Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(10):1927-1934. doi:10.7150/jca.17930 This issue Cite
Research Paper
A critical reappraisal for the value of tumor size as a prognostic variable in rectal adenocarcinoma
1. Department of Colorectal Surgery, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
2. Department of General Surgery, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
3. Institute of Toxicology, College of Medicine, National Taiwan University, Taipei, Taiwan
4. Department of Radiation Oncology, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
5. Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
6. Department of Biotechnology, Hungkuang University, Taichung, Taiwan
Received 2016-10-15; Accepted 2017-2-19; Published 2017-7-4
Abstract
Background and Objectives: To investigate critical prognostic factors for local recurrence in patients with rectal adenocarcinoma.
Methods: We enrolled 221 consecutive patients who had histologically confirmed adenocarcinoma of the rectum and underwent surgery in our hospital between January 2000 and December 2014. Total mesorectal excision was performed in all patients undergoing a sphincter-sparing procedure or abdominal perineal resection of rectal cancer. To evaluate prognostic factors for local recurrence, we performed univariate and multivariate Cox regression analyses of the local recurrence rate in all patients. Overall survival rates were calculated using the Kaplan-Meier method, and Kaplan-Meier survival curves were compared using the log-rank test.
Results: After the inclusion of only model variables of local recurrence with the highest or lowest univariate risk, a tumor size of <5 cm, a negative circumferential margin, well-to-moderately differentiated adenocarcinoma, low anterior resection, not receiving adjuvant RT, pathological T1-T3 stages, and upper- and middle-third rectal cancers were identified as strong prognostic factors with hazard ratios of 0.18, 0.20, 0.03, 0.01, 0.25, 0.18 and 0.18, respectively (95% confidence intervals [CIs], 0.06-0.58, 0.05-0.82, 0.03-0.38, 0.04-0.23, 0.05-0.64,0.09-0.70 and 0.06-0.54, respectively). After the multivariate Cox regression analysis of the local recurrence rate, a pathological tumor size of ≥5 cm was identified as the only prognostic risk factor (95% CI, 0.03-0.66; P = 0.013). The 5-year local recurrence rates among the patients having tumors measuring <5 cm and ≥5 cm in size were 1.40% and 23.00%, respectively (log-rank, P = 0.0001). The 5-year overall survival rates in the patients having tumors measuring <5 cm and ≥5 cm in size were 82.60% and 71.20%, respectively (log-rank, P = 0.001).
Conclusion: A pathological tumor size of ≥5 cm is an independent prognostic factor for local recurrence in rectal adenocarcinoma.
Keywords: rectal adenocarcinoma, total mesorectal excision, local recurrence
Introduction
In Taiwan, mortality from colorectal cancer (CRC) has consistently increased over the years [1]. Surgical resection is the primary treatment modality for CRC, and the most powerful method for assessing prognosis following a potentially curative surgery is the pathological analysis of the resected specimen. However, although colon and rectal cancers share many features, some crucial differences are present between these two cancers, which include the tendency of rectal cancer (but not of colon cancer) to recur locally [2, 3]. Local recurrence of rectal cancer is common (15%-45%) after a standard surgery and is often catastrophic [2-5]. Moreover, local recurrence is difficult to cure, and the associated symptoms are debilitating. Therefore, preventing or predicting local recurrence is one of the main goals in rectal cancer treatment.
Most rectal tumors are carcinomas [6]. Other histological types such as neuroendocrine neoplasms, hamartomas, mesenchymal tumors, and lymphomas are relatively unusual. Of the carcinomas, >90% are adenocarcinomas [6, 7]. However, most studies have included colon and rectal cancers with mixed pathological types instead of the same anatomical site or all adenocarcinomas [7-9]. Until now, the most powerful method for assessing prognosis following a potentially curative surgery for CRC is the pathological analysis of the resected specimen [7, 10, 11]. Although parameters that determine the pathological stage are the strongest predictors of postoperative outcomes, other clinical and histological features may influence the prognosis regardless of the stage [11, 12]. The actual prognostic factors of local recurrence in rectal adenocarcinoma following a surgery cannot be adequately extrapolated. In this study, we recruited Asian patients with rectal adenocarcinoma who underwent surgery.
The assessment of prognosis in patients with rectal adenocarcinoma is crucial with respect to surveillance and selection of neoadjuvant or adjuvant therapy [13-16]. Therefore, this study investigated critical prognostic factors for local recurrence in patients with rectal adenocarcinoma.
