Impact Factor
ISSN: 1837-9664
J Cancer 2016; 7(5):587-594. doi:10.7150/jca.13687 This issue Cite
Research Paper
C-Met as a Molecular Marker for Esophageal Squamous Cell Carcinoma and Its Association with Clinical Outcome
1. School of Medicine, Shandong University, Jinan, China;
2. Department of Radiation Oncology, Zhejiang Cancer Hospital, Hangzhou, China;
3. First Clinical Medical School, Wenzhou Medical University, Wenzhou, China;
4. Department of Pathology, Zhejiang Cancer Hospital, Hangzhou, China;
5. Department of Radiation Oncology, Shandong University Affiliated Shandong Cancer Hospital and Institute, Jinan, Shandong, China.
Received 2015-8-29; Accepted 2016-1-9; Published 2016-3-18
Abstract
Background: Epidermal growth factor receptor (EGFR), c-Met, and human epidermal growth factor receptor 2 (HER2) are overexpressed in a variety of human cancers, and may serve as biomarkers for disease prognosis. We examined whether high expression of these molecular markers correlates with poor disease prognosis in esophageal squamous cell cancer (ESCC). Materials and Methods: Expression of EGFR, c-Met, and HER2 protein was detected by immunohistochemistry (IHC) in 180 paraffin-embedded tissue samples from stage IIB-IIIC ESCC patients. The overall survival (OS) rates were calculated according to the Kaplan-Meier method, and the log-rank test was used to evaluate differences between survival curves. The Cox proportional hazards model was used for univariate and multivariate analyses. Results: The median survival of all patients was 46 months. There was no significant difference in OS in terms of HER2 and EGFR status (P = 0.177 and P=0.061, respectively). However, there was a significant difference in OS between c-Met high expression patients and c-Met low expression or negative patients (median: 41.9 months vs. 56.7 months; P = 0.001). Multivariate analysis also showed that, of the covariates analyzed, c-Met high expression was the only prognostic factor for OS (HR: 0.459 [95 % confidence interval: 0.287-0.733]; P = 0.001). Patients with ESCC that had concurrent overexpression of EGFR and c-Met had significantly worse survival than ESCC that displayed overexpression of either EGFR or c-Met individually or that did not have overexpression of either protein (P=0.000). Conclusions: Overexpression of HER2 and EGFR individually is not significantly associated with poor prognosis in ESCC. High expression of c-Met may be indicative of a poorer prognosis in ESCC. In order to promote efficient and rapid development of therapeutic methods in ESCC, further studies are necessary to explore the role of c-Met.
Keywords: Esophageal squamous cell carcinoma, Epidermal growth factor receptor, C-MET, Human epidermal growth factor receptor 2.
Introduction
Esophageal cancer is the sixth most common cause of cancer deaths worldwide and the incidence of this disease ranks fifth highest among malignant cancers in China1. Esophageal squamous cell carcinoma (ESCC) is the most common esophageal cancer in China, accounting for more than 90% of cases. The majority of individuals presenting with ESCC are diagnosed with advanced disease, due to the late emergence of clinical symptoms. Although these patients may benefit from perioperative sequential or concurrent chemoradiotherapy (CRT), the prognosis is still quite poor, with 5-year survival rates around 16%-39%2. The treatment of locally advanced ESCC remains a challenge, and oncologists and researchers are evaluating potential targeted-therapy approaches.
Molecular markers specific to ESCC remain unknown, and identification of targetable molecules for ESCC therapy is of great importance. Epidermal growth factor receptor (EGFR), a transmembrane glycoprotein belonging to the HER family of receptor tyrosine kinases, is overexpressed in 36.6%-80% of ESCC patients, and a promising candidate for targeted therapy3. EGFR participates in cellular differentiation and proliferation5, and EGFR overexpression correlates with tumor invasion and lymph node metastasis6-8. Overexpression of EGFR has been found in many human malignancies, including cancers of the head and neck, lung cancer, breast cancer, colorectal cancer, and esophageal cancer9. A number of studies have shown that increased EGFR expression is associated with poor survival among patients with esophageal cancer6, 8-11. However, other studies report contradictory findings4.
