Impact Factor
ISSN: 1837-9664
J Cancer 2013; 4(9):697-702. doi:10.7150/jca.6248 This issue Cite
Short Research Communication
Novel Functional Assay for Spindle-Assembly Checkpoint by Cyclin-Dependent Kinase Activity to Predict Taxane Chemosensitivity in Breast Tumor Patient
1. Sysmex Corporation, 4-4-4, Takatsukadai, Nishi-ku, Kobe 651-2271, Japan
2. Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
3. Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
4. Department of Breast and Endocrine Surgery, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita 565-0871, Japan
5. Breast Cancer Translational Research Laboratory, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
6. Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
Received 2013-3-15; Accepted 2013-7-23; Published 2013-11-14
Abstract
Taxanes are among the drugs most commonly used for preoperative chemotherapy for breast cancer. Taxanes induce mitotic arrest and subsequent apoptosis. The spindle-assembly checkpoint (SAC) is known to be activated during mitosis, along with cyclin-dependent kinase-1 (CDK1), and is required for taxane-induced cell death. We hypothesized that CDK1 activity predicts response to taxane-containing chemotherapy.
This study included breast cancer patients who received preoperative chemotherapy— taxane-containing treatment followed by anthracycline-based treatment—and then underwent surgery. Before starting taxane-containing chemotherapy, patients underwent fine-needle aspiration biopsy, and the biopsy samples were incubated in paclitaxel solution to measure CDK activity. Clinical were evaluated after taxane therapy, and pathological resposes were evaluated after completion of all preoperative chemotherapy. Thirty five patients were eligible for analysis of clinical response to taxane-containing therapy. Twenty-six patients had taxane-sensitive and 9 taxane-resistant tumors.
Using a cut-off of CDK activity determined by the ROC analysis, patients were classified into SAC function and dysfunction groups. Univariate logistic regression analysis with clinicopathologic parameters showed that only CDK-based SAC functionality was significantly correlated with clinical response (P =0.017). No significant correlation was observed between SAC functionality and pathologic response.
CDK-based SAC functionality significantly predicted clinical response (P =.0072, overall agreement = 71.4%), and this is a unique mechanism-based marker for predicting taxane chemosensitivity. Further, large prospective study is needed to determine CDK-based SAC functionality could be developed as a predictive biomarker.
Keywords: chemosensitivity prediction, cyclin-dependent kinase, preoperative chemotherapy, spindle-assembly checkpoint, taxane
Introduction
Preoperative chemotherapy is well accepted and increasingly used to treat for patients with stage II-III breast cancer.1 There are several advantages of preoperative chemotherapy over postoperative chemotherapy—for instance, with preoperative chemotherapy, the clinical effects of the chemotherapy on tumor burden can be monitored using imaging, the pathologic response can be directly measured by examination of tumors removed after chemotherapy, and the chance of receiving breast-conserving surgery is improved. The clinical outcome of patients treated with preoperative chemotherapy has been shown to be similar to that of patients treated with postoperative chemotherapy in terms of survival and overall disease progression.2
Development of methods to predict tumor response to particular types of preoperative chemotherapy might be of great benefit to patients with primary breast cancer because it could spare them unnecessary treatment and the associated toxic effects. To date, two main biological approaches have been used to try to predict the sensitivity of breast cancer to chemotherapy—genomic expression analysis (multiple genomic expression analysis or gene expression signatures) 3-8 and cultivation of resected tumor tissues with chemotherapeutic agents (so-called chemotherapy sensitivity and resistance assays [CSRAs]).9-12 However, neither expression profiling nor CSRAs fully meet the requirements for clinical application yet—the robustness of the predictive performance of multiple genomic analyses among various cohorts studies remains uncertain, and CSRAs have not been prospectively evaluated in clinical trials.
