Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(11):2051-2059. doi:10.7150/jca.18981 This issue Cite
Research Paper
Adjuvant Therapeutic Modalities Following Three-field Lymph Node Dissection for Stage II/III Esophageal Squamous Cell Carcinoma
1. Departments of Radiation Oncology and Chemotherapy, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China, 32500
2. Department of thoracic surgery, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China, 325000.
* authors contribute equally
Received 2016-12-30; Accepted 2017-4-10; Published 2017-7-5
Abstract
Background: The rates of locoregional and distant recurrence for esophageal squamous cell carcinoma (ESCC) patients underwent radical esophagectomy remain high. The purpose of this study is to explore an optimal postoperative therapeutic modality by investigating the efficacy of various adjuvant therapies in the treatment of ESCC.
Methods: We retrospectively reviewed 408 ESCC patients underwent thoracic esophagectomy and 3-field lymph node dissection from 2010 to 2015. Patients were classified into surgery alone (Group S), adjuvant chemotherapy (Group CT) and postoperative chemotherapy plus radiotherapy (Group CRT), respectively. Univariate and multivariate Cox regression analyses were used to analyze prognostic factors and survival.
Results: The overall survival (OS) and disease-free survival (DFS) were similar among groups. Postoperative CT and CRT both were beneficial for patients with positive lymph nodes, particularly for those with 3 or more lymph nodes involvement and metastasis in the middle thoracic segment compared with surgery alone. The 3-year OS and DFS for patients with 3 or more lymph nodes involvement were 30.8%, 53.7%, 50.5% and 19.9%, 41.6%, 34.0% for Group S, CT, and CRT, respectively (p=0.04; p=0.004, respectively). There was no notable difference in OS and DFS between the adjuvant Group CT and CRT (p=0.42; p=0.49, respectively). Postoperative CRT significantly reduced the rates of distant metastasis and overall recurrence for patients with positive lymph nodes (p=0.042; p=0.01, respectively). Number of metastatic lymph nodes, extent of resection, and AJCC stage were independent predictors of survival. Grade 1-2 myelosuppression was experienced significantly more frequently by patients in Group CRT than those in Group CT (P=0.03). Late toxicities were rare and manageable overall.
Conclusions: Postoperative CT and CRT both were associated with better survival for patients with positive lymph nodes, particularly for those with 3 or more lymph nodes involvement and metastasis in the middle thoracic segment. Postoperative CRT was significantly more effective at reducing the rates of distant metastasis and overall recurrence for patients with positive lymph nodes.
Keywords: Esophageal squamous cell carcinoma, Chemotherapy, Chemoradiotherapy, Lymph node metastasis, Survival.
Introduction
Esophageal cancer is the 8th most common cancer and 6th leading cause of cancer mortality in the word [1, 2]. The major pathological types of this cancer are adenocarcinomas in Europe and United States [3], and squamous cell carcinoma in Asia [2]. Three-field lymph node dissection is now considered as a standard procedure in the treatment of thoracic esophageal carcinoma, however, the use of surgery alone still results in high rates of locoregional recurrence and distant metastasis [4, 5]. It has been reported that the presence of lymph node metastasis and the number of involved nodes are important prognostic factors for survival after surgery [6-8]. Patients with local regional lymph node metastasis had worse prognosis than those without [8-10]. Even after radical surgery, the 5-year overall survival (OS) of patients with lymph node positive esophageal squamous cell carcinoma (ESCC) was only 14.7-38% [8-10]. Therefore, combined-modality therapy that included adjuvant chemotherapy and radiotherapy were used to reduce the rate of recurrence and to improve the survival outcome after surgery.
The reported efficacy of postoperative adjuvant radiotherapy in the treatment of thoracic ESCC was conflicting [11-13]. Only a few retrospective studies with small sample size comparing postoperative chemoradiotherapy (CRT) with surgery alone had been reported in the literature [14, 15]. Whether adjuvant CRT can improve the survival of patients with esophageal carcinoma is still under debate. In fact, there is no generally accepted strategy for postoperative treatment of ESCC patients in China, and the treatment regimens are mainly based on tumor stage, the doctors' and/or patients' preferences. The purpose of this study is to explore optimal postoperative therapeutic modalities in the treatment of ESCC by retrospectively reviewing the outcomes of adjuvant treatment in ESCC patients at our institute from 2010 to 2015.
Materials and Methods
Patients
The eligibility criteria for this study were as follows: patients had undergone radical esophagectomy with 3-field lymph node dissection and were pathologically confirmed with stage II ⁄ III thoracic ESCC; Liver and kidney function and results of blood tests were normal. Heart and lung function were not obviously damaged, and patients were supposed to be able to tolerate chemotherapy; Eastern Cooperative Oncology Group performance status <2. The exclusion criteria were as follows: patients with preoperative chemotherapy or preoperative radiotherapy; with a type of esophageal cancer other than squamous cell carcinoma (SCC) or with cancer at other site; with a history of cancer at any other site; and those lost to follow-up.
According to the type of postoperative adjuvant treatments, patients were classified into three groups: surgery alone (Group S), adjuvant chemotherapy alone (Group CT) and postoperative concurrent chemoradiotherapy (Group CRT). This study was approved by the Institutional Review Board and performed at the 1st Affiliated Hospital of Wenzhou Medical University. The informed consents from all the patients were obtained.