The cell surface receptor c-Met (mesenchymal-epithelial transition factor, MET) is the receptor for hepatocyte growth factor (HGF). C-Met overexpression in Asian ESCC patients is about 34%- 69.2%12, 13, which differs from patients in western countries, where overexpression of c-Met is observed in less than 10% of cases14. HGF and c-Met have been reported as significant factors relating to lymph node stage and distant metastasis12, 13. It was reported that c-Met was involved in a number of human tumors, including gastric15, ovarian16, colorectal17, and renal cancer18. C-Met was overexpressed in 34%-54% of esophageal adenocarcinoma and had a significant association with disease survival19, but the correlation between c-Met status and clinical outcome in ESCC remains unclear.
The human epidermal growth factor receptor 2 (HER2) protein also belongs to the HER family of receptors, and has attracted much attention in gastric and gastroesophageal junction (EGJ) adenocarcinomas20. HER2 expression has a prognostic significance in patients with EGJ cancer 21. The rate of high expression of HER2 in adenocarcinoma of the esophagus (15%-30%) is higher than in ESCC (5%-13%) 4, 22-24. Some other studies also indicated that HER2 overexpression is associated with poorer survival8, especially in patients with ESCC4, 24. However, these studies did not assess the concurrent overexpression of EGFR, c-Met and HER2; moreover, the classification of expression of these biomarkers was not standardized. In this study, we evaluate the use of these proteins as potential biomarkers in ESCC, as identification of actionable biomarkers will promote efficient and rapid development of therapeutic methods for ESCC.
Material and Methods
Patients and samples
Clinical data and paraffin-embedded tissue samples of histologically confirmed stage IIB-IIIC ESCC were collected from 180 patients who underwent esophagectomy at Zhejiang Cancer Hospital between January 2007 and December 2012. Median follow-up time was 46.4 months. Information on patient age, gender, smoking, alcohol use, tumor loction, tumor size, differentiation, stage of disease, venous or nerve invasion, and therapy was obtained from medical records. Patients did not receive any targeted therapy. This study was approved by the ethical committee of the Zhejiang Cancer Hospital, and written informed consent was obtained from all patients to use surgically resected samples for research. Surgically resected samples were formalin-fixed and paraffin-embedded by standard techniques.
Immunohistochemistry (ICH)
Formalin-fixed, paraffin-embedded tumor tissue samples were cut into 4 μm sections and mounted on adhesion microscope slides. Immunohistochemical (IHC) staining for EGFR and HER2 was performed by automation using a Ventana BenchMark ULTRA instrument with the following antibodies: Primary rabbit anti-EGFR antibody (dilution, ready to use[no need dilution]; Cat.no. 790-4347; Clone no. 5B7; Ventana) and Primary rabbit anti-HER/neu antibody (dilution, ready to use; Cat.no. 790-4493; Clone no. 4B5; Ventana). IHC staining of c-Met was performed manually using primary rabbit anti-c-Met antibody (dilution, ready to use; Cat. no. 2A-0547; Clone no. EP1454Y) at the Zhejiang Cancer Hospital. Deparaffinized tissue sections were immersed for 10 minutes in 100% methanol. Antigen retrieval was performed in DAKO PT-link, followed by 5 minutes incubation in 0.3% hydrogen peroxide to block endogenous peroxidase. After antigen retrieval, tissue sections were incubated with c-Met primary antibody for 90 minutes in a moist chamber at room temperature. Sections were then soaked with PBS for 5 minutes, and then were incubated in a moist chamber at room temperature with a polymer helper for 20 minutes. Slides were soaked again in PBS buffer, and then incubated with polymer conjugated horseradish peroxidase linked anti-mouse/rabbit secondary antibody in a moist chamber for 30 minutes at room temperature. The slides were then stained using 3, 3'-diaminobenzidine (DAB [Doke]) for 3 minutes and subsequently counterstained with hematoxylin. The positive controls were tissues known to positively express the target antigen and the negative controls were performed with rabbit pre-immuno-serum (Figure S1).
Immunohistochemistry assessment
The results of the immunohistochemical staining were blindly scored by two experienced pathologists who had no information regarding the samples' clinical data. The expression of c-Met and EGFR were judged by following criteria: H-score assessment was based on a combination of the percentage and the intensity of the stained tumor cells. Each individual intensity level (0-3) was multiplied by the percentage of cells(0%-100%), and all values were added to generate the final IHC score, with an H-score range of 0 to 300. The X-tile software (version 3.6.1, 2003-2005; Yale University, New Haven, CT, USA) was used to determine the optimal cut-off for high expression staining by dichotomizing patients according to H-score value and clinical outcome. Assessment for HER2 status was performed by IHC following the modified scoring system used in the ToGA trial25 (It was recommended in NCCN Guidelines Version 3.2015).