Taxanes (paclitaxel and docetaxel) are among the drugs most commonly used for preoperative chemotherapy for breast cancer.13 Taxanes specifically bind to tubulin and stabilize microtubule polymerization, resulting in arrest of the cell cycle at the G2/M phase and consequently cell death, mostly via apoptotic pathways. In the G2/M phase, the spindle-assembly checkpoint (SAC) is normally activated to secure the mitotic process.14 Various mitotic checkpoint regulators are the determinants of paclitaxel response.15,16 In a previous report, we showed that activation of the SAC is required for paclitaxel-induced cell death.17 Cyclin-dependent kinase-1 (CDK1), which is known to be a master kinase for cell cycle processes, also plays a key role in the mitotic process. The activity of CDK1 is maximized when the SAC is activated. Premature activation of CDK1 is known to be required for apoptosis induction by paclitaxel.18,19 Thus, we previously hypothesized that the functionality of the SAC can be assessed by measuring CDK1 activity and that CDK1 activity can be used as a predictor of paclitaxel sensitivity.20 Preclinical in vitro and in vivo experiments using breast cancer cell lines and xenograft mouse models showed that in cell lines sensitive to paclitaxel, CDK1 activity increased significantly after paclitaxel incubation, whereas in cell lines resistant to paclitaxel, CDK1 activity was unchanged after paclitaxel incubation, supporting our hypothesis.20 On the other hand, previous study reported that CDK2 activity, critical in the transition of the cell cycle at G1/S phase, do not alter at 24 hr after paclitaxel treatment to the baseline in breast cancer cell lines 21. Our preclinical in vivo experiment agrees with the above observation of CDK2 activity levels20 and CDK2 activity was used to normalize the activity of CDK1. Therefore, we hypothesized that the relative activity of CDK1 normalized by CDK2 activity (CDK 1/2-AC) could be a parameter for paclitaxel sensitivity.
We validated the results of our preclinical study. We conducted a prospective study to determine whether CDK activity predicts taxane sensitivity in breast cancer patients. We previously established a sophisticated technology, termed cell-cycle profiling (C2P) technology, to quantify the kinase activity and expression level of multiple proteins relevant to the cell cycle, including CDKs, in a simultaneous and multiparallel way.22 Using the C2P technology, we successfully predicted breast cancer recurrence in Japanese and Caucasian cohorts by measuring CDK1 and CDK2 specific activities.23,24 In this study, we used the C2P technology to assess CDK activity and analyzed the relationship between CDK activity and clinical response of breast cancer to taxane-containing chemotherapy.
Materials and Methods
Patient Enrollment and Selection for Analysis
Patients in this study were enrolled in an ongoing pharmacogenomic marker discovery program (LAB-99-402) conducted in patients with primary breast cancer at The University of Texas MD Anderson Cancer Center. LAB-99-402 is open to patients with a newly discovered palpable breast lump who are scheduled to undergo diagnostic core or fine-needle aspiration biopsy. In addition to enrollment in LAB-99-402 between 2004 and 2007, inclusion criteria for the study reported herein were diagnosis of primary invasive breast cancer and eligibility for preoperative chemotherapy. Any previous chemotherapy for the current breast cancer excluded a patient from our study. Of the patients in LAB99-402, a total of 60 patients met the inclusion criteria for our study.
Of the 60 patients, 7 patients were excluded from analysis of clinical response to taxane-containing chemotherapy, respectively, because clinical outcome of taxane therapy was not available. Our study ultimately enrolled 52 patients for analysis of clinical response to taxane-containing therapy.
This study was approved by the institutional review boards of MD Anderson Cancer Center and Sysmex Corporation. All patients gave informed consent for voluntary participation.
Study Design and Evaluation of Response to Taxane-containing Chemotherapy
This was a prospective study in breast cancer patients who received 6 months of preoperative chemotherapy consisting of paclitaxel-containing treatment (80 mg/m2 weekly) or docetaxel-containing treatment (100 mg/m2 every 3 weeks) for the initial 3 months followed by fluorouracil-epirubicin-cyclophosphamide or fluorouracil-doxorubicin-cyclophosphamide for the second 3 months. Before starting preoperative chemotherapy, patients were asked to undergo fine-needle aspiration biopsy of the primary breast tumor. Also, the longest diameter of the primary tumor and its orthogonal diameters were measured by ultrasonography.
At the completion of taxane treatment, the clinical response was evaluated and classified according to the following definitions: Complete response (CR) was defined as the absence of all clinical evidence of active tumor in the breast and lymph nodes. Partial response (PR) was defined as a more than 50% reduction in the product of the perpendicular diameters of the measurable lesion(s) without progression of any lesion or appearance of any new disease. Stable disease (SD) was defined as no change or reduction in tumor measurements insufficient to qualify as a PR. Progression of disease (PD) was defined as a more than 25% increase in the size of any tumor and/or the appearance of new lesions. After completing preoperative chemotherapy, patients underwent breast-conserving surgery or mastectomy.
