Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(10):1769-1773. doi:10.7150/jca.17803 This issue Cite
Short Research Communication
Increased Transgenerational Intestinal Tumorigenesis in Offspring of Ionizing Radiation Exposed Parent APC1638N/+ Mice
1. Department of Biochemistry and Molecular & Cellular Biology and Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC USA
2. Department of Pathology, Georgetown University Medical Center, Washington, DC USA.
Received 2016-10-3; Accepted 2017-2-19; Published 2017-7-1
Abstract
The purpose of the study was to assess transgenerational intestinal tumorigenic effects of low dose ionizing radiation employing a well-characterized mouse model of human colorectal cancer. Mice (6 to 8 weeks old APC1638N/+ mice; n=20 per study group) were exposed to whole-body 25 cGy x-rays and mated 2 days post-irradiation. Intestinal tumorigenesis in male and female F1 mice from No Parents Irradiated (NPI), Both Parents Irradiated (BPI), and Male Parent Irradiated (MPI) groups were compared 210 days after birth. Male and female Direct Parent Irradiated (DPI) groups were additional controls for male and female F1 groups respectively. Data showed higher intestinal tumor frequency (± standard error of the mean) in male and female F1 from BPI (male: 7.81 ± 0.91; female: 5.45 ± 0.36) as well as from MPI (male: 6.30 ± 0.33; female: 4.45 ± 0.33) mice relative to F1 from NPI mice (male: 4.2 ± 0.48; female: 3.35 ± 0.37). Compared to male and female DPI (male: 5.55 ± 0.40; female: 3.60 ± 0.22), tumor frequency in F1 mice of BPI and MPI, though higher, was not statistically significant except for DPI vs. BPI in male mice. Additionally, both BPI and MPI showed increased frequency of larger tumors relative to NPI. In summary, our observations demonstrated that the APC1638N/+ mice due to its low spontaneous tumor frequency could serve as an effective model to study risk of transgenerational carcinogenesis in gastrointestinal tissues after exposure to clinically relevant low doses of ionizing radiation.
Keywords: Mouse model, Transgenerational, Intestinal tumor, Colorectal cancer, Ionizing radiation.
Introduction
It is estimated that low dose radiation exposure from computed tomography (CT) scan is linked to about 2% of all cancers in the USA and with increasing medical imaging procedures, cancer risk could be higher from cumulative radiation dose [1]. Radiation is a risk factor for colorectal cancer (CRC) [2] and CRC has a familial transmission trend [3]. We explored whether parental low dose radiation exposure increased intestinal tumorigenesis in the offspring of a mouse model of human CRC. Radiation even at low doses is known to cause changes in germline cells that may be transmitted to the next generation [4]. Studies in the offspring of Chernobyl accident survivors showed a two-fold increase in germline mutation frequency and high levels of base-pair substitution, which raises the possibility of enhanced transgenerational cancer risk [5]. However, epidemiological data from atom bomb survivors were not conclusive on transgenerational colorectal cancer (CRC) risk [6]. Furthermore, data on transgenerational CRC risk from low dose medical radiation exposures is not available. Therefore, we undertook this study on transgenerational intestinal tumorigenesis to test the hypothesis that parental radiation exposure will lead to increased risk of intestinal tumors in the offspring. We used one (APC1638N/+) of the adenomatous polyposis coli (APC) mutant mouse models, which are known to closely simulate human colorectal carcinogenic processes both with and without radiation exposure [7], to investigate transgenerational effects of low-dose radiation on intestinal tumorigenesis. We believe ours is the first report showing higher frequency of intestinal tumors and of larger tumors in the offspring of x-ray irradiated (25 cGy) parental mice relative to the offspring of sham-irradiated parents.
Materials and methods
Animal housing, breeding, and genotyping
Five mice per cage were housed in a temperature (22 °C) and humidity (50%) controlled room with 12 h dark and light cycle and standard certified rodent diet and filtered water was provided ad libitum. Animal maintenance and experimental procedures were performed in compliance with Georgetown University's Institutional Animal Care and Use Committee (IACUC) approved protocol. We followed the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, National Research Council, and U.S. National Academy of Sciences for our research. Male APC1638N/+ mice in C57BL/6J background were obtained from Mouse Model for Human Cancer Consortium (National Cancer Institute, Frederick, MD), were bred with female C57BL/6J mice, and were genotyped using tail DNA samples as per protocol described previously [8]. Male and female offspring heterozygous for APC were used in the tumorigenesis studies.