Statistical analysis
The overall survival rates were calculated by the Kaplan-Meier method, and the log-rank test was used to evaluate the differences between survival curves. The Cox proportional hazards model was used for univariate analysis and multivariate analysis. The multivariate Cox proportional hazards model analysis was performed by backward elimination with a stay level of 0.10. All P-values reported are two-tailed, and a P-value of < 0.05 was considered statistically significant. Statistical analyses were performed using IBM® SPSS® Statistics version 21 (IBM, Armonk, NY, USA).
Results
Patients, follow-up, and IHC H-scores
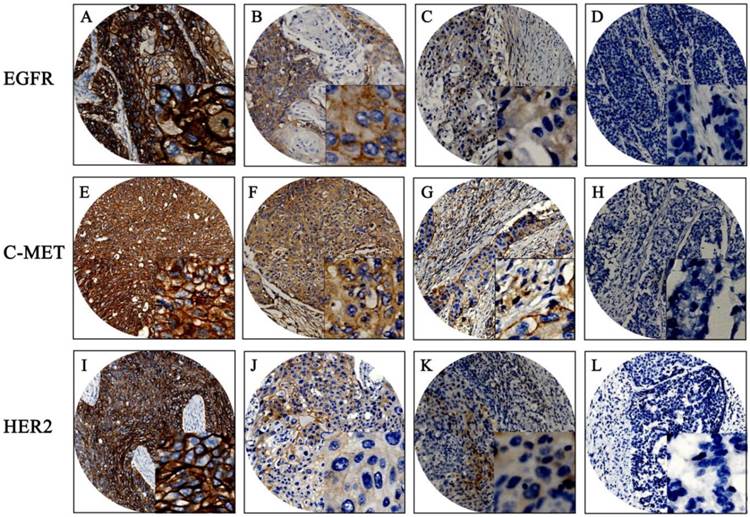
The baseline clinical characteristics of 180 patients are given in Table S1. Mean patient age was 59 years, with a range of 37 to 80 years. Median patient survival was 46 months. Positive expression of EGFR, c-Met, and HER2 was detected in 94.4%, 87.2%, and 11.1% of the patient population, respectively. EGFR and c-Met proteins were mainly located in cell membranes and cytoplasm; HER2 proteins were mainly located in cell membranes. The mean H-score for EGFR expression in this population was 169 with a standard deviation of ±90; the mean H-score of c-Met was 158 ± 73. X-tile generated cut points were as follows: EGFR had an H-score cut point of 170 with 101 (56.1%) cases having a high score; c-Met had an H-score cut point of 160 with 84 (46.7%) cases having a high score. Representative examples of different staining scores are shown in Figure 1.
EGFR
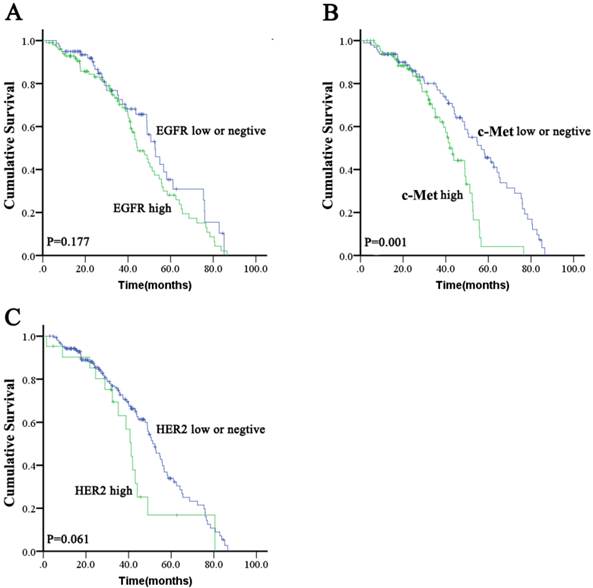
High expression of EGFR was significantly correlated with tumor poor differentiation (P = 0.038). There were no other significant differences in baseline clinical characteristics between ESCC with high EGFR expression and those with low expression or negative EGFR expression (Table S1). There was no significant difference in OS (median: 44.5 months [95 % CI: 37.8-51.2 months] vs. 52.7 months [95 % CI: 46.0-59.4 months]; P = 0.177; Figure 1A) between the high EGFR and low/negative EGFR expressing groups. The proportions of patients who received chemotherapy and radiotherapy were not significantly different between high EGFR expression or low/negative EGFR expression groups (46.5 % vs. 41.8 % for chemotherapy [P = 0.548] and 52.5 % vs. 48.1% for radiotherapy [P = 0.652]).