Measurement of CDK Activity in Tumor Samples Incubated with Paclitaxel
It is known that fine-needle aspiration (FNA) samples contain in tumor cells, few lymphocytes and stromal cells.25 Therefore, in this study, tumor samples were obtained by FNA before the administration of any preoperative chemotherapy. Following the sample collection, the samples were immediately incubated in paclitaxel solution containing 100 nM paclitaxel in medium with 2% FBS for 24 hr at 37ºC. This has been reported to be the lowest cytotoxic concentration of paclitaxel that was clinically relevant 26, and exposure to 100 nM paclitaxel for 24 hr blocked a majority of the cells, which are sensitive to paclitaxel, into the mitotic phase in in vitro experiment.20 After incubation, samples were washed and pellets were stored at -80ºC until the measurements of CDK1 and CDK2 enzymatic activities. The CDK activity was measured by previously reported methods.22 On the other hand, in regard to CDK expression, the amount of tumor samples obtained by FNA was not enough for the measurement of CDK expression.
Statistical Analysis
Summary statistics were provided in the form of frequency and percentages for categorical variables (e.g., estrogen receptor status, pathologic subtype) and in the form of median and average for continuous variables (e.g., age).
The ratio of CDK1 activity to CDK2 activity (CDK1/2-Ac) was used as the index of SAC functionality. The cut-off values of CDK1/2-AC were deduced by receiver operating characteristic (ROC) analysis. Patients with clinical CR or PR were defined as having sensitive disease. Each response rate was calculated by dividing the number of sensitive cases by all examined cases.
Cross-tabulations of categorical variables (e.g., response to taxane-containing chemotherapy vs. SAC functionality) were provided, and associations were assessed using Fisher's exact test. Univariate logistic regression was used to assess the utility of SAC functionality in predicting clinical and pathologic response to taxane-containing chemotherapy. All tests were two-sided, and P values of .05 or less were considered statistically significant. Statistical analysis was carried out using MedCalc version 10 (MedCalc Software, Belgium).
Results
Patient Demographics
The demographic, clinical, and pathologic features for patients in the clinical response prediction and pathologic response prediction analysis sets are listed in Table 1. Of the enrolled 52 patients for analysis, there were some cases with no CDK2 activity measurable in our study. One possible explanation is that the biopsy samples might have low or zero percent of cycling cells when both CDK1 and CDK2 were not detectable. Another possibility is that CDK1 or other kinase might take over CDK2 function in the cells whose CDK2 activity was not detectable but CDK1 activity was detectable, suggesting that CDK2 might not be essential for cell cycle in these breast cancer cells. The latter hypothesis is supported by a previous report that CDK1 can compensate for CDK2 loss in most cell type.27 In either case, patients who showed no CDK2 activity were excluded from the analysis because we will not be able to obtain a CDK1/2-AC ratio. Therefore, for prediction of clinical response, 17 patients were excluded, leaving 35 of the initial 52 patients available for the final analysis.
Patient Demographic and Clinicopathologic Features*
| Patients for Clinical Response Prediction (N = 35) | |
|---|---|
| Age, years | |
| Median | 51.0 |
| Mean | 50.0 |
| < 50 | 17 (48.6) |
| ≥ 50 | 18 (51.4) |
| ER status | |
| Negative | 15 (42.9) |
| Positive | 20 (57.1) |
| PgR status | |
| Negative | 14 (40.0) |
| Positive | 21 (60.0) |
| HER2 status | |
| Negative | 34 (97.1) |
| Positive | 1 (2.9) |
| Stage | |
| II | 27 (77.1) |
| III | 12 (22.9) |
| Nuclear grade | |
| 1 | 4 (11.4) |
| 2 | 10 (28.6) |
| 3 | 20 (57.1) |
| Unknown | 1 (2.9) |
| Pathologic subtype | |
| IDC | 27 (77.2) |
| ILC | 4 (11.4) |
| Unknown | 4 (11.4) |
| Regimen | |
| Paclitaxel-containing | 26 (74.3) |
| Docetaxel-containing | 9 (25.7) |
*All values are number (percentage) of patients unless otherwise indicated.
Prediction of Clinical Response to Taxane-containing Chemotherapy
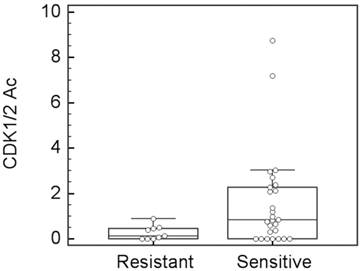
In the analysis set for clinical response (35 patients), 26 patients showed sensitivity to taxane-containing preoperative chemotherapy (2 CR and 24 PR), and 9 patients showed resistance (7 SD and 2 PD). These patients were defined as the sensitive and resistant groups, respectively. The distribution of the CDK1/2-Ac values between the sensitive and resistant groups was separated (analysis of variance, P = .077, Figure 1). This tendency was strongly in agreement with our preclinical findings that cell lines sensitive to paclitaxel had increased CDK1 activity after paclitaxel incubation, whereas cell lines resistant to paclitaxel had no obvious CDK1 activity alteration after paclitaxel incubation.20 The ROC analysis showed a prediction capability of CDK1/2-Ac, within an area under the curve of 0.720 when the cut-off was set to be 0.55 (P = .0087, 95% confidence interval: 0.54-0.86).