Surgical procedure
All patients included in our study underwent curative resection by total or subtotal thoracic esophagectomy and 3-field lymph node dissection, which included the subcarinal, paraesophageal, pulmonary ligament, diaphragmatic, paracardial, and left gastric artery lymph nodes. Esophageal reconstruction was performed using stomach, colon, or jejunum. Pathological staging and tumor location were uniformly defined according to the 7th edition of American Joint Committee on Cancer (AJCC) Guidelines [16]. Regional lymph nodes (N) extend from periesophageal cervical nodes to celiac nodes. N1, N2, and N3 indicated metastasis in 1 to 2, 3-6, and in ≥7 regional lymph nodes, respectively.
Chemotherapy
The first cycle of adjuvant chemotherapy was started at the 3rd to 4th week after operation. The chemotherapy regimen mainly consisted of intravenous infusion of cisplatin (75 mg/m2) averaged on days 1-3 plus paclitaxel (135 mg/ m2) on day 1 for a 21-day cycle. Another alternative chemotherapy regimen was comprised of 5-fluorouracil (5-FU) and cisplatin. Paclitaxel would also be replaced by 5-FU if patients were allergic. Cisplatin could be changed into nedaplatin. Supportive care and symptomatic treatment were provided during chemotherapy.
Chemoradiation therapy
Radiation was given by conformal fields with T-shaped target volume that included the bilateral supraclavicular area, mediastinum, and subcarinal area for lesions in the upper thoracic segment of the esophagus. The superior boundary of the middle thoracic segment was the upper edge of the first thoracic vertebra. The upper border of the lower thoracic segment was 3 cm above the upper edge of the gross tumor based on preoperative computed tomography (CT) images. The inferior border of the midlower thoracic segment was 3-4 cm below the lower edge of the gross tumor, as identified on preoperative CT images. The field contained the related drainage areas of the lymph nodes in the mediastinum and the primary esophageal tumor bed. A total dose of 50 Gy was delivered at 2.0 Gy per fraction over a 5-week period. Radiotherapy was given at a 6 MV X-Ray linear accelerator. Radiation was started day 1st when the patient in Group CRT received the first cycle of concurrent chemotherapy the same as described above.
Follow-up
Follow-up was performed every 3 months for the first two years, then every 6 months for the next 3 years, annually thereafter. Follow-up examinations consisted of physical examination, a complete blood count measurement, liver function test, chest CT scan, esophagogram, and abdominal CT scan or ultrasound. Positron emission tomography, endoscopy, bone scintigraphy, and/or cerebral CT were performed if clinically indicated. Any histologic evidence, unequivocal radiologic (computed tomography, magnetic resonance imaging, and bone scintigraphy) suspicious lesions of tumor was regarded as recurrent disease. Toxicities were graded according to National Cancer Institute common terminology criteria for adverse events (CTCAE version 3.0).
Statistical analysis
Disease-free survival (DFS) was calculated from the date of surgery to the time of first local or distant recurrence, or death from any cause. Overall survival (OS) was measured from the date of surgery to death or the last follow-up visit. Survival curves were estimated using Kaplan-Meier method and comparisons were made using the log-rank test. Categorical variables were compared using the chi-square test. Cox regression was used to estimate hazard ratios (HRs). For multivariate analysis of prognostic factors, separate Cox proportional hazards regression models were utilized to estimate the relationship between each variables and OS or DFS. A probability (p) value of < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS version 22.0 (IBM, Armonk, NY, U.S.A.).
Results
Clinical characteristics of the study population
Table 1 lists the characteristics of available patients and comparisons among different treatment modalities. There were total of 408 patients met the selection criteria in our study: 191(46.8%) received surgery alone, 83 (20.3%) received postoperative chemotherapy and 134 (32.9%) received postoperative concurrent CRT, respectively. Patients in Group CRT were younger than those in the other two groups (p=0.001). The rest of the characteristics did not differ significantly among three groups (P>0.05).