c-Met
There was no significant difference in baseline characteristics between high c-Met expressing patients and low/negative c-Met expressing patients (Table S1). There was a significant difference in OS (median: 41.9 months [95 % CI: 37.8-46.0 months] vs. 56.7 months [95 % CI: 45.5-67.9 months]; P = 0.001; Figure 1B) between the high c-Met and low/negative c-Met expressing groups. The proportions of patients who received chemotherapy or radiotherapy were not significantly different between the high c-Met and low/negative c-Met expressing groups (38.1% vs. 50% for chemotherapy [P = 0.247] and 50% vs. 51.0% for radiotherapy [P = 1.000]).
HER2
There was no significant difference in baseline characteristics between HER2 positive patients and HER2 negative patients (Table S1). A non-significant trend toward decreased OS (median: 41.2 months [95 % CI, 36.7-45.7 months] vs. 52.4 months [95 % CI, 47.3-57.5 months]; P = 0.061; Figure 1C) was found in the HER2 positive group compared to the HER2 negative group. The proportions of patients who received chemotherapy were not significantly different between HER2 positive and HER2 negative patients (57.1 % vs. 42.8 %; P = 0.247), but the proportions of patients who received radiotherapy were significantly different between HER2 positive and HER2 negative patients (28.6% vs. 53.5%; P = 0.038).
Immunohistochemistry results of EGFR, C-MET, and HER2. Representative examples: (A, E, I) strong staining; (B, F, J) moderate staining; (C, G, K) weak staining; (D, H, L) negative staining. Original magnification: 100x and 400x (overlay insert).
Prognostic significance of co-overexpression of HER2, EGFR, and c-Met
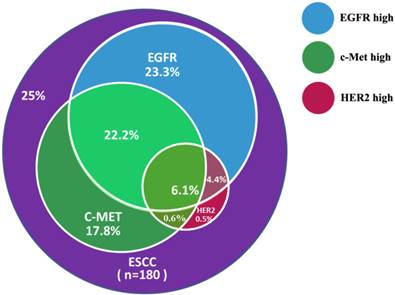
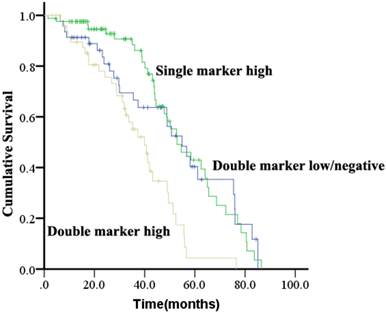
Patients were evaluated for concurrent overexpression of HER2, EGFR, and c-Met (Figure 3). Simultaneous overexpression of HER2, EGFR, and c-Met was detected in 11 out of 180 patients (6.1%). Patients with high expression of both EGFR and c-Met had significantly worse OS compared with those with high expression of either EGFR or c-Met alone, or high expression of neither (P = 0.000; Figure 4). The percentage of c-Met positive tumors that were EGFR positive was not significantly different than the percentage of c-Met low/negative tumors that were EGFR positive (60.7% vs. 52.1%; P = 0.292). Similarly, the percentage of c-Met positive tumors that were HER2 positive was not significantly different than the percentage of c-Met low/negative tumors that were HER2 positive (14.3% vs. 9.4%; P = 0.214). In contrast, the percentage of EGFR overexpressing tumors that were HER2 positive was significantly different than the percentage of EGFR low/negative tumors that were HER2 positive (18.8 vs. 2.5%; P = 0.001).
Univariate and multivariate analyses
Univariate Cox proportional hazards analysis revealed that high c-Met expression was a significant prognostic factor for OS (P = 0.003). Multivariate Cox proportional hazards analysis revealed that high c-Met expression was the only significant prognostic factor for OS among the covariates analyzed (HR: 0.459 [95 % CI, 0.287-0.733]; P = 0.001; Table 1).