On the basis of this cut-off value, 18 patients having higher CDK1/2-Ac values were classified as the SAC function group, and 17 patients having lower CDK1/2-Ac values were classified as the SAC dysfunction group. CDK-based SAC functionality was a significant predictor of clinical response to taxane-containing chemotherapy (chi-squared test, P = .0072, sensitivity = 65.4%, specificity = 88.9%, positive predictive value [PPV] = 94.4%, negative predictive value [NPV] = 47.1%, and overall agreement = 71.4%, Table 2). Comparison of the point estimation of the clinical response rate between the SAC function and SAC dysfunction groups evaluated with chi-squared test showed a difference (P = .0155, Table 3).
A box-whisker plot for taxane clinical response vs. CDK1/2-Ac. Analysis of variance test shows P = 0.077.
Performance of CDK-based SAC Functionality in Predicting Clinical Response to Taxane-containing Chemotherapy
| Clinical Response to Taxane-containing Chemotherapy | ||||
|---|---|---|---|---|
| Sensitive | Resistant | Total | ||
| CDK-based SAC Functionality | Function | 17 | 1 | 18 (51.4%) |
| Dysfunction | 9 | 8 | 17 (48.6%) | |
| Total | 26 (74.3%) | 9 (25.7%) | 35 | |
Statistical Analysis for Difference in Response Rate between SAC Function and Dysfunction Groups
| Response rate | Statistics | |||
|---|---|---|---|---|
| CDK-based SAC functionality | Function | 94.4% | Chi square p= | 0.0155 |
| Dysfunction | 52.9% | Difference = | 41.50% | |
| Total | 74.3% | (95% CI: 9.065 to 67.21) |
Logistic Regression Modeling of Utility of CDK-based SAC Functionality and Other Clinicopathologic Parameters in Predicting Clinical Response
Many clinicopathologic parameters were examined in this study. CDK-based functional SAC was the only clinicopathologic factor that correlated significantly with clinical response (Table 4). Nuclear grade were moderately correlated with clinical response. Since all patients with estrogen-receptor-positive or progesterone-receptor-positive status were sensitive to taxanes, odds ratios could not be calculated for those factors. HER2 status was not included in the analysis since there was only 1 HER2-positive patient in this analysis set.
Univariate Logistic Regression Model for Clinical Response to Taxane-containing Chemotherapy
| Variable | Segments | Odds Ratio | 95% CI | P |
|---|---|---|---|---|
| Age | <50 vs. ≥50 (year) | 1.25 | 0.273-5.73 | 0.774 |
| ER status | Positive vs. negative | NA | NA | - |
| PgR status | Positive vs. negative | NA | NA | - |
| Stage | III vs. II | 1.059 | 0.213-5.27 | 0.944 |
| Nuclear grade | 3 vs. 2/1 | 0.264 | 0.046-1.53 | 0.137 |
| Pathologic subtype | ILC vs. IDC | 1.05 | 0.093-11.83 | 0.969 |
| SAC functionality | Function vs. dysfunction | 15.11 | 1.62-140.6 | 0.017 |
Discussion
We found in this study that SAC function correlated with clinical response to preoperative taxane-containing chemotherapy (P = .0072, PPV = 94.4%, NPV = 47.1%, and overall agreement = 71.4%). Logistic regression analysis with conventional clinicopathologic factors showed that SAC functionality was the only factor significantly associated with clinical response. These findings indicate that patients with SAC function have statistically significantly higher probability of clinical response to taxane treatment (94.4%; 17 of 18 patients) than the patients with SAC dysfunction (52.9%; 9 of 17 patients, P =0.015). On the other hand, the overall rate of resistance to taxane chemotherapy in this cohort was 25.7% (9 of 35 patients), and the resistant population was enriched more than 1.8-fold (47.0% vs. 25.7%) by segregating patients on the basis of SAC dysfunction. Moreover, it is possible that improvement of the accuracy of predicting non-responder by SAC contributes to avoid non-essential medication and to improve the quality of life in breast cancer patients. For the patients with SAC dysfunction, other preoperative chemotherapies could be considered as an alternative.