Comparison of patient characteristics by treatment assignment
| Characteristic | No. of patients (%) | |||||
|---|---|---|---|---|---|---|
| Total (n=408) | Group S (n=191) | Group CT (n=83) | Group CRT (n=134) | χ2 value | p value | |
| Sex | 2.544 | 0.694 | ||||
| Male | 360(88.2) | 169(88.5) | 75(90.3) | 116(86.6) | ||
| Female | 48(11.8) | 22(11.5) | 8(9.7) | 18(13.4) | ||
| Age at diagnosis (y) | 13.951 | 0.001 | ||||
| <60 | 201(49.3) | 89(46.6) | 42(50.6) | 90(67.2) | ||
| ≥60 | 207(50.7) | 102(53.4) | 41(49.4) | 44(32.8) | ||
| Smoking | 1.943 | 0.379 | ||||
| Yes | 253(62.0) | 121(63.4) | 46(55.4) | 86(64.2) | ||
| No | 155(38.0) | 70(36.6) | 37(44.6) | 48(35.8) | ||
| Tumor location | 4.734 | 0.316 | ||||
| Upper thoracic segment | 34(8.3) | 18(9.4) | 8(9.6) | 8(6.0) | ||
| Middle thoracic segment | 242(59.3) | 114(59.7) | 42(50.6) | 86(64.2) | ||
| Lower thoracic segment | 132(32.4) | 59(30.9) | 33(39.8) | 40(29.9) | ||
| Tumor differentiation | 2.264 | 0.687 | ||||
| Grade I | 88(21.6) | 38(19.9) | 22(26.5) | 28(20.9) | ||
| Grade II | 201(49.3) | 94(49.2) | 37(44.6) | 70(52.2) | ||
| Grade III | 119(29.2) | 59(30.9) | 24(28.9) | 36(26.9) | ||
| Depth of invasion (%) | 0.930 | 0.920 | ||||
| T2 | 118(28.9) | 51(26.7) | 25(30.1) | 42(31.3) | ||
| T3 | 252(61.8) | 122(63.9) | 50(60.2) | 80(59.7) | ||
| T4 | 38(9.3) | 18(9.4) | 8(9.6) | 12(9.0) | ||
| No. of positive lymph nodes | 10.063 | 0.122 | ||||
| N0 | 137(33.6) | 75(39.3) | 22(26.5) | 40(29.9) | ||
| N1 | 167(40.9) | 78(40.8) | 35(42.2) | 54(40.3) | ||
| N2 | 86(21.1) | 34(17.8) | 20(24.1) | 32(23.9) | ||
| N3 | 18(4.4) | 4(2.1) | 6(7.2) | 8(6.0) | ||
| Extent of resection (%) | 0.034 | 0.983 | ||||
| R0 | 370(90.7) | 173(90.6) | 75(90.4) | 122(91.0) | ||
| R1 | 38(9.3) | 18(9.4) | 8(9.6) | 12(9.0) | ||
| 7th AJCC stage | 7.410 | 0.493 | ||||
| IIA | 50(12.3) | 32(16.8) | 6(7.2) | 12(9.0) | ||
| IIB | 121(29.7) | 54(28.3) | 25(30.1) | 42(31.3) | ||
| IIIA | 130(31.9) | 60(31.4) | 28(33.7) | 42(31.3) | ||
| IIIB | 62(15.2) | 26(13.6) | 14(16.9) | 22(16.4) | ||
| IIIC | 45(11.0) | 19(9.9) | 10(12.0) | 16(11.9) | ||
Abbreviations: AJCC=American Joint Committee on Cancer (2009 criteria); S=surgery alone; CT=chemotherapy alone; CRT=chemotherapy plus radiation therapy;
Treatment compliance
There were 191 (88.0%) patients in Group CT and CRT together received chemotherapy consisted of paclitaxel and platinum (cisplatin or nedaplatin) every 21 days, and 12% received combinations of 5-fu and cisplatin every 21 days. Cisplatin was used in 83.4% of platinum-based chemotherapy. The median number of chemotherapy cycles were 4 (range, 2-6 cycles) and 3 (range, 2-4 cycles) in Group CT and CRT, respectively. There were five patients (6%) in Group CT excluded from the treatment protocol after two cycles of planned chemotherapy due to toxicities (3 patients) and patients' refusal (2 patients). In Group CRT, twelve patients (9%) discontinued treatments as a result of poor tolerance (10 patients) and infection (2 patients). Only one patient dropped out of the radiotherapy protocol due to severe radiation reaction.
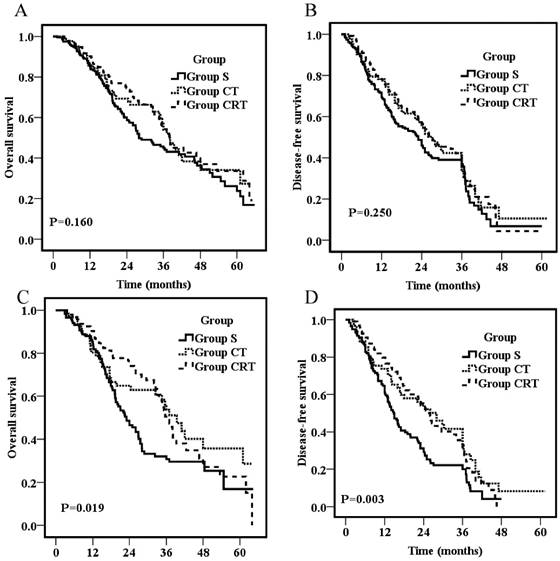
Overall survival for all patients (A) or patients with positive lymph nodes (C); Disease-free survival for all patients (B) or patients with positive lymph nodes (D).
Survival
The follow-up duration was similar for different groups with a median follow-up for all patients was 48.3 months. Overall survival rates for the entire population were 87% at 1 year, 49.8% at 3 years, and 29.3% at 5 years, respectively, with a median OS of 36 months. As shown in figure 1A, there was no significant difference on OS among groups (p=0.16) with 1-year, 3-year and 5-year OS of 85.3%, 85.5%, 90.2%; 44.9%, 53.9%, 54.3%; and 23.8%, 27.3%, 33.6% for Group S, CT, and CRT, respectively. The median 1- and 3-year DFS for all the patients were 75.2% and 40.3%, respectively. There was also no significant difference on DFS among groups (p=0.25) as shown in figure 1B with an overall 1- and 3- year DFS of 71.7% ,78.3% ,78.3%, and 35.7%, 42.4%,41.2% for Group S, CT and CRT, respectively.