Discussion
Our study investigated concurrent overexpression of HER2, EGFR, and c-Met and the prognostic impact and clinicopathological features associated with overexpression of EGFR, c-Met, and HER2 in ESCC. It has been reported that elevated expression of EGFR, c-Met, and HER2 is frequently associated with poor prognosis6, 7, 12, 13, 26. However, high expression of EGFR \was not a significant prognostic factor in our study. In our study, only high c-Met expression was a significant and independent prognostic factor; this is consistent with recent studies12, 13. High expression of HER2 displayed a non-significant trend toward being a prognostic factor in our study; this suggests potential agreement with recent studies21, 27.
Results of univariate and multivariate analyses for ESCC overall survival (n = 180).
| Factors | P-value. | HR | 95.0% CI for HR | |
|---|---|---|---|---|
| Univariate analyses | Lower | Upper | ||
| Age (≤65 vs. >65 years) | 0.251 | 0.766 | 0.487 | 1.207 |
| Gender (male vs. female) | 0.549 | 0.789 | 0.364 | 1.712 |
| Smoking | 0.663 | 1.152 | 0.609 | 2.179 |
| Alcohol use | 0.871 | 0.950 | 0.544 | 1.766 |
| Tumor location | 0.977 | |||
| Middle esophagus vs. Lower esophagus | 0.845 | 0.861 | 0.191 | 3.888 |
| Upper esophagus vs. Lower esophagus | 0.962 | 1.012 | 0.625 | 1.638 |
| Tumor size | 0.502 | 1.047 | 0.916 | 1.196 |
| Differentiation | 0.972 | |||
| Grade 2 vs. Grade1 | 0.816 | 0.915 | 0.433 | 1.934 |
| Grade 3 vs. Grade1 | 0.890 | 0.964 | 0.576 | 1.613 |
| Stage (IIA-IIIA vs. IIIB-IIIC) | 0.444 | 1.221 | 0.732 | 2.036 |
| Venous or nerve invasion (no invasion vs. invasion) | 0.363 | 1.253 | 0.771 | 2.037 |
| EGFR (H-score > median vs. ≤ median) | 0.677 | 0.903 | 0.560 | 1.457 |
| c-Met (H-score > median vs. ≤ median) | 0.003 | 0.481 | 0.297 | 0.778 |
| HER2 (positive vs. negative) | 0.138 | 0.631 | 0.344 | 1.159 |
| Postoperative radiotherapy (no radiotherapy vs. radiotherapy ) | 0.155 | 0.698 | 0.426 | 1.145 |
| Postoperative chemotherapy (no chemotherapy vs. chemotherapy ) | 0.980 | 1.006 | 0.606 | 1.673 |
| Multivariate analysis | ||||
| EGFR (H-score > median vs. ≤ median ) | 0.421 | 0.831 | 0.530 | 1.304 |
| c-Met (H-score > median vs. ≤ median) | 0.001 | 0.459 | 0.287 | 0.733 |
| HER2 (positive vs. negative) | 0.205 | 0.685 | 0.381 | 1.230 |
| Postoperative radiotherapy (no radiotherapy vs. radiotherapy ) | 0.146 | 0.706 | 0.441 | 1.129 |
| Postoperative chemotherapy (no chemotherapy vs. chemotherapy ) | 0.940 | 0.982 | 0.614 | 1.157 |
Kaplan-Meier survival analysis for overall survival according to EGFR, c-Met, or HER2 status in ESCC tumors.
Concurrent overexpression rates of EGFR, c-Met, and HER2 in ESCC samples.
Kaplan-Meier analysis of overall survival analysis according to concurrent overexpression status of EGFR and c-Met in ESCC.
There are several potential reasons that our findings related to high expression of EGFR were not consistent with those from previous studies8-10. These studies used different primary anti-EGFR antibodies and different diagnostic criteria than we employed in our study. Moreover, the target populations were different, as most of the patient populations in the former studies consisted of patients with adenocarcinoma and ESCC, whereas our study was only conducted in patients with ESCC. Our results agree with a recent study which reported that there was no significant relationship between EGFR IHC scores and disease prognosis4. Our results that high EGFR expression associates with the state of tumor differentiation are consistent with a recent report concerning Chinese patients28. Similarly, Delektorskaya and associates reported that amplification of EGFR gene (≥2.2) correlated with low degree of tumor differentiation (P = 0.006)8.
