowing us to use their facilities.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dowling P, Hughes DJ, Larkin AM, Meiller J, Henry M, Meleady P. et al. Elevated levels of 14-3-3 proteins, serotonin, gamma enolase and pyruvate kinase identified in clinical samples from patients diagnosed with colorectal cancer. Clinica chimica acta; international journal of clinical chemistry. 2015;441:133-41
2. Fatima S, Shi X, Lin Z, Chen GQ, Pan XH, Wu JC. et al. 5-Hydroxytryptamine promotes hepatocellular carcinoma proliferation by influencing beta-catenin. Molecular oncology. 2016;10:195-212
3. Frobe A, Cicin-Sain L, Jones G, Soldic Z, Lukac J, Bolanca A. et al. Plasma free serotonin as a marker for early detection of breast cancer recurrence. Anticancer research. 2014;34:1167-9
4. Soll C, Jang JH, Riener MO, Moritz W, Wild PJ, Graf R. et al. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology. 2010;51:1244-54
5. Soll C, Riener MO, Oberkofler CE, Hellerbrand C, Wild PJ, DeOliveira ML. et al. Expression of serotonin receptors in human hepatocellular cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:5902-10
6. Rand M, Reid G. Source of 'serotonin' in serum. Nature. 1951;168:385
7. Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W. et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104-7
8. Starlinger P, Assinger A, Haegele S, Wanek D, Zikeli S, Schauer D. et al. Evidence for serotonin as a relevant inducer of liver regeneration after liver resection in humans. Hepatology. 2014;60:257-66
9. Ruddell RG, Oakley F, Hussain Z, Yeung I, Bryan-Lluka LJ, Ramm GA. et al. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. The American journal of pathology. 2006;169:861-76
10. Nocito A, Dahm F, Jochum W, Jang JH, Georgiev P, Bader M. et al. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterology. 2007;133:608-18
11. Lang PA, Contaldo C, Georgiev P, El-Badry AM, Recher M, Kurrer M. et al. Aggravation of viral hepatitis by platelet-derived serotonin. Nature medicine. 2008;14:756-61
12. Bruix J, Sherman M, Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-36
13. Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A. et al. Resection of hepatocellular cancer </=2 cm: results from two Western centers. Hepatology. 2013;57:1426-35
14. Abdel-Razik A, Elhelaly R, Elzehery R, El-Diasty A, Abed S, Elhammady D. et al. Could serotonin be a potential marker for hepatocellular carcinoma? A prospective single-center observational study. European journal of gastroenterology & hepatology. 2016;28:599-605
15. Deb C, Lakshmi C, Ray C, Ray MR. Decrease in platelet serotonin level in uterine cervix cancer patients. Haematologia. 2001;31:147-52
16. Muller K, Gilbertz KP, Meineke V. Serotonin and ionizing radiation synergistically affect proliferation and adhesion molecule expression of malignant melanoma cells. Journal of dermatological science. 2012;68:89-98
17. Ingeberg S, Jacobsen P, Fischer E, Bentsen KD. Platelet aggregation and release of ATP in patients with hepatic cirrhosis. Scandinavian journal of gastroenterology. 1985;20:285-8
18. Laffi G, Cinotti S, Filimberti E, Ciabattoni G, Caporale R, Marra F. et al. Defective aggregation in cirrhosis is independent of in vivo platelet activation. Journal of hepatology. 1996;24:436-43
19. Laffi G, Cominelli F, Ruggiero M, Fedi S, Chiarugi VP, La Villa G. et al. Altered platelet function in cirrhosis of the liver: impairment of inositol lipid and arachidonic acid metabolism in response to agonists. Hepatology. 1988;8:1620-6
20. Laffi G, Marra F, Gresele P, Romagnoli P, Palermo A, Bartolini O. et al. Evidence for a storage pool defect in platelets from cirrhotic patients with defective aggregation. Gastroenterology. 1992;103:641-6
21. Boneu B, Bugat R, Boneu A, Eche N, Sie P, Combes PF. Exhausted platelets in patients with malignant solid tumors without evidence of active consumption coagulopathy. European journal of cancer & clinical oncology. 1984;20:899-903