Patients and Methods
Study Patients
We enrolled 221 consecutive patients who had histologically confirmed adenocarcinoma of the rectum and underwent surgery at the Taipei Medical University-Wan Fang Hospital between January 2000 and December 2014. All enrolled patients were Taiwanese (Asian population). In addition, we included patients who received neoadjuvant treatments such as neoadjuvant concurrent chemoradiotherapy (CCRT; n = 31). Inclusion criteria comprised undergoing neoadjuvant CCRT for rectal adenocarcinoma, receiving a diagnosis of stage II or III rectal adenocarcinoma, and having tumors with a large circumference (>1/2). After rectal surgery, the mean number of total harvested lymph nodes was 18 (standard deviation [SD], 9). The mean follow-up period was 80 months (SD, 37 months). Clinical and pathological data were reviewed to evaluate prognostic factors for the local recurrence of rectal adenocarcinoma. Adjuvant radiotherapy (RT) and chemotherapy (CT) are indicated for pT3, pT4, or lymph node-positive rectal cancer in our hospital [13]. Upper-, middle-, and lower-third rectal adenocarcinomas were defined as the tumor margins 11-15 cm from, 6-10 cm from, and within 5 cm of the anal verge, respectively, as measured through rigid sigmoidoscopy. All tumor sizes in our study were measured by professional pathologists by using the three-dimensional maximal diameter, and not only the horizontal or vertical tumor extent. Our protocols were reviewed and approved by the institutional review board at our hospital (TMU-JIRB No. 201503041).
Surgery and Follow-up
Total mesorectal excision (TME) was performed in all patients undergoing a sphincter-sparing procedure or an abdominal perineal resection (APR) of rectal cancer. TME included high ligation of the inferior mesentery artery and vein; mobilization of the sigmoid colon, descending colon, or splenic flexure; and mobilization of the rectum through sharp dissection with diathermy or scissors under direct vision in the avascular plane between the visceral fascia of the mesorectum and the parietal fascia of the pelvis, as described by Heald et al. [17].
Pathological staging of the disease was performed according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 6th edition. Following the surgery, all patients were enrolled in a surveillance program designed to detect local recurrence and distant disease. Clinic visits were scheduled every 3 months for the first 2 years and then at 6-month intervals for 3 years. At each visit, pelvic examination was performed and the carcinoembryonic antigen level was measured. Abdominal ultrasound or computed tomography was performed every 6 months. Colonoscopy was performed after 1 and 3 years. If the patients did not follow-up at our outpatient department, we contacted them by telephone or mail. Any symptom potentially related to local tumor recurrence was investigated through digital rectal examination, colonoscopy, and computed tomography or magnetic resonance imaging. Recurrence was confirmed through biopsy.
Statistical Analysis
The primary endpoint of the study was confirmation of local recurrence. Patients lost to follow-up were censored from the time of last follow-up. Patients with confirmed recurrence (confirmed by pathological findings) were compared with those without confirmed recurrence. Continuous variables were expressed as medians (ranges) and compared using the Mann-Whitney U test, whereas categorical variables (percentages) were compared using the chi-squared test or Fisher exact test, when indicated. Pearson's correlation analysis was performed to determine whether the pathological tumor size is useful for predicting the local recurrence of rectal cancer, and receiver operating characteristic (ROC) curve analysis was conducted to confirm the predictive value of factors identified as predictors of local recurrence. Multivariate analysis was performed using Cox regression analysis for long-term follow-up (different time, censored data), with only model variables having the highest or lowest (P < 0.05) univariate risk being included. Statistical significance was defined as P < 0.05, whereas results were described with a hazard ratio (HR) and 95% confidence interval (CI). All P values were two-tailed. The cumulative proportion of local recurrence and survival rate was calculated using the Kaplan-Meier method. Kaplan-Meier survival curves were compared using the log-rank test. Statistical analyses were performed using SPSS, Version 13.0, for Windows (SPSS Inc., Chicago, IL).
Characteristics of patients with rectal cancer with or without local recurrence following surgery
| Local Recurrence (n, %) | No Recurrence (n, %) | P value | |
|---|---|---|---|
| Men | 8 (6.20%) | 121 (93.80%) | 0.788 |
| Women | 7 (6.25%) | 85 (93.75%) | |
| Age ≤ 65 years | 7 (7.22%) | 90 (92.78%) | 1.00 |