Our finding that c-Met was an independent prognostic factor for poorer OS suggests that c-Met may be a candidate for targeted therapy in ESCC. Ozawa and associates13 examined 104 surgically obtained ESCC specimens and reported that patients with high c-Met expression had significantly worse survival; this is in agreement with our study. Their study also declared that elevated expression of c-Met significantly correlated with tumor depth and pathological stage13, but these associations were not apparent in our study. Similarly, it was reported that overexpression of c-Met associated with worse prognosis (P = 0.011)12, and that there was no significant association between c-Met expression and clinical features except for patient sex and tumor location. Our study showed that high expression of c-Met was significantly correlated with worse survival, but its role in ESCC needs to be further verified with prospective study.
Elevated HER2 expression in esophagogastric cancers varies widely, ranging from 2%-45%25, 29, 30. In this study, we observed an 11.1% HER2 positive rate, which is similar to recent report indicating a low rate of HER2 positivity among ESCC30. We observed a non-significant trend for HER2 positivity to be a prognostic factor for OS in ESCC patients (P = 0.061). The discrepancies between our study and other studies may be that the most common esophogastric cancer in Chinese populations is ESCC, rather than adenocarcinoma21, 27. There was a statistically significant different in the proportions of patients who received postoperative radiotherapy between the HER2 positive group and HER2 negative group, which may confound our results. Although it has been reported that HER2 overexpression correlates with tumor invasion and lymph node metastasis25, this association was not observed in our study, possibly due to the low incidence of HER2 positive ESCC and the small sample size of our study. HER2 has been well established as an actionable biomarker in other cancers, specifically in breast cancer, but its role in ESCC needs to be further verified.
Approximately 75% of the ESCC patients in our present study demonstrate overexpression of one or more receptors that can be a target of molecular targeted therapy. In our study, concurrent overexpression of EGFR and HER2 was observed in 10.6% patients, a rate which was similar to a previous report31. We discovered that patients with concurrent overexpression of EGFR and c-Met had significantly worse survival. To the best of our knowledge, this study is the first investigation to report the clinical significance of concurrent overexpression of EGFR and c-Met in ESCC. Patients with high EGFR expression had higher rates of HER2 positivity, but there was no significant difference in HER2 positivity between high c-Met expressing and low/negative c-Met expressing patients.
Targeted therapy against EGFR, c-Met, and HER2 is being evaluated in ESCC. Although clinical trials evaluating anti-EGFR antibodies, such as erlotinib or gefitinib, in combination with chemotherapy as first-line treatment failed to show any benefit in non-selected esophageal cancer patients, the phase II trial of icotinib as a second-line treatment for EGFR-overexpressing patients is ongoing (NCT01855854). For c-Met, phase I/II trials of the targeted therapy AMG 337 in advanced stomach or esophageal cancer patients with c-Met overexpression is ongoing (NCT02344810). Therefore, overexpression of c-Met in ESCC patients may have clinical significance, especially if the results of this trial indicate an advantage in targeting c-Met in these patients. For HER2, the ToGA study24 evaluated the efficacy and safety of trastuzumab in combination with cisplatin and a fluoropyrimidine in HER2-neu-positive gastric and EGJ adenocarcinoma. A significant improvement in median OS was reported with combination treatment with trastuzumab plus chemotherapy compared to chemotherapy alone in patients with HER2-neu overexpression (13.8 months vs. 11 months, respectively; P =. 046). Although trastuzumab combined with chemotherapy is a standard first-line treatment for HER2-positive ESCC patients, no targeted therapy has been established as standard of care for the ESCC patients. In gastroesophageal cancer, the phase II trial of lapatinib vs. lapatinib plus capecitabine as a second-line treatment for patients with HER2-overexpressing ESCC was closed prematurely due to futility32. Although the proportion of HER2-positive tumors is small in ESCC patients, it is necessary to further confirm the role of anti-HER2 targeted therapy in HER2-positive ESCC patients, and the results of our study support further study in this area.
We acknowledge several limitations of our study. Firstly, the diagnostic criteria for EGFR and c-Met status were not standardized. Secondly, due to the lack of the fresh human tumor tissues of these patients, the western blotting have not be done to validate the expression levels of EGFR, C-MET, and HER2. Thirdly, we evaluated EGFR, c-Met overexpression by protein expression only, using IHC, and did not evaluate gene amplification. In terms of clinical utility, the standardized methods and diagnostic criteria regarding EGFR and c-Met should be investigated in future prospective clinical trials. Since clinical trials often use IHC as diagnostic criteria for esophageal cancer patient classification, the correlation between protein overexpression (as indicated by IHC) and the efficacy of each targeted therapy may define the success of clinical development for each agent, and may be impacted by tumor heterogeneity.