22. Mannucci PM, Cattaneo M, Canciani MT, Maniezzo M, Vaglini M, Cascinelli N. Early presence of activated ('exhausted') platelets in malignant tumors (breast adenocarcinoma and malignant melanoma). European journal of cancer & clinical oncology. 1989;25:1413-7
23. Aryal B, Shimizu T, Kadono J, Furoi A, Komokata T, Inoue M. et al. A Switch in the Dynamics of Intra-Platelet VEGF-A from Cancer to the Later Phase of Liver Regeneration after Partial Hepatectomy in Humans. PloS one. 2016;11:e0150446
24. Hashiguchi T, Arimura K, Matsumuro K, Otsuka R, Watanabe O, Jonosono M. et al. Highly concentrated vascular endothelial growth factor in platelets in Crow-Fukase syndrome. Muscle & nerve. 2000;23:1051-6
25. Lesurtel M, Soll C, Humar B, Clavien PA. Serotonin: a double-edged sword for the liver? The surgeon: journal of the Royal Colleges of Surgeons of Edinburgh and Ireland. 2012;10:107-13
26. Chauhan A, Adams DH, Watson SP, Lalor PF. Platelets: No longer bystanders in liver disease. Hepatology. 2016;64:1774-84
27. Mann DA, Oakley F. Serotonin paracrine signaling in tissue fibrosis. Biochimica et biophysica acta. 2013;1832:905-10
28. Jungwirth N, Haeberle L, Schrott KM, Wullich B, Krause FS. Serotonin used as prognostic marker of urological tumors. World journal of urology. 2008;26:499-504
29. Ordinas A, Escolar G, Cirera I, Vinas M, Cobo F, Bosch J. et al. Existence of a platelet-adhesion defect in patients with cirrhosis independent of hematocrit: studies under flow conditions. Hepatology. 1996;24:1137-42
30. Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA. et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Annals of surgery. 2006;243:229-35
31. Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. British journal of pharmacology. 2004;141:1285-93
32. Ohman L, Simren M. Intestinal microbiota and its role in irritable bowel syndrome (IBS). Current gastroenterology reports. 2013;15:323
33. Caranobe C, Sie P, Fernandez F, Pris J, Moatti S, Boneu B. Abnormal platelet serotonin uptake and binding sites in myeloproliferative disorders. Thrombosis and haemostasis. 1984;51:349-53
34. Shehta A, Han HS, Ahn S, Yoon YS, Cho JY, Choi YR. Post-resection recurrence of hepatocellular carcinoma in cirrhotic patients: Is thrombocytopenia a risk factor for recurrence? Surgical oncology. 2016;25:364-9
35. Siddiqui EJ, Shabbir MA, Mikhailidis DP, Mumtaz FH, Thompson CS. The effect of serotonin and serotonin antagonists on bladder cancer cell proliferation. BJU international. 2006;97:634-9
36. Burtin C, Scheinmann P, Salomon JC, Lespinats G, Canu P. Decrease in tumour growth by injections of histamine or serotonin in fibrosarcoma-bearing mice: influence of H1 and H2 histamine receptors. British journal of cancer. 1982;45:54-60
37. Orvoine RH. Influence of various hexoses and vasoactive agents on osmotically induced oncolysis. Cancer research. 1981;41:5044-8
38. Starlinger P, Alidzanovic L, Schauer D, Brugger P, Sommerfeldt S, Kuehrer I. et al. Platelet-stored angiogenesis factors: clinical monitoring is prone to artifacts. Disease markers. 2011;31:55-65
Author contact
![]() Corresponding author: Teruto Hashiguchi; Department of Laboratory and Vascular Medicine, Graduate School of Medical and Dental Sciences, Kagoshima University, 8-35-1, Sakuragaoka, Kagoshima 890-8520, Japan. Telephone: (+81)-99-275-5437; Fax: (+81)-99-275-5437. Email: terutohakufm.kagoshima-u.ac.jp
Corresponding author: Teruto Hashiguchi; Department of Laboratory and Vascular Medicine, Graduate School of Medical and Dental Sciences, Kagoshima University, 8-35-1, Sakuragaoka, Kagoshima 890-8520, Japan. Telephone: (+81)-99-275-5437; Fax: (+81)-99-275-5437. Email: terutohakufm.kagoshima-u.ac.jp

 Global reach, higher impact
Global reach, higher impact