| Age > 65 years | 8 (6.45%) | 116 (93.55%) | |
| Pathological stage I | 0 (0.00%) | 48 (100%) | 0.063 |
| Pathological stage II | 7 (9.86%) | 64 (90.14%) | |
| Pathological stage III | 8 (7.84%) | 93 (92.16%) | |
| Adjuvant CT (-) | 6 (5.36%) | 106 (94.64%) | 0.433 |
| Adjuvant CT (+) | 9 (8.26%) | 100 (91.74%) | |
| Neoadjuvant CCRT (-) | 13 (6.46%) | 178 (93.54%) | 0.243 |
| Neoadjuvant CCRT (+) | 3 (9.68%) | 28 (90/32%) | |
| Pathological tumor size < 5 cm | 4 (2.78%) | 139 (97.22%) | 0.003 |
| Pathological tumor size ≥ 5 cm | 11 (14.10%) | 67 (85.90%) | |
| Adjuvant RT (-) | 9 (4.46%) | 193 (95.57%) | 0.001 |
| Adjuvant RT (+) | 6 (31.58%) | 13 (68.42%) | |
| APR (-) | 9 (4.46%) | 193 (95.57%) | 0.001 |
| APR (+) | 6 (31.58%) | 13 (68.42%) | |
| Upper-third rectum | 0 (0.00%) | 56 (100%) | 0.003 |
| Middle-third rectum | 5 (7.65%) | 93 (93.35%) | |
| Lower-third rectum | 10 (14.93%) | 57 (85.07%) | |
| WD adenocarcinoma | 2 (4.65%) | 41 (95.35%) | 0.046 |
| MD adenocarcinoma | 12 (6.90%) | 162 (93.10%) | |
| PD adenocarcinoma | 1 (50.00%) | 1 (50.00%) | |
| LVI negative | 6 (4.58%) | 125 (95.42%) | 0.150 |
| LVI positive | 8 (10.39%) | 69 (89.61%) | |
| CRM negative | 10 (5.00%) | 190 (95.00%) | 0.052 |
| CRM positive | 6 (28.57%) | 15 (71.43%) | |
| Pathological T1-3 | 12 (5.71%) | 198 (94.29%) | 0.016 |
| Pathological T4 | 3 (27.27%) | 8 (72.73%) | |
| Pathological N0 | 9 (7.14%) | 117 (92.86%) | 1.0 |
| Pathological N1-2 | 6 (6.32%) | 89 (93.68%) |
APR, abdominal perineal resection; RT, radiotherapy; CT, chemotherapy; CRM: circumferential margin; WD, well-differentiated; MD, moderately differentiated; PD, poorly differentiated; LVI, lymphatic vascular invasion; CCRT, concurrent chemoradiotherapy
Results
We enrolled 221 patients with rectal adenocarcinoma. The characteristics of these patients with or without local recurrence following surgery are presented in Table 1. Of the 221 patients, 129 were men and 92 were women. The mean age of the patients was 66 years (SD, 12 years; range, 30-95 years). No significant difference was observed in age, sex, pathological AJCC stages, open surgery, laparoscopic surgery, neoadjuvant CCRT, adjuvant CT, pathological stages, pathological N stages, lymphatic vascular invasion, or circumferential margins (CRMs) between the two groups (Table 1). Moreover, all distal surgical margins in the study were free, and the mean margin distance from the distal edge of the tumor was 2.27 cm (SD, 1.52 cm). In the ROC curve analysis, the sensitivity and specificity of the tumor size (cutoff, 5 cm) were 73.30% and 79.10%, respectively; the area under the curve was 0.79 (Supplemental Figure 1). Local recurrence was significantly higher in patients having tumors measuring ≥5 cm in size, having lower-third rectal cancers, having poorly differentiated adenocarcinoma, having pathological T4 stage, receiving adjuvant RT, and undergoing APR. Specifically, the local recurrence rates observed in the patients having tumors measuring ≥5 cm in size, receiving adjuvant RT, undergoing APR, having lower-third rectal cancers, having poorly differentiated adenocarcinoma, and having pathological T4 stage were 21.43%, 14.10%, 31.58%, 31.58%, 14.93%, 50.00%, and 27.27%, respectively. In addition, the characteristics of patients with different pathological tumor sizes following surgery are presented in Table 2. As indicated in Table 2, the proportion of patients undergoing APR (68.42%), having a positive CRM (66.67%), and having pathological T4 stage (72.73%) was significantly higher among the patients having tumors measuring ≥5 cm in size. To examine prognostic factors for local recurrence, we performed a univariate Cox regression analysis of the local recurrence rate in the patients with rectal adenocarcinoma (Table 3). After including only model variables of local recurrence having the highest or lowest univariate risk, we observed that a tumor size of <5 cm, a negative CRM, well-to-moderately differentiated adenocarcinoma, low anterior resection (LAR), not receiving adjuvant RT, and upper- and middle-third rectal cancers were strong prognostic factors. In the univariate analysis, the HRs of local recurrence for a tumor size of <5 cm, a negative CRM, well-to-moderately differentiated adenocarcinoma, LAR, not receiving adjuvant RT, pathological T1-T3 stages, and upper- and middle-third rectal cancers were 0.18, 0.20, 0.03, 0.01, 0.25, 0.18, and 0.18, respectively (95% CI, 0.06-0.58, 0.05-0.82, 0.03-0.38, 0.04-0.23, 0.05-0.64, 0.09-0.70, and 0.06-0.54, respectively; Table 3). However, after the execution of a multivariate Cox regression analysis of the local recurrence rate in the patients with rectal cancer, a pathological tumor size of ≥5 cm was identified as the only prognostic risk factor (Table 4). The HR of local recurrence for a pathological tumor size of <5 cm was 0.14 (95% CI, 0.03-0.66; P = 0.013).