Schematic representation of the experimental design. Group I - No Parents Irradiated or NPI, Group II - Both Parents Irradiated or BPI, Group III - Male Parent Irradiated or MPI, Group IV: Direct Parent Irradiated (DPI) male, Group V: Direct Parent Irradiated (DPI) female. While Group I, II, and III were F1 generation from NPI, BPI, and MPI mice, Group IV and V were male and female parents irradiated separately and used as controls.
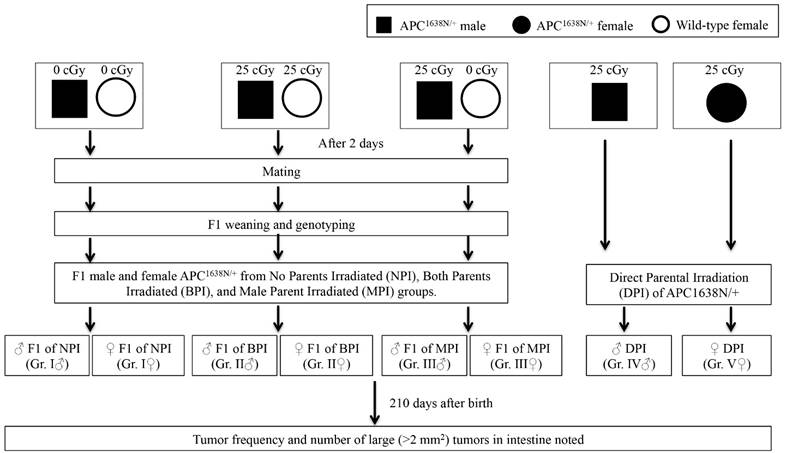
Experimental design and irradiation
Experimental design is schematically presented in Figure 1. Briefly, six to eight weeks old male APC1638N/+ mice and female wild type C57BL/6J mice were exposed to 25 cGy x-rays using a X-RAD 320 irradiator (1.56 cGy/sec, 320 KV, 12.5 mA; Precision x-ray Inc., North Branford, CT, USA) and 48 h post-exposure mice were set up for breeding to obtain F1 generation offspring. The 48 h time point for this first study on radiation-induced transgenerational intestinal tumorigenesis was chosen due to the fact that abnormal spermatogonia count declined and normal spermatogonia count restored in testes ~24 h after low dose radiation exposure [9]. Also, a radiation dose of 25 cGy is at the lower end of the dose spectrum expected to be delivered in testes during radiotherapy of sub-diaphragmatic cancer [10] and is reported to not affect post-exposure paternal sperm quality [9, 11]. Sham irradiated age-matched male APC1638N/+ and female C57BL/6J mice were also set up for breeding and these offspring were used as control. For additional control, six to eight weeks old male and female APC1638N/+ mice exposed to 0.25 cGy x-rays were followed directly along with the F1 mice for tumorigenesis. The F1 mice from No Parents Irradiated (NPI) were Group I, from Both Parents Irradiated (BPI) were Group II, and from Male Parent Irradiated (MPI) were Group III. There were two additional groups - Group IV for male direct parent irradiated (♂ DPI), and Group V for female direct parent irradiated (♀ DPI). Therefore, there were four study groups of male (Group I, II, II, and IV) and four of female (Group I, II, III, and V) mice for comparison. Newborn (F1) male and female offspring were separated and genotyped after weaning, and APC1638N/+ mice were housed under standard conditions described earlier.