In conclusion, our study indicated that the high expression of HER2 and EGFR are not significant prognostic indicators in ESCC, while high expression of c-Met may indicate a poorer prognosis than low/negative c-Met expression. In order to promote efficient and rapid development of therapeutic methods in ESCC, further studies are necessary to explore the role of c-Met as an actionable biomarker.
Abbreviations
CRT: Chemoradiotherapy; EGFR: Epidermal growth factor receptor; ESCC: Esophageal Squamous Cell Carcinoma; EGJ: gastroesophageal junction; HGF: hepatocyte growth factor; HER2: human epidermal growth factor receptor 2; IHC: Immunohistochemistry; OS: Overall Survival.
Supplementary materials
Supplemental Table S1, and Figure S1.
Acknowledgements
This work was supported by a grant from Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (to Yaping Xu). This work was presented in the 16th World Conference on Lung Cancer (IASLC 2015) in Denver, CO, USA (Abstract ID# 2495).
Competing Interests
The authors have no conflicts of interest to disclose.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90
2. Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R. et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086-92
3. Shang L, Liu HJ, Hao JJ, Jiang YY, Shi F, Zhang Y. et al. A panel of overexpressed proteins for prognosis in esophageal squamous cell carcinoma. PloS one. 2014;9:e111045
4. Kato H, Arao T, Matsumoto K, Fujita Y, Kimura H, Hayashi H. et al. Gene amplification of EGFR, HER2, FGFR2 and MET in esophageal squamous cell carcinoma. Int J Oncol. 2013;42:1151-8
5. Hanawa M, Suzuki S, Dobashi Y, Yamane T, Kono K, Enomoto N. et al. EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int J Cancer. 2006;118:1173-80
6. Jiang D, Li X, Wang H, Shi Y, Xu C, Lu S. et al. The prognostic value of EGFR overexpression and amplification in Esophageal squamous cell Carcinoma. BMC cancer. 2015;15:377
7. Li JC, Zhao YH, Wang XY, Yang Y, Pan DL, Qiu ZD. et al. Clinical significance of the expression of EGFR signaling pathway-related proteins in esophageal squamous cell carcinoma. Tumour Biol. 2014Jan;35:651-7
8. Delektorskaya VV, Chemeris GY, Zavalishina LE, Ryazantseva AA, Grigorchuk AY, Kononets PV. et al. Squamous cell carcinoma of the esophagus: evaluation of the status of epidermal growth factor receptors (EGFR and HER-2) by immunohistochemistry and in situ hybridization. Bull Exp Biol Med. 2010;149:615-20
9. Zhang W, Zhu H, Liu X, Wang Q, Zhang X, He J. et al. Epidermal growth factor receptor is a prognosis predictor in patients with esophageal squamous cell carcinoma. Ann Thorac Surg. 2014;98:513-9
10. Fukai Y, Masuda N, Kato H, Fukuchi M, Miyazaki T, Nakajima M. et al. Correlation between laminin-5 gamma2 chain and epidermal growth factor receptor expression in esophageal squamous cell carcinomas. Oncology. 2005;69:71-80
11. Yu WW, Guo YM, Zhu M, Cai XW, Zhu ZF, Zhao WX. et al. Clinicopathological and prognostic significance of EGFR over-expression in esophageal squamous cell carcinoma: a meta-analysis. Hepato-gastroenterology. 2011;58:426-31
12. Xu Y, Peng Z, Li Z, Lu M, Gao J, Li Y. et al. Expression and clinical significance of c-Met in advanced esophageal squamous cell carcinoma. BMC cancer. 2015;15:6
13. Ozawa Y, Nakamura Y, Fujishima F, Felizola SJ, Takeda K, Okamoto H. et al. c-Met in esophageal squamous cell carcinoma: an independent prognostic factor and potential therapeutic target. BMC cancer. 2015;15:451
14. Jardim DL, de Melo Gagliato D, Falchook GS, Janku F, Zinner R, Wheler JJ. et al. MET aberrations and c-Met inhibitors in patients with gastric and esophageal cancers in a phase I unit. Oncotarget. 2014;5:1837-45