Characteristics of patients with rectal cancer having different pathological tumor sizes following surgery
| Pathological tumor size < 5 cm (n, %) | Pathological tumor size ≥ 5 cm (n, %) | P value | |
|---|---|---|---|
| Upper-third rectum | 40 (75.47%) | 16 (24.53%) | 0.368 |
| Middle-third rectum | 50 (56.18%) | 39 (43.82%) | |
| Lower-third rectum | 44 (65.67%) | 23 (34.33%) | |
| Adjuvant CT (-) | 71 (63.39%) | 41 (36.61%) | 0.778 |
| Adjuvant CT (+) | 72 (66.06%) | 37 (33.94%) | |
| Sphincter-saving procedure | 137 (67.82%) | 65 (32.18%) | 0.002 |
| APR | 6 (31.58%) | 13 (68.42%) | |
| Adjuvant RT (-) | 110 (59.17%) | 59 (40.83%) | 0.869 |
| Adjuvant RT (+) | 33 (63.46%) | 19 (36.54%) | |
| CRM negative | 112 (56.00%) | 88 (44.00%) | 0.012 |
| CRM positive | 7 (33.33%) | 14 (66.67%) | |
| Neoadjuvant CCRT (-) | 122 (64.21%) | 68 (35.79%) | 0.288 |
| Neoadjuvant CCRT (+) | 21 (63.74%) | 10 (32.2%) | |
| Pathological T1-3 | 140 (66.67%) | 70 (33.33%) | 0.001 |
| Pathological T4 | 3 (27.27%) | 8 (72.73%) | |
| Pathological N0 | 81 (64.29%) | 45 (35.71%) | 0.888 |
| Pathological N1-2 | 62 (65.26%) | 33 (34.74%) | |
| WD adenocarcinoma | 31 (72.09%) | 12 (27.91%) | 0.100 |
| MD adenocarcinoma | 111 (63.79%) | 63 (36.21%) | |
| PD adenocarcinoma | 0 (0.00%) | 2 (100.00%) | |
| LVI negative | 85 (64.89%) | 46 (35.11%) | 0.763 |
| LVI positive | 52 (67.53%) | 25 (32.47%) |
APR, abdominal perineal resection; RT, radiotherapy; CT, chemotherapy; CRM: circumferential margin; WD, well-differentiated; MD, moderately differentiated; PD, poorly differentiated; LVI, lymphatic vascular invasion; CCRT, concurrent chemoradiotherapy
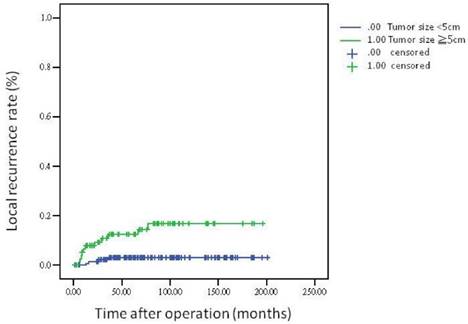
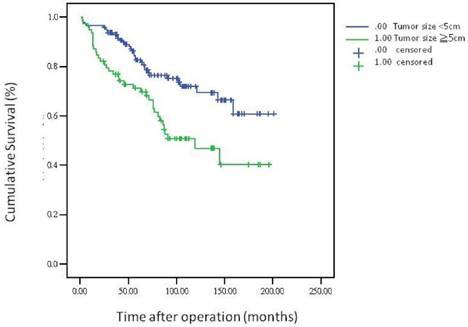
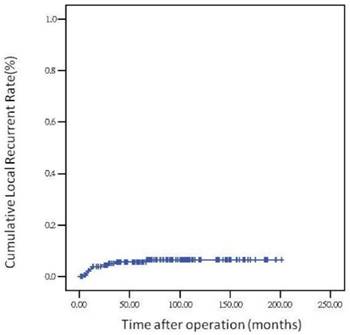
The 5-year overall survival rate, disease-free survival rate, and local recurrence rate among the patients with rectal cancer were 79.90%, 82.91%, and 6.79%, respectively. The cumulative curve of local recurrence among patients having tumors measuring ≥5 cm in size was steep within the first 2 years after surgery, almost reached a plateau after 2 years, and remained unchanged after 75 months (Figure 1). In addition, the cumulative proportion of overall survival among patients having tumors measuring <5 cm or ≥5 cm in size was calculated using the Kaplan-Meier method, and Kaplan-Meier survival curves were compared using the log-rank test (Figure 2). As presented in Figure 2, the 5-year overall survival rates among the patients having tumors measuring <5 cm and ≥5 cm in size were 82.60% and 71.20%, respectively (log-rank, P = 0.001), and the 5-year local recurrence rates among these patients were 1.40% and 23.00%, respectively (log-rank, P = 0.0001). Furthermore, as illustrated in Figure 3, the cumulative curve of local recurrence among all the patients with rectal cancer exhibited a trend similar to the curve in Figure 1. No local recurrence was observed for 75 months after surgery.