Tumor counting
Mice (n=20 per study group) were euthanized as per approved protocol at 210 days of age. Small intestine and colon were surgically removed, and gently flushed using phosphate-buffered saline (PBS). Small intestine was further divided into three sub-sections - duodenum, jejunum, and ilium. All tissue sections were opened longitudinally on a PBS soaked absorbent paper. Small intestinal and colonic tumors were counted under a dissecting scope (Leica MZ6, Buffalo Grove, IL) by three independent observers blinded to the treatments groups as described previously [12]. A built-in scale in the dissecting scope was used to measure tumor size and the number of tumors measuring >2 mm2 in each animal was noted. Tumor data from multiple radiation exposure experiments were pooled to achieve statistical significance.
Statistical analysis
We employed Shapiro-Wilk test to determine normality of data distribution in all the study groups. Using test statistics (p-value of <0.05), skewness and kurtosis measures with standard errors, and histograms), we concluded that the data were not approximately normally distributed. Subsequently, we performed non-paramatric Levene's test to determine equality of variances of the data and test statistics with p-value <0.05 show inequality of variances in the data. Although variances were unequal, we had equal sample size (n=20 mice) per study group and therefore, we performed Welch's one-way analysis of variance (ANOVA) with Games-Howell post hoc test on our data set. The test allowed us to determine statistical significance (p<0.05) of tumor frequency and size among various study groups. Statistical significance of gender differences in tumor frequency was determined using Wilcoxon matched-pairs test, and p<0.05 was considered significant. We used IBM SPSS Statistics for Macintosh v22.0 (IBM Corp., Armonk, NY) for statistical tests. Error bars represent mean ± standard error of the mean.
Results
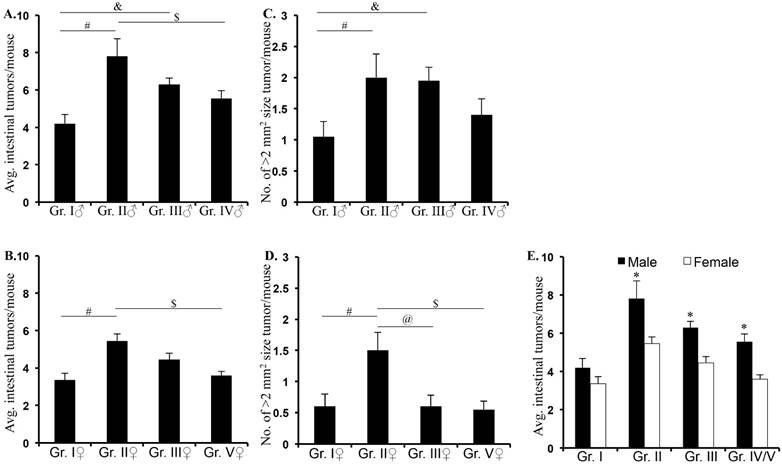
F1 male and female APC1638N/+ mice from Both Parents Irradiated (BPI - male: 7.81 ± 0.91, p<0.0001; female: 5.45 ± 0.36, p<0.001) and Male Parent Irradiated (MPI - male: 6.30 ± 0.33, p<0.004; female: 4.45 ± 0.33, p<0.05) groups displayed significantly higher intestinal tumor frequency (± standard error of the mean) relative to the No Parents Irradiated (NPI; male: 4.20 ± 0.48; female: 3.35 ± 0.37) group (Figure 2A and B; Supplementary table 1). Higher intestinal tumor frequency in BPI and MPI relative to Direct Parent Irradiated (DPI; male: 5.55 ± 0.40; female: 3.60 ± 0.22) APC1638N/+ mice was not statistically significant except for the BPI vs. DPI (p<0.003) in male mice. This suggests that even though offspring are not exposed, they are at risk of developing radiation-related intestinal tumorigenesis. Comparison of frequency of intestinal tumors with >2 mm2 size in male F1 APC1638N/+ mice from BPI (2.0 ± 0.38, p<0.04) and MPI (1.95 ± 0.21, p<0.04) revealed significantly higher number of larger tumors relative to NPI (1.05 ± 0.24; Figure 2C). In female mice, significantly higher number of large tumors was observed in BPI (1.5 ± 0.28) group relative to MPI (0.6 ± 0.18; p<0.02) and NPI (0.6 ± 0.19; p<0.04) groups (Figure 2D). Representative H&E stained images showing tumor size differences in male (Supplementary figure 1) and female (Supplementary figure 2) groups are presented in Supplementary Material. We showed that in male F1, both BPI and MPI and in female F1, only BPI showed higher frequency of large tumor relative to NPI suggesting a gender bias in the contribution of germ cells to tumorignesis. Comparison of tumor frequency in male and female F1 showed significantly greater tumorigenesis in male relative to female mice in BPI and MPI groups (Figure 2E; *denotes p<0.05).