For patients with positive nodal involvement, both postoperative chemotherapy and CRT provided a significant survival advantage compared with surgery alone (p=0.019; p=0.003, respectively; Fig 1C&D). The 3-year OS was 30.8%, 53.7%, and 50.5% for Group S, CT, and CRT, respectively. The survival rates of Group S were significantly lower than those of Group CT (p=0.04) and Group CRT (p=0.01), but no significant difference was found between Group CT and CRT (p=0.69). Similarly, the 3-year DFS were 19.9%, 41.6%, and 34.0% for Group S, CT, and CRT, respectively. The DFS of Group S were significantly lower than those of Group CT (p=0.01) and Group CRT (p=0.003), but no significant difference was found between Group CT and CRT (p=0.64).
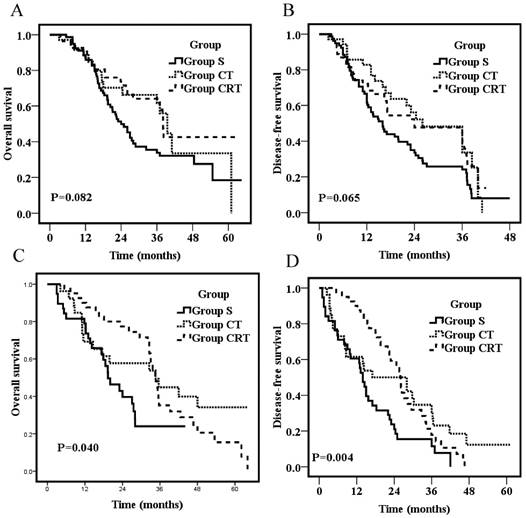
On stratification according to the number of lymph nodes, subgroup analyses of patients with metastasis of N1 (1 or 2 locoregional lymph nodes) showed no survival difference among the three groups (p=0.082; p=0.065, respectively; Fig 2A&B). However, for patients with metastasis of N2 + N3 (3 or more locoregional lymph nodes), patients of Group S had significantly worse OS and DFS than those in the other two groups (p=0.04; p=0.004, respectively; Fig 2C&D). The 3-year OS were 22.0%, 44.9%, and 35.2% for Group S, CT, and CRT, respectively. The 3-year DFS was 11.8%, 30.8%, and 17.7% for Group S, CT, and CRT, respectively. OS and DFS were not significantly different between Group CT and Group CRT (p=0.42; p=0.49, respectively; Fig 2C&D).
Overall survival for patients with metastasis of N1 (1 or 2 locoregional lymph nodes) (A) or patients with metastasis of N2+N3 (3 or more locoregional lymph nodes) (C); Disease-free survival for patients with metastasis of N1 (1 or 2 locoregional lymph nodes) (B) or patients with metastasis of N2+N3 (3 or more locoregional lymph nodes) (D).
Overall survival for patients with tumors in the middle thoracic segments (A) or patients with tumors in the lower thoracic segments (C); Disease-free survival for patients with tumors in the middle thoracic segments (B) or patients with tumors in the lower thoracic segments (D).
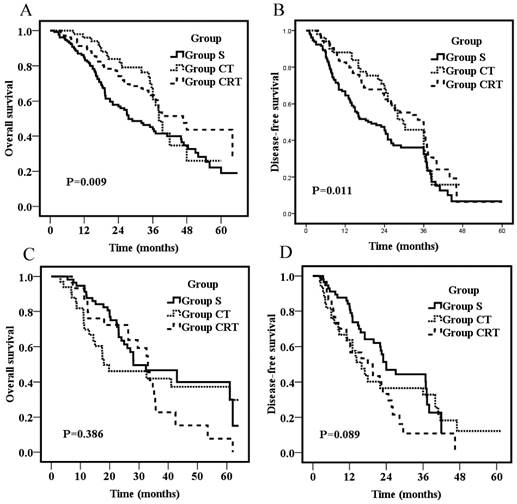
To explore the influence of tumor location on survival, the effects of adjuvant treatments on patients with tumors in the middle and lower thoracic segments (the number of patients who had tumors in the upper thoracic segments was too small to do subgroup analyses) were compared. For patients who had tumors in the middle thoracic region, the 3-year OS were 43.9%, 61.0%, and 63.4% for Group S, CT, and CRT, respectively. The survival rates of Group S were significantly lower than those of Group CT (P=0.03) and Group CRT (P=0.007), but no significant difference was found between Group CT and CRT (P=0.59; Fig 3A). Likewise, the 3-year DFS were 32.4%, 45.7%, and 45.8% for Group S, CT, and CRT, respectively. The DFS of Group S were significantly lower than those of Group CT (P=0.04) and Group CRT (P=0.006), but no significant difference was found between Group CT and CRT (P=0.23; Fig 3B). For patients who had tumors in the lower thoracic region, OS and DFS were not significantly different among groups (p=0.386; p=0.089, respectively; Fig 3C&D).