Univariate Cox regression analysis of local recurrence rate in patients with rectal cancer
| HR | 95% CI | P value | |
|---|---|---|---|
| Pathological tumor size < 5cm (RG: ≥5 cm) | 0.18 | 0.06-0.58 | 0.004 |
| CRM negative (RG: CRM positive) | 0.20 | 0.05-0.82 | 0.025 |
| WD adenocarcinoma (RG: PD adenocarcinoma) | 0.03 | 0.03-0.38 | 0.006 |
| MD adenocarcinoma (RG: PD adenocarcinoma) | 0.05 | 0.01-0.41 | 0.005 |
| LVI negative (RG: LVI positive) | 0.40 | 0.14-1.17 | 0.094 |
| LAR (RG: APR) | 0.01 | 0.04-0.23 | 0.001 |
| Pathological T1-3 (RG: pathological T4) | 0.18 | 0.05-0.64 | 0.008 |
| Pathological N0 (RG: pathological N1-2) | 1.02 | 0.36-2.86 | 0.975 |
| No adjuvant RT (RG: adjuvant RT) | 0.25 | 0.09-0.70 | 0.008 |
| No adjuvant CT (RG: adjuvant CT) | 0.64 | 0.22-1.79 | 0.394 |
| No neoadjuvant CCRT (RG: neoadjuvant CCRT) | 0.62 | 0.17-2.20 | 0.458 |
| Age ≤ 65 years (RG: >65 years) | 1.07 | 0.39-2.96 | 0.892 |
| Men (RG: Women) | 0.83 | 0.30-2.30 | 0.712 |
| Upper- and middle-third rectum (RG: lower-third rectum) | 0.18 | 0.06-0.54 | 0.002 |
HR, hazard ratio; CI, confidence interval; RG, reference group; RT, radiotherapy; CT, chemotherapy; APR, abdominal perineal resection; LAR, lower anterior resection; CRM: circumferential margin; WD, well-differentiated; MD, moderately differentiated; PD, poorly differentiated; LVI, lymphatic vascular invasion; CCRT, concurrent chemoradiotherapy
Multivariate Cox regression analysis of local recurrence rate in patients with rectal cancer
| HR | 95% CI | P value | |
|---|---|---|---|
| Men versus Women | 1.83 | 0.48-5.02 | 0.371 |
| Age ≤ 65 years | 0.89 | 0.22-3.67 | 0.871 |
| Pathological tumor size < 5 cm | 0.14 | 0.03-0.66 | 0.013 |
| Upper- and middle-third rectum | 0.41 | 0.08-2.23 | 0.304 |
| LAR | 0.34 | 0.05-2.46 | 0.323 |
| No adjuvant RT | 0.33 | 0.08-1.47 | 0.147 |
| No adjuvant CT | 1.96 | 0.45-8.62 | 0.372 |
| No neoadjuvant CCRT | 0.35 | 0.04-2.84 | 0.323 |
| Pathologic T1-3 | 0.34 | 0.05-2.23 | 0.260 |
| LVI negative | 0.24 | 0.05-7.11 | 0.068 |
| CRM negative | 0.31 | 0.09-2.45 | 0.342 |
| WD adenocarcinoma | 0.37 | 0.02-10.15 | 0.558 |
| MD adenocarcinoma | 0.28 | 0.02-4.79 | 0.381 |
HR, hazard ratio; CI, confidence interval; RT, radiotherapy; CT, chemotherapy; APR, abdominal perineal resection; LAR, lower anterior resection; CCRT, concurrent chemoradiotherapy
Local recurrence rate according to tumor size
Overall survival according to tumor size
Local recurrence rate over time
Discussion
The tumor-node-metastasis (TNM) staging system of the AJCC and Union for International Cancer Control is the preferred staging system for CRC [18]. In general, the most critical indicator of outcomes after CRC resection is the pathological AJCC stage at presentation [18]. The 5-year survival rates stratified by tumor stage for rectal cancer by using the AJCC staging criteria differ significantly; nonetheless, the AJCC stage is not a prognostic factor for local recurrence [18]. Notably, the extent of tumor invasion, but not tumor size, is considered in the AJCC pathological staging system [18]. Among the various factors that have been thoroughly studied for CRC, tumor size [19-21] and gross tumor configuration [20, 22] have been determined to not exert a significant impact on prognosis. However, Kornprat et al. suggested that tumor size can be an adverse prognostic factor for colon cancer but not for rectal cancer [23]. They also reported that an overall tumor size of >4.5 cm was an independent predictor of poor outcomes; nevertheless, the optimal cutoff point for size indicative of adverse prognosis varied with the anatomical location in the colon, decreasing from the right to the left [23]. Nevertheless, the study of Kornprat et al. included a smaller sample size of patients with rectal cancer (n = 146) than that in our study (n = 221), and the number of patients with rectal cancer having tumors measuring ≥5 cm in size was unclear in their study. Moreover, the total number of patients with colon and rectal cancers having tumors measuring ≥5 cm in size in their study was lower than that in our study (n = 37). Therefore, the cutoff value for tumor size in their study was 3.4 cm. The optimal cutoff value with respect to local recurrence prediction appeared to vary among patients with rectal adenocarcinoma. All data in the study of Kornprat et al. were obtained from the CRC Database of the Institute of Pathology, Medical University of Graz, Graz, Austria.