15. Betts G, Valentine H, Pritchard S, Swindell R, Williams V, Morgan S. et al. FGFR2, HER2 and cMet in gastric adenocarcinoma: detection, prognostic significance and assessment of downstream pathway activation. Virchows Arch. 2014;464:145-56
16. Mhawech-Fauceglia P, Afkhami M, Pejovic T. MET/HGF Signaling Pathway in Ovarian Carcinoma: Clinical Implications and Future Direction. Patholog Res Int. 2012;2012:960327
17. Samame Perez-Vargas JC, Biondani P, Maggi C, Gariboldi M, Gloghini A, Inno A. et al. Role of cMET in the development and progression of colorectal cancer. Int J Mol Sci. 2013;14:18056-77
18. Matsumoto K, Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001;59:2023-38
19. Mesteri I, Schoppmann SF, Preusser M, Birner P. Overexpression of CMET is associated with signal transducer and activator of transcription 3 activation and diminished prognosis in oesophageal adenocarcinoma but not in squamous cell carcinoma. Eur J Cancer. 2014;50:1354-60
20. Hechtman JF, Polydorides AD. HER2/neu gene amplification and protein overexpression in gastric and gastroesophageal junction adenocarcinoma: a review of histopathology, diagnostic testing, and clinical implications. Arch Pathol Lab Med. 2012;136:691-7
21. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-97
22. Reichelt U, Duesedau P, Tsourlakis M, Quaas A, Link BC, Schurr PG. et al. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol. 2007;20:120-9
23. Schoppmann SF, Jesch B, Friedrich J, Wrba F, Schultheis A, Pluschnig U. et al. Expression of Her-2 in carcinomas of the esophagus. Am J Surg Pathol. 2010;34:1868-73
24. Dreilich M, Wanders A, Brattstrom D, Bergstrom S, Hesselius P, Wagenius G. et al. HER-2 overexpression (3+) in patients with squamous cell esophageal carcinoma correlates with poorer survival. Dis Esophagus. 2006;19:224-31
25. Hofmann M, Stoss O, Shi D. et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805
26. Moelans CB, van Diest PJ, Milne AN, Offerhaus GJ. Her-2/neu testing and therapy in gastroesophageal adenocarcinoma. Patholog Res Int. 2011;2011:674182
27. Sato-Kuwabara Y, Neves JI, Fregnani JH, Sallum RA, Soares FA. Evaluation of gene amplification and protein expression of HER-2/neu in esophageal squamous cell carcinoma using Fluorescence in situ Hybridization (FISH) and immunohistochemistry. BMC cancer. 2009;9:6
28. GANG LIN, XIAO-JIANG SUN, QIAN-BO HAN. et al. Epidermal growth factor receptor protein overexpression and gene amplification are associated with aggressive biological behaviors of esophageal squamous cell carcinoma. Oncology letters. 2015 in press
29. Almhanna K, Meredith KL, Hoffe SE, Shridhar R, Coppola D. Targeting the human epidermal growth factor receptor 2 in esophageal cancer. Cancer Control. 2013;20:111-6
30. Cappellesso R, Fassan M, Hanspeter E. et al. HER2 status in gastroesophageal cancer: a tissue microarray study of 1040 cases. Hum Pathol. 2015;46:665-72
31. Kawaguchi Y, Kono K, Mimura K. et al. Targeting EGFR and HER-2 with cetuximab- and trastuzumab-mediated immunotherapy in oesophageal squamous cell carcinoma. Br J Cancer. 2007;97:494-501
32. Lorenzen S, Riera Knorrenschild J, Haag GM, Pohl M. et al. Lapatinib versus lapatinib plus capecitabine as second-line treatment in human epidermal growth factor receptor 2-amplified metastatic gastro-oesophageal cancer: a randomised phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Eur J Cancer. 2015;51:569-76
Author contact
![]() Corresponding author: Jin-Ming Yu M.D., Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong Academy of Medical Sciences, Jiyan Road 440, Jinan, Shandong Province, 250117, China. Email: sdyujinmingcom.
Corresponding author: Jin-Ming Yu M.D., Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong Academy of Medical Sciences, Jiyan Road 440, Jinan, Shandong Province, 250117, China. Email: sdyujinmingcom.

 Global reach, higher impact
Global reach, higher impact