Failure analysis of patients with positive lymph nodes
The failure patterns of patients with positive lymph nodes were detailed in Table 2. Local recurrence rates were similar among the three groups (p=0.10). However, Group CRT had significantly fewer cases of hematogenous metastasis and overall recurrence (P=0.04; p=0.01, respectively).
Univariate and multivariate analyses of prognostic factors
Univariate analysis showed that OS and DFS were significantly associated with sex, tumor differentiation, number of positive lymph nodes, extent of resection and AJCC stage, but they were not significantly associated with age at diagnosis, tumor location, and depth of invasion (Table 3). Multivariate analysis showed that number of positive lymph nodes, extent of resection and AJCC stage were independent prognostic factors (Table 4).
Failure patterns of patients with positive lymph nodes
| No. of patients (%) | |||||
|---|---|---|---|---|---|
| Group S | Group CT | Group CRT | |||
| Failure Pattern | (n=116) | (n=61) | (n=94) | χ2 value | p value |
| Local recurrence, total | 42(37.1) | 20(32.8) | 22(23.4) | 4.578 | 0.101 |
| Supraclavicular | 12(10.3) | 5(8.2) | 4(4.3) | ||
| Mediastinum | 21(18.1) | 9(14.8) | 10(10.6) | ||
| Abdominal cavity | 7(6.0) | 5(8.2) | 5(5.3) | ||
| Tumor bed | 7(6.0) | 4(6.6) | 3(3.2) | ||
| Hematogenous metastasis | 38(32.5) | 13(21.3) | 17(18.1) | 6.33 | 0.042 |
| Mixed | 10(8.6) | 2(3.3) | 2(2.1) | 4.49 | 0.101 |
| Overall | 70(60.3) | 31(50.8) | 37(39.4) | 9.148 | 0.01 |
Abbreviations: AJCC=American Joint Committee on Cancer (2009 criteria); S=surgery alone; CT=chemotherapy alone; CRT=chemotherapy plus radiation therapy.
Univariate analysis of factors predictive of survival
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| median dfs | χ2 value | p value | median os | χ2 value | p value | |
| Sex | ||||||
| Male | 24.00 | 5.451 | 0.020 | 35.13 | 5.679 | 0.017 |
| Female | 36.30 | 42.00 | ||||
| Age at diagnosis (y) | ||||||
| <60 | 24.30 | 0.218 | 0.640 | 35.27 | 1.180 | 0.277 |
| >=60 | 26.00 | 38.17 | ||||
| Tumor location | ||||||
| Upper thoracic segment | 8.00 | 2.110 | 0.348 | 11.10 | 3.626 | 0.163 |
| Middle thoracic segment | 26.20 | 38.00 | ||||
| Lower thoracic segment | 22.00 | 32.33 | ||||
| Tumor differentiation | ||||||
| Grade I | 24.00 | 15.839 | <0.001 | 36.00 | 15.297 | <0.001 |
| Grade II | 29.03 | 41.00 | ||||
| Grade III | 19.60 | 26.30 | ||||
| Depth of invasion (%) | ||||||
| T2 | 34.00 | 5.080 | 0.079 | 41.00 | 2.462 | 0.292 |
| T3 | 23.10 | 35.50 | ||||
| T4 | 23.00 | 32.50 | ||||
| No. of positive lymph nodes | ||||||
| N0 | 36.7 | 37.780 | <0.001 | 47.0 | 23.577 | <0.001 |
| N1 | 20.0 | 36.0 | ||||
| N2 | 23.0 | 33.0 | ||||
| N3 | 10.0 | 13.0 | ||||
| Extent of resection (%) | ||||||
| R0 | 26.0 | 13.403 | <0.001 | 38.00 | 21.921 | <0.001 |
| R1 | 15.6 | 23.03 | ||||
| 7th AJCC stage | ||||||
| IIA | 36.90 | 27.125 | <0.001 | 61.00 | 21.529 | <0.001 |
| IIB | 36.00 | 41.00 | ||||
| IIIA | 23.00 | 37.00 | ||||
| IIIB | 21.90 | 29.50 | ||||
| IIIC | 16.17 | 25.00 | ||||
Abbreviations: DFS=disease-free survival; OS=overall survival; AJCC=American Joint Committee on Cancer (2009 criteria)
Multivariate analysis of prognostic factors for survival
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| Variables | median dfs | HR (95% CI) | p | median os | HR (95% CI) | p |
| No. of lymph node metastases | 1.377(1.203,1.575) | <0.001 | 1.128(1.087,1.645) | 0.032 | ||
| N0 | 36.7 | 47.0 | ||||
| N1 | 20.0 | 36.0 | ||||
| N2 | 23.0 | 33.0 | ||||
| N3 | 10.0 | 13.0 | ||||
| Extent of resection | 1.692(1.187,2.413) | 0.004 | 2.100(1.448,3.047) | <0.001 | ||
| R0 | 26.0 | 38.00 | ||||
| R1 | 15.6 | 23.03 | ||||
| 7th AJCC stage | 1.130(1.043,1.339) | 0.013 | 1.242(1.108,1.392) | <0.001 | ||
| IIA | 36.90 | 61.00 | ||||
| IIB | 36.00 | 41.00 | ||||
| IIIA | 23.00 | 37.00 | ||||
| IIIB | 21.90 | 29.50 | ||||
| IIIC | 16.17 | 25.00 |
Abbreviations: DFS=disease-free survival; OS=overall survival; HR = hazard ratio; CI = confidence interval. AJCC=American Joint Committee on Cancer (2009 criteria).