Because data regarding the pathological type were not available in the database, the study of Kornprat et al. may have included other pathological types, such as neuroendocrine neoplasms, hamartomas, mesenchymal tumors, and lymphomas, in their rectal cancers, instead of including only rectal adenocarcinoma. Furthermore, they did not provide details regarding surgical procedures; local control and survival could have been affected by different surgical procedures [24-26]. In our study, TME was performed in all the patients with rectal adenocarcinoma. TME has been associated with improved local control and survival rates [27-30]. The local recurrence rate following the inclusion of a TME process with an APR or a sphincter-sparing procedure ranged from 4% to 7% [27-30], which is very similar to the local recurrence rate in our study (6.79%). Kornprat et al. did not report on the local recurrence rate because their endpoint was cancer-specific survival and progression-free survival. Taken together, the findings of Kornprat et al. indicate that tumor size may be an adverse prognostic factor for colon cancer but not for rectal cancer. In our study, a pathological tumor size of ≥5 cm was identified to be an independent prognostic factor for local recurrence in patients with rectal adenocarcinoma who underwent TME. To the best of our knowledge, this study used the largest sample size of patients with rectal adenocarcinoma who underwent TME and is the first to demonstrate a pathological tumor size of ≥5 cm as an independent prognostic factor for local recurrence in patients with rectal adenocarcinoma who underwent TME.
According to the TNM classification, the T stage reflects vertical tumor penetration within or beyond the bowel wall, whereas data regarding the biological significance of tumor extent, obtained by measuring the maximum tumor diameter, are scant and contradictory [18]. Our data indicate a significant association of tumor size with the T classification and the AJCC stage instead of the N stage (Table 2). We assumed that a narrow pelvic space having extensive peritoneal covering of the rectum might act as an internal stress for a large-size tumor that consequently leads to high microscopic tumor seeding to distal areas, and its removal through surgery can be difficult [31, 32]. Theoretically, a surgeon should remove 3-5 cm of the mesorectum beyond the primary tumor in the TME procedure [33]. No tumor implants were observed beyond 4 cm from the distal edge of the tumor within the mesorectum [34, 35]. Moreover, no tumor implants were observed beyond 1 cm of the tumor in patients with T1 or T2 lesions [35]. However, the optimal distal edge for tumors measuring ≥5 cm in size remains unclear. In our study, all margins were free, and the mean margin of 2.27 cm from the distal edge of the tumor might not be adequate for rectal adenocarcinomas measuring ≥5 cm in size. We determined that a pathological tumor size of ≥5 cm is an independent prognostic factor for local recurrence in rectal adenocarcinoma. Therefore, tumor size should be considered in the AJCC staging system for more effective estimation of rectal cancer outcomes. In addition, evaluating the optimal distal edge for tumors measuring ≥5 cm in size is crucial in the future.
Regional lymph node involvement is one of the strongest predictors of outcomes following surgical resection of CRC, second only to distant metastasis. Nodal spread is an indicator for adjuvant therapy for both colon and rectal cancers. Regarding nodal disease, Adachi et al. reported a high number of positive nodes in patients with CRC measuring >6 cm in size (42% vs. 22%) [36]. However, they reported that tumor size was not an independent predictor of local lymphatic spread. These findings are compatible with those of our study (Table 2). The only difference between their and our data is that our data are specific to rectal adenocarcinoma. Adequate lymph nodes were harvested in our study, and the mean number of harvested lymph nodes was 18. We did not observe tumor size to be an independent predictor of local lymphatic spread.
As presented in Table 3, the HRs of local recurrence for a tumor size of <5 cm, a negative CRM, well-to-moderately differentiated adenocarcinoma, LAR, not receiving adjuvant RT, pathological T1-T3 stages, and upper- and middle-third rectal cancers were 0.18, 0.20, 0.03, 0.01, 0.25, 0.18 and 0.18, respectively (95% CI, 0.06-0.58, 0.05-0.82, 0.03-0.38, 0.04-0.23, 0.05-0.64,0.09-0.70 and 0.06-0.54, respectively; Table 3). However, after the execution of a multivariate Cox regression analysis of the local recurrence rate in the patients with rectal cancer, a pathological tumor size of ≥5 cm was identified as the only prognostic risk factor (Table 4). Moreover, the HR of local recurrence for a pathological tumor size of <5 cm was 0.14 (95% CI, 0.03-0.66; P = 0.013). The variables of LAR, CRM, differentiated tumors, adjuvant RT, pathological T stages, and lower-third rectal cancers had selection bias in the retrospective data. After the multivariate analysis, only the pathological tumor size of ≥5 cm was identified as an independent predictor. In clinical practice, 68.42% of patients with rectal adenocarcinoma receive APR when the tumor size is ≥5 cm (Table 2). The sphincter-sparing rate was low in the group of patients having tumors measuring ≥5 cm in size (Table 2). A high number of patients with rectal adenocarcinoma measuring ≥5 cm had a positive CRM (66.67%; Table 2). In rectal cancer, the quality of the surgical technique and the status of the CRM are the most crucial predictive factors for both local and distant recurrence as well as survival [25, 37, 38]. In our study, TME was performed with adequate surgical clearance around the penetrating edge of the tumor, which reduced the rate of local relapse. With this approach, all mesorectal soft tissues encasing the rectum, including the mesentery and all regional lymph nodes, can be removed intact, and the circumferential surface is the mesorectal fascia. However, the sample size of the patients with a positive CRM in our study was small; thus, we could not make a scientific conclusion. In addition, CRM positivity is used as an indicator for adjuvant RT or CT in our hospital, regardless of the local tumor extent, particularly for rectal cancer. Adjuvant treatments might also mask the effect of local recurrence observed for CRM-positive patients after the multivariate analysis. In this study, only 10 patients had a large tumor size (≥5 cm) and received neoadjuvant CCRT (Table 2). According to our previous study, the survival rate, disease-free survival rate, and sphincter-sparing rate in patients with rectal cancer improved after the addition of neoadjuvant CCRT [13]. Therefore, we suggest that neoadjuvant CCRT can be beneficial for patients with rectal cancer having large-size tumors, and local control and overall survival following neoadjuvant CCRT might be promising in future clinical trials.