Discussion
Although human and animal studies have linked radiation exposure to colorectal carcinogenesis [13, 14] and CRC has a major hereditary component [15], characterization of transgenerational CRC risk after parental radiation exposure either in human or in experimental animals has not been done to date. Our data reveal that low dose radiation exposure to parents significantly increased intestinal tumor frequency in F1 of APC1638N/+ mice. We also observed greater number of large tumors in F1 mice of irradiated parents (BPI and MPI) relative to F1 mice of unirradiated parents (NPI) and irradiated parents (DPI) themselves.
Intestinal tumor frequency and size in F1 mice. Intestinal tumor frequency was higher in male (A) and female (B) F1 mice from irradiated parents (BPI and MPI) relative to F1 from unirradiated parent (NPI) and directly irradiated parents (DPI). Larger tumor size was observed in male (C) and female (D) F1 from irradiated (BPI and MPI) relative to unirradiated (NPI) and directly irradiated (DPI) parents. Transgenerational intestinal tumorigenesis was significantly higher in FI male relative to F1 female mice from BPI and MPI groups (E). Although, it was similar in F1 male and female from NPI, intestinal tumorigenesis showed male preponderance in DPI group (E). Symbols #, &, and $ denotes significant difference.
Ionizing radiation is a known genotoxic agent and currently, probability of exposure to low dose medical radiation has increased because diagnostic imaging procedures especially CT scan has increased more than 6-fold over the past decades [16]. Such low dose exposures from multiple procedures could lead to a cumulative dose beyond acceptable limits with adverse health consequences in exposed person as well as in their offspring. Radiation exposure has been reported to cause genetic/epigenetic alterations in germ cell DNA, and post-meiotic cells are suggested to be specifically sensitive to the effects of ionizing radiation (reviewed in [17]). In the current study, the goal was to understand transgenerational intestinal tumorigenic risk using BPI and MPI groups. We used MPI because evidences in literature suggest greater susceptibility of male germ cells to radiation-induced changes relative to female germ cells [17]. Indeed, we observed a similar increase in tumorigenesis in the F1 from BPI and MPI groups which we believe is indicative of a predominant role of male germ line and conforms to published data [17]. We also observed a gender bias in radiation-induced intestinal tumorigenesis with higher frequency and larger size of tumors in male relative to female F1 mice, and male preponderance of CRC has been reported in the literature [18]. Considering that radiation-induced increased mutation rate has been reported to be transmitted equally to the male and female offspring in mice [4], it is believed that the postnatal environment, endogenous as well as exogenous, could be playing a role in greater tumorigenesis in male relative to female [19, 20]. While we have provided important initial evidence suggesting transgenerational intestinal carcinogenesis after radiation exposure, we acknowledge that more studies using well-controlled transgenerational parameters (reviewed in [17]) as well as CRC relevant surrogate biological end-points such as mutation frequency, microsatellite instability in tumor susceptible genes will be required to develop mechanistic understanding of transmission of heritable alterations to offspring from irradiated parents.
In conclusion, our findings demonstrated that the APC1638N/+ due to its low spontaneous tumor frequency could serve as an effective model to study transgenerational tumorigenesis in gastrointestinal tissues involving different radiation doses and dose rates, either paternal or maternal irradiation, and varying post-exposure wait period before mating. Our study, we believe, will provide the foundation not only for research in animal models but also for long-term follow-up studies in human patients exposed to low dose radiation to assess transgenerational CRC risk.
Supplementary Material
Supplementary figures and table.