Toxic reactions
| No. of patients (%) | ||||
|---|---|---|---|---|
| Group CT | Group CRT | |||
| Toxicity | (n=83) | (n=134) | χ2 value | p value |
| Gastrointestinal reaction | ||||
| Grade 1-2 | 15(18.1) | 27(20.1) | 0.14 | 0.71 |
| Grade 3-4 | 2(2.4) | 4(3.0) | 0.06 | 1.0 |
| Myelosuppression | ||||
| Grade 1-2 | 24(28.9) | 58(43.2) | 4.50 | 0.03 |
| Grade 3-4 | 12(14.5) | 33(24.6) | 3.22 | 0.07 |
Abbreviations: CT=chemotherapy alone; CRT=chemotherapy plus radiation therapy.
Toxicities of postoperative chemotherapy and chemoradiotherapy
No significant difference in the incidences of gastrointestinal disorder and Grade 3-4 myelosuppression was found between the 2 groups (p>0.05). Grade 1-2 myelosuppression was experienced significantly more frequently by patients in Group CRT than those in Group CT (P=0.03) (Table 5). Late toxicities were rare and manageable overall.
Discussion
High rates of local-regional and distant recurrence resulted in the death of resected esophageal cancer patients have leaded to intense exploration on the application of multidisciplinary approaches in the treatment of esophageal cancer. National Comprehensive Cancer Network (NCCN) guidelines recommend preoperative neoadjuvant CRT for patients with locally advanced esophageal cancer. However, no additional postoperative adjuvant treatment was recommended unless for postive margins. Yet, even patients underwent extensive operation with unfavourable prognosis. Most existing literatures on adjuvant treatments were hindered by their retrospective nature or small numbers of ESCC included. Therefore, no clear consensuses in the treatment of postoperative ESCC had been reached.
In this study, the retrospective outcomes of adjuvant treatments suggested that paclitaxel-based adjuvant chemotherapy and CRT both significantly increased OS and DFS compared to surgery alone in lymph node positive ESCC patients, particularly in patients with three or more lymph nodes involvement and with tumors in the middle thoracic region. Postoperative CRT decreased the rates of distant metastasis and overall recurrence for patients with positive lymph nodes. Furthermore, this study showed that no significant difference in survival was found between postoperative CT and CRT Groups.
Only a few studies are available on the efficacy of postoperative chemotherapy. Ando et al conducted a randomized controlled trial and compared postoperative adjuvant chemotherapy with surgery alone. Their report demonstrated that adjuvant chemotherapy with cisplatin and fluorouracil improved 5-year DFS in patients with ESCC, especially in subgroup patients with lymph node metastasis. However, no significant difference was found in 5-year OS between two groups [9]. Similarly, a non-randomized prospective study conducted by Lee et al suggested that postoperative chemotherapy might prolong DFS in lymph node-positive, curatively resected esophageal cancer patients. This study failed to demonstrate OS benefit in the adjuvant group either [17]. Recently, Lyu et al retrospectively reviewed 349 ESCC patients with positive lymph node metastasis and showed that postoperative adjuvant chemotherapy with more than four cycles of taxane-based regimens prolonged overall survival [18]. Meanwhile, a meta-analysis indicated ESCC patients with stage III-IV diseases could benefit from adjuvant chemotherapy on 3-year OS [19]. Our study suggested that paclitaxel-based adjuvant chemotherapy increased OS and DFS compared to surgery alone in lymph node positive ESCC patients. These together showed that postoperative adjuvant chemotherapy might improve survival for certain small fraction of ESCC patients according to their pathological stages or their status of lymph node metastasis.
With regard to postoperative CRT, there also have been limited numbers of literatures exploring its use in ESCC patients. A prospectively, non-randomly trial conducted by Hung-Chang Liu et al showed that postoperative concurrent chemoradiotherapy (CCRT) with weekly cisplatin significantly increased OS for patients with locally advanced esophageal cancer compared with radiotherapy alone (30.9 mo vs 20.7 mo; 95% CI, 27.5-36.4 vs 15.2-26.1) [20]. Po-Kuei Hsu et al retrospectively reviewed 290 ESCC patients and found that postoperative CRT provided a survival benefit for patients with positive lymph node involvement (CRT versus S group: median OS 29.0 versus 16.0 months, 3-year OS rate 48.6% versus 16.8%; p=0.003) [21]. Junqiang Chen and coworkers also reported a significant survival advantage with postoperative CRT in node-positive ESCC patients compared with radiotherapy alone (p=0.03) [22]. In addition to survival benefits, the study of Junqiang Chen and our study both demonstrated lower frequency of distant and overall recurrence in Group CRT compared with Group S, indicating that surgery plus chemoradiation is more efficient for distant control than surgery alone.