Regarding the recurrence pattern, we observed that more than 80% of patients with local recurrence of rectal adenocarcinoma underwent TME within 2 years (Figures 1 and 3). However, we did not observe local recurrence after 75 months. This phenomenon implies that physicians should closely follow-up patients with rectal cancer for recurrence within the first 2 years; the recurrence rate would reach a plateau after 75 months. In addition, we calculated the cumulative proportion of the overall survival rate in the patients having tumors measuring <5 cm or ≥5 cm in size by using the Kaplan-Meier method, and Kaplan-Meier survival curves were compared using the log-rank test (Figure 2). As presented in Figure 2, the 5-year overall survival rates among the patients having tumors measuring <5 cm and ≥5 cm in size were 82.60% and 71.20%, respectively (log-rank, P = 0.001). In our experience, local recurrence resulted in difficulty in re-resection and local infection induced irreversible sepsis, causing death. Thus, aggressive treatment for rectal adenocarcinoma measuring ≥5 cm in size is warranted for the prevention of local recurrence.
Conclusion
A pathological tumor size of ≥5 cm is an independent prognostic factor for local recurrence in rectal adenocarcinoma. More than 80% of patients with rectal adenocarcinoma having local recurrence underwent TME within 2 years. Furthermore, local recurrence of rectal adenocarcinoma requiring TME was not observed after 75 months.
Supplementary Material
Supplementary figure 1.
Abbreviations
CRC, colorectal cancer; RT, radiotherapy; CT, chemotherapy; CCRT, concurrent chemoradiotherapy; LAR, low anterior resection; TME, total mesorectal excision; AJCC, American Joint Committee on Cancer; CRM, circumferential margin.
Competing Interests
The authors have declared that no competing interests exist.
References
1. Su SY, Huang JY, Jian ZH, Ho CC, Lung CC, Liaw YP. Mortality of colorectal cancer in Taiwan, 1971-2010: temporal changes and age-period-cohort analysis. International journal of colorectal disease. 2012;27:1665-72
2. Larsen SG, Wiig JN, Tretli S, Giercksky KE. Surgery and pre-operative irradiation for locally advanced or recurrent rectal cancer in patients over 75 years of age. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2006;8:177-85
3. Koyama Y, Moriya Y, Hojo K. Effects of extended systematic lymphadenectomy for adenocarcinoma of the rectum-significant improvement of survival rate and decrease of local recurrence. Japanese journal of clinical oncology. 1984;14:623-32
4. Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:5644-50
5. Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Annals of surgical oncology. 2007;14:447-54
6. Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE. et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47:141-6
7. Knox RD, Luey N, Sioson L, Kedziora A, Clarkson A, Watson N. et al. Medullary colorectal carcinoma revisited: a clinical and pathological study of 102 cases. Annals of surgical oncology. 2015;22:2988-96
8. Secco GB, Fardelli R, Campora E, Lapertosa G, Gentile R, Zoli S. et al. Primary mucinous adenocarcinomas and signet-ring cell carcinomas of colon and rectum. Oncology. 1994;51:30-4
9. Minsky BD, Mies C, Rich TA, Recht A, Chaffey JT. Colloid carcinoma of the colon and rectum. Cancer. 1987;60:3103-12
10. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR. et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Archives of pathology & laboratory medicine. 2000;124:979-94
11. Griffin MR, Bergstralh EJ, Coffey RJ, Beart RW Jr, Melton LJ 3rd. Predictors of survival after curative resection of carcinoma of the colon and rectum. Cancer. 1987;60:2318-24
12. Katoh H, Yamashita K, Wang G, Sato T, Nakamura T, Watanabe M. Prognostic significance of preoperative bowel obstruction in stage III colorectal cancer. Annals of surgical oncology. 2011;18:2432-41
13. Chen CH, Wei PL, Hsieh MC, Lin EK, Chiou JF, Lu YJ. et al. The outcomes of therapeutic decision in lower 3rd rectal cancer patients. Medicine (Baltimore). 2016;95:e4638
14. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C. et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:1926-33
15. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R. et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. The New England journal of medicine. 2004;351:1731-40
16. Kim TW, Lee JH, Ahn JH, Kang YK, Lee KH, Yu CS. et al. Randomized trial of postoperative adjuvant therapy in Stage II and III rectal cancer to define the optimal sequence of chemotherapy and radiotherapy: 10-year follow-up. International journal of radiation oncology, biology, physics. 2011;81:1025-31