Acknowledgements
This study is supported in part by NASA Grant# NNX13AD58G and NNX15AI21G. We are thankful to Steve Strawn and Pelagie Ake for animal husbandry and administrative support.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lin EC. Radiation risk from medical imaging. Mayo Clin Proc. 2010;85:1142-6 quiz 1146
2. Pawel D, Preston D, Pierce D, Cologne J. Improved estimates of cancer site-specific risks for A-bomb survivors. Radiat Res. 2008;169:87-98
3. Kline RP. Recognizing hereditary colorectal cancer. JAAPA. 2014;27:14-8
4. Barber R, Plumb MA, Boulton E, Roux I, Dubrova YE. Elevated mutation rates in the germ line of first- and second-generation offspring of irradiated male mice. Proc Natl Acad Sci U S A. 2002;99:6877-82
5. Dubrova YE, Nesterov VN, Krouchinsky NG. et al. Human minisatellite mutation rate after the Chernobyl accident. Nature. 1996;380:683-6
6. Little MP, Goodhead DT, Bridges BA, Bouffler SD. Evidence relevant to untargeted and transgenerational effects in the offspring of irradiated parents. Mutat Res. 2013;753:50-67
7. Suman S, Kumar S, Moon BH. et al. Relative Biological Effectiveness of Energetic Heavy Ions for Intestinal Tumorigenesis Shows Male Preponderance and Radiation Type and Energy Dependence in APC(1638N/+) Mice. Int J Radiat Oncol Biol Phys. 2016;95:131-8
8. Trani D, Datta K, Doiron K, Kallakury B, Fornace AJJ. Enhanced intestinal tumor multiplicity and grade in vivo after HZE exposure: mouse models for space radiation risk estimates. Radiat Environ Biophys. 2010;49:389-96
9. Hasegawa M, Wilson G, Russell LD, Meistrich ML. Radiation-induced cell death in the mouse testis: relationship to apoptosis. Radiat Res. 1997;147:457-67
10. Winther JF, Boice JDJ, Christensen J. et al. Hospitalizations among children of survivors of childhood and adolescent cancer: a population-based cohort study. Int J Cancer. 2010;127:2879-87
11. Mughal SK, Myazin AE, Zhavoronkov LP, Rubanovich AV, Dubrova YE. The dose and dose-rate effects of paternal irradiation on transgenerational instability in mice: a radiotherapy connection. PLoS One. 2012;7:e41300
12. Datta K, Suman S, Kallakury BV, Fornace AJJ. Heavy ion radiation exposure triggered higher intestinal tumor frequency and greater beta-catenin activation than gamma radiation in APC(Min/+) mice. PLoS One. 2013;8:e59295
13. Cragle DL, McLain RW, Qualters JR. et al. Mortality among workers at a nuclear fuels production facility. Am J Ind Med. 1988;14:379-401
14. Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160:381-407
15. Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695-723
16. Brenner DJ, Hall EJ. Computed tomography-an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-84
17. Nomura T. Transgenerational carcinogenesis: induction and transmission of genetic alterations and mechanisms of carcinogenesis. Mutat Res. 2003;544:425-32
18. Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128:1668-75
19. Mohr U, Dasenbrock C, Tillmann T. et al. Possible carcinogenic effects of X-rays in a transgenerational study with CBA mice. Carcinogenesis. 1999;20:325-32
20. Martinelli M, Scapoli L, Cura F. et al. Colorectal cancer susceptibility: apparent gender-related modulation by ABCB1 gene polymorphisms. Journal of Biomedical Science. 2014;21:1-8
Author contact
![]() Corresponding author: Kamal Datta, M.D., Associate Professor, Department of Biochemistry and Molecular & Cellular Biology, Georgetown University, Research Building, Room E518, 3970 Reservoir Rd., NW, Washington, DC 20057, USA. Phone: 202-687-7956, Fax: 202-687-3140, Email: kd257edu
Corresponding author: Kamal Datta, M.D., Associate Professor, Department of Biochemistry and Molecular & Cellular Biology, Georgetown University, Research Building, Room E518, 3970 Reservoir Rd., NW, Washington, DC 20057, USA. Phone: 202-687-7956, Fax: 202-687-3140, Email: kd257edu

 Global reach, higher impact
Global reach, higher impact