The current study also found that postoperative CT and CRT both were associated with improved survival rates for patients with tumors in the middle thoracic regions but not for those with tumor in the lower region. Similarly, a previous study suggested postoperative radiotherapy was particularly beneficial for patients with positive lymph nodes in the upper (supraclavicular and upper mediastinal) region or both the upper and lower (mediastinal and abdominal) regions but not for those with only lower-region node disease. (p < 0.05) [12]. This study also demonstrated a better survival rate for patients with metastases in the lower region than those with metastases in the upper region or both the upper and lower regions (p < 0.0001). One possible explanation is that the middle and lower mediastinum and upper abdominal areas could be well exposed to achieve a relatively more thorough lymph node dissection. On the other hand, lymph node dissection in the lower neck and upper mediastinal regions is difficult due to the complex anatomy in those regions. Thus, postoperative chemotherapy and radiotherapy can reduce the recurrence rate of subclinical lesions which might be left behind by incomplete dissection and can reduce the incidence of latent distant metastasis. This was in line with our results, in which patients in Group S had the largest number of distant and overall recurrences.
Our study showed there was no notable difference in OS and DFS between the adjuvant CT and CRT Groups. Similarly, a previous prospective randomized clinical trial conducted by Tachibana et al reported that postoperative radiotherapy administered concurrently with cisplatin and 5-fluorouracil chemotherapy did not provide a survival benefit compared with chemotherapy alone [23]. To our knowledge, there were no other randomized reports exploring the effects and toxicities of postoperative CRT compared with chemotherapy alone since then. In this study, mild myelosuppression was significantly more common in the Group CRT than in the Group CT. No significant difference in the incidence of gastrointestinal disorder and severe myelosuppression was found between the 2 groups. Similarly, many studies showed that the incidence of complications was high in the CRT group and most complications due to CRT were chemotherapy-related [22, 24, 25]. However, paclitaxel-based adjuvant chemotherapy was in general well tolerated and no treatment-related death was observed in our study. Most of the complications were manageable and could be reversed by supportive care.
Multivariate analysis in the present study showed that the number of metastatic lymph nodes, extent of resection, and AJCC stage were independent prognostic factors for OS and DFS. These findings were consistent with the published literatures [26-29].
Owing to the retrospective nature of our study and the small number of patient size, it was difficult to make a definite conclusion about the best treatment of thoracic ESCC patients. Our analysis suggested that postoperative chemotherapy and CRT have similar survival rate and both may benefit ESCC patients with positive lymph nodes, especially those with metastasis of 3 or more locoregional lymph nodes and with tumors in the middle thoracic segment. Postoperative CRT was significantly more effective at reducing the rates of distant metastasis and overall recurrence for patients with positive lymph nodes. Mild myelosuppression was more common with CRT than with chemotherapy alone, but patients could tolerate CRT. Multicenter randomized trials with large sample size are needed to confirm our results. Further investigation in lymph node positive resected ESCC to compare the effects of postoperative CRT with chemotherapy alone is warranted.
Abbreviations
DFS: disease-free survival; OS: overall survival; AJCC: American Joint Committee on Cancer (2009 criteria); CT: computed tomography; CRT: chemotherapy plus radiation therapy; ESCC: esophageal squamous cell carcinoma; 5-FU: 5-fluorouracil; NCCN: National Comprehensive Cancer Network; HRs: hazard ratios; CCRT: concurrent chemoradiotherapy.
Acknowledgements
The study was partially founded by National Natural Science Foundation of China (11675122), Natural Science Foundation of Zhejiang Province (grant number LY16H160047 and Y17H160199), and Health Science and Technology Funding of Zhejiang Provincial Health Department (2015KYB241).
Ethics approval and consent to participate
This study was approved by the Regional Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University.
Authors' contributions
LL and LZ carried out data analyses and drafted the manuscript. BL, HS, MS did the follow-up; DX performed the surgery. XJ, CX designed, coordinated, and supervised the study and critically reviewed and discussed the manuscript. All authors have read and approved the final version of the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127:2893-2917
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69-90
3. Daly JM, Fry WA, Little AG. et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. Journal of the American College of Surgeons. 2000;190:562-572 discussion 572-3
4. Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. Journal of the American College of Surgeons. 2004;198:205-211
5. Bhansali MS, Fujita H, Kakegawa T. et al. Pattern of recurrence after extended radical esophagectomy with three-field lymph node dissection for squamous cell carcinoma in the thoracic esophagus. World J Surg. 1997;21:275-281