17. Heald RJ. Total mesorectal excision is optimal surgery for rectal cancer: a Scandinavian consensus. The British journal of surgery. 1995;82:1297-9
18. Edge S, Byrd D, Compton C. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 7th edition ed: Springer, New York. 2010
19. Chapuis PH, Dent OF, Fisher R, Newland RC, Pheils MT, Smyth E. et al. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. The British journal of surgery. 1985;72:698-702
20. Newland RC, Dent OF, Lyttle MN, Chapuis PH, Bokey EL. Pathologic determinants of survival associated with colorectal cancer with lymph node metastases. A multivariate analysis of 579 patients. Cancer. 1994;73:2076-82
21. Takahashi Y, Tucker SL, Kitadai Y, Koura AN, Bucana CD, Cleary KR. et al. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg. 1997;132:541-6
22. Michelassi F, Ayala JJ, Balestracci T, Goldberg R, Chappell R, Block GE. Verification of a new clinicopathologic staging system for colorectal adenocarcinoma. Annals of surgery. 1991;214:11-8
23. Kornprat P, Pollheimer MJ, Lindtner RA, Schlemmer A, Rehak P, Langner C. Value of tumor size as a prognostic variable in colorectal cancer: a critical reappraisal. American journal of clinical oncology. 2011;34:43-9
24. Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J. et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821-8
25. Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D. et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707-11
26. Cawthorn SJ, Parums DV, Gibbs NM, A'Hern RP, Caffarey SM, Broughton CI. et al. Extent of mesorectal spread and involvement of lateral resection margin as prognostic factors after surgery for rectal cancer. Lancet. 1990;335:1055-9
27. Arbman G, Nilsson E, Hallbook O, Sjodahl R. Local recurrence following total mesorectal excision for rectal cancer. The British journal of surgery. 1996;83:375-9
28. MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457-60
29. Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery-the clue to pelvic recurrence? The British journal of surgery. 1982;69:613-6
30. Maurer CA, Renzulli P, Kull C, Kaser SA, Mazzucchelli L, Ulrich A. et al. The impact of the introduction of total mesorectal excision on local recurrence rate and survival in rectal cancer: long-term results. Annals of surgical oncology. 2011;18:1899-906
31. Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908). CA: a cancer journal for clinicians. 1971;21:361-4
32. Rouanet P, Mourregot A, Azar CC, Carrere S, Gutowski M, Quenet F. et al. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Diseases of the colon and rectum. 2013;56:408-15
33. Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J. et al. Guidelines 2000 for colon and rectal cancer surgery. Journal of the National Cancer Institute. 2001;93:583-96
34. Scott N, Jackson P, al-Jaberi T, Dixon MF, Quirke P, Finan PJ. Total mesorectal excision and local recurrence: a study of tumour spread in the mesorectum distal to rectal cancer. The British journal of surgery. 1995;82:1031-3
35. Hida J, Yasutomi M, Maruyama T, Fujimoto K, Uchida T, Okuno K. Lymph node metastases detected in the mesorectum distal to carcinoma of the rectum by the clearing method: justification of total mesorectal excision. Journal of the American College of Surgeons. 1997;184:584-8
36. Adachi Y, Yasuda K, Kakisako K, Sato K, Shiraishi N, Kitano S. Histopathologic criteria for local excision of colorectal cancer: multivariate analysis. Annals of surgical oncology. 1999;6:385-8
37. Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:303-12
38. Gosens MJ, Klaassen RA, Tan-Go I, Rutten HJ, Martijn H, van den Brule AJ. et al. Circumferential margin involvement is the crucial prognostic factor after multimodality treatment in patients with locally advanced rectal carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:6617-23
Author contact
![]() Corresponding author: Szu-Yuan Wu, M.D., M.P.H., Ph.D., Attending Physician, Department of Radiation Oncology, Taipei Medical University - Wan Fang Medical Center, NO.111, Section 3, Hsing-Long Rd, Taipei 116, Taiwan, R.O.C. E-mail: szuyuanwu5399com
Corresponding author: Szu-Yuan Wu, M.D., M.P.H., Ph.D., Attending Physician, Department of Radiation Oncology, Taipei Medical University - Wan Fang Medical Center, NO.111, Section 3, Hsing-Long Rd, Taipei 116, Taiwan, R.O.C. E-mail: szuyuanwu5399com

 Global reach, higher impact
Global reach, higher impact