6. Hofstetter W, Correa AM, Bekele N. et al. Proposed modification of nodal status in AJCC esophageal cancer staging system. The Annals of thoracic surgery. 2007;84:365-373 discussion 374-5
7. Shimada H, Okazumi S, Matsubara H. et al. Impact of the number and extent of positive lymph nodes in 200 patients with thoracic esophageal squamous cell carcinoma after three-field lymph node dissection. World J Surg. 2006;30:1441-1449
8. Xiao ZF, Yang ZY, Miao YJ. et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radiat Oncol Biol Phys. 2005;62:82-90
9. Ando N, Iizuka T, Ide H. et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study-JCOG9204. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003;21:4592-4596
10. Xiao ZF, Yang ZY, Liang J. et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. The Annals of thoracic surgery. 2003;75:331-336
11. Schreiber D, Rineer J, Vongtama D. et al. Impact of postoperative radiation after esophagectomy for esophageal cancer. Journal of thoracic oncology. 2010;5:244-250
12. Chen J, Pan J, Zheng X. et al. Number and location of positive nodes, postoperative radiotherapy, and survival after esophagectomy with three-field lymph node dissection for thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:475-482
13. Martin JT, Worni M, Zwischenberger JB. et al. The role of radiation therapy in resected T2 N0 esophageal cancer: a population-based analysis. The Annals of thoracic surgery. 2013;95:453-458
14. Bedard EL, Inculet RI, Malthaner RA, Brecevic E, Vincent M, Dar R. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer. 2001;91:2423-2430
15. Rice TW, Adelstein DJ, Chidel MA. et al. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. The Journal of thoracic and cardiovascular surgery. 2003;126:1590-1596
16. Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721-1724
17. Lee J, Lee KE, Im YH. et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin in lymph node-positive thoracic esophageal squamous cell carcinoma. The Annals of thoracic surgery. 2005;80:1170-1175
18. Lyu X, Huang J, Mao Y. et al. Adjuvant chemotherapy after esophagectomy: is there a role in the treatment of the lymph node positive thoracic esophageal squamous cell carcinoma? J Surg Oncol. 2014;110:864-868
19. Zhang SS, Yang H, Xie X. et al. Adjuvant chemotherapy versus surgery alone for esophageal squamous cell carcinoma: a meta-analysis of randomized controlled trials and nonrandomized studies. Diseases of the esophagus. 2014;27:574-584
20. Liu HC, Hung SK, Huang CJ. et al. Esophagectomy for locally advanced esophageal cancer, followed by chemoradiotherapy and adjuvant chemotherapy. World journal of gastroenterology. 2005;11:5367-5372
21. Hsu PK, Huang CS, Wang BY, Wu YC, Hsu WH. Survival benefits of postoperative chemoradiation for lymph node-positive esophageal squamous cell carcinoma. The Annals of thoracic surgery. 2014;97:1734-1741
22. Chen J, Pan J, Liu J. et al. Postoperative radiation therapy with or without concurrent chemotherapy for node-positive thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;86:671-677
23. Tachibana M, Yoshimura H, Kinugasa S. et al. Postoperative chemotherapy vs chemoradiotherapy for thoracic esophageal cancer: a prospective randomized clinical trial. Eur J Surg Oncol. 2003;29:580-587
24. Zheng B, Zheng W, Zhu Y, Lin XY, Xu BH, Chen C. Role of adjuvant chemoradiotherapy in treatment of resectable esophageal carcinoma: a meta-analysis. Chinese medical journal. 2013;126:1178-1182
25. Coia LR, Engstrom PF, Paul AR, Stafford PM, Hanks GE. Long-term results of infusional 5-FU, mitomycin-C and radiation as primary management of esophageal carcinoma. Int J Radiat Oncol Biol Phys. 1991;20:29-36
26. Duan H, Zhang X, Wang FX. et al. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World journal of gastroenterology. 2015;21:5591-5597
27. Hirahara N, Matsubara T, Hayashi H, Takai K, Fujii Y, Tajima Y. Impact of inflammation-based prognostic score on survival after curative thoracoscopic esophagectomy for esophageal cancer. Eur J Surg Oncol. 2015;41:1308-1315
28. Han LH, Jia YB, Song QX, Wang JB, Wang NN, Cheng YF. Prognostic significance of preoperative lymphocyte-monocyte ratio in patients with resectable esophageal squamous cell carcinoma. Asian Pacific journal of cancer prevention. 2015;16:2245-2250
29. Dexter SP, Sue-Ling H, McMahon MJ, Quirke P, Mapstone N, Martin IG. Circumferential resection margin involvement: an independent predictor of survival following surgery for oesophageal cancer. Gut. 2001;48:667-670
Author contact
![]() Corresponding authors: Dr. Xiance Jin, PhD., Department of Radiotherapy and Chemotherapy, The First Affiliated Hospital of Wenzhou Medical University, No.2 Fuxue Lane, Wenzhou, China, 325000. Phone: (0086)15957704166, Fax: 0086-577-55578999-664166; E-mail: jinxc1979com and Dr. Congying Xie, PhD, Department of Radiotherapy and Chemotherapy, The First Affiliated Hospital of Wenzhou Medical University, No.2 Fuxue Lane, Wenzhou, China, 325000. Phone: (0086)13867711881; Fax: 0086-577-55578999-611881; E-mail: wzxiecongyingcom
Corresponding authors: Dr. Xiance Jin, PhD., Department of Radiotherapy and Chemotherapy, The First Affiliated Hospital of Wenzhou Medical University, No.2 Fuxue Lane, Wenzhou, China, 325000. Phone: (0086)15957704166, Fax: 0086-577-55578999-664166; E-mail: jinxc1979com and Dr. Congying Xie, PhD, Department of Radiotherapy and Chemotherapy, The First Affiliated Hospital of Wenzhou Medical University, No.2 Fuxue Lane, Wenzhou, China, 325000. Phone: (0086)13867711881; Fax: 0086-577-55578999-611881; E-mail: wzxiecongyingcom

 Global reach, higher impact
Global reach, higher impact