CDK activity measured by our method successfully predicted sensitivity to taxane-based chemotherapy, strongly suggesting that this CDK-based assessment may reflect the functionality of the SAC in human tumor tissues since SAC activation is closely related to taxane sensitivity.17 This SAC functionality assessment via CDK activity is a unique mechanism-based rational approach to predict sensitivity to taxane-based chemotherapy. Several other rational approaches to sensitivity prediction are so far known.28 These rational approaches, including ours, should ideally be developed alongside the development of multigene predictors to maximize clinical benefit for patients.
CDK-based assessment of SAC functionality was not associated to pCR (P > .1, data not shown). Pathologic review was performed after the completion of all preoperative chemotherapy, including anthracycline-based regimens. Given that the hypothesis underpinning this work is that CDK1 activity predicts response specifically to taxane-containing chemotherapy, it may not be surprising that CDK activity did not predict pCR at the end of all preoperative chemotherapy, which included both taxane-containing and other regimens. Although CDK-based SAC functionality did not show obvious association with pCR to taxane-based chemotherapy, a high NPV was observed (NPV = 86.7%, data not shown), suggesting that patients with SAC dysfunction may be resistant to taxane-containing chemotherapy. Since the same tendency was observed in the prediction of clinical response, the SAC functionality assessment may be suitable for prediction of resistance to taxane-containing chemotherapy.
It is difficult to define the chemotherapy benefit on the treatment of HER2-negative breast cancer, however, recent reports on the clinical benefit of adding 3-weeks treatment with taxanes to anthracycline-based postoperative chemotherapy in HER2-negative patients have indicated little or no benefit from taxanes.29 Our CDK1-based method of assessing SAC functionality may be useful for identifying which subset of patients with HER2-negative breast cancer actually would benefit from postoperative chemotherapy.
In conclusion, we have shown that SAC functionality assessment by CDK activity is a novel method for predicting the sensitivity of HER2-negative breast cancer to taxane-containing preoperative chemotherapy. We are currently conducting a large prospective clinical study in breast cancer patients using Mammotome biopsy to clinically validate the findings of this study.
Acknowledgements
We thank Dr. Tameo Iwasaki and Mr. Kaoru Asano of Sysmex Corporation, for intensive discussions and for supporting this project. We thank Ms. Stephanie Deming, ELS, Department of Scientific Publications, MD Anderson Cancer Center, for editorial review.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Gralow JR, Burstein HJ, Wood W. et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26:814-9
2. Mauri D, Pavlidis N, Ioannidis JPA. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188-94
3. Hess KR, Anderson K, Symmans WF. et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol. 2006;24:4236-44
4. Chang JC, Wooten EC, Tsimelzon A. et al. Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet. 2003;362:362-9
5. Paik S, Tang G, Shak S. et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726-34
6. Iwao-Koizumi K, Matoba R, Ueno N. et al. Prediction of docetaxel response in human breast cancer by gene expression profiling. J Clin Oncol. 2005;23:422-31
7. Sotiriou C, Powles TJ, Dowsett M. et al. Gene expression profiles derived from fine needle aspiration correlate with response to systemic chemotherapy in breast cancer. Breast Cancer Res. 2002;4:R3
8. Thuerigen O, Schneeweiss A, Toedt G. et al. Gene expression signature predicting pathologic complete response with gemcitabine, epirubicin, and docetaxel in primary breast cancer. J Clin Oncol. 2006;24:1839-45
9. Schrag D, Garewal HS, Burstein HJ. et al. American Society of Clinical Oncology technology assessment: chemotherapy sensitivity and resistance assays. J Clin Oncol. 2004;22:3631-8
10. Samson DJ, Seidenfeld J, Ziegler K, Aronson N. Chemotherapy sensitivity and resistance assays: a systematic review. J Clin Oncol. 2004;22:3618-30
11. Mi Z, Holmes FA, Hellerstedt B. et al. Feasibility assessment of a chemoresponse assay to predict pathologic response in neoadjuvant chemotherapy for breast cancer patients. Anticancer Res. 2008;28:1733-40
12. Liedtke C, Packeisen J, Hess KR. et al. Systematic analysis of in vitro chemosensitivity and mib-1 expression in molecular breast cancer subtypes. Eur J Cancer. 2012;48:2066-74
13. McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785:96-132
14. Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379-93
15. Swanton C, Marani M, Pardo O. et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498-512
16. Whitehurst AW, Bodemann BO, Cardenas J. et al. Synthetic lethal screen identification of chemosensitizer loci in cancer cells. Nature. 2007;446:615-819
17. Sudo T, Nitta M, Saya H, Ueno NT. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004;64:2502-8
18. Shen SC, Huang TS, Jee SH, Kuo ML. Taxol-induced p34cdc2 kinase activation and apoptosis inhibited by 12-O-tetradecanoylphorbol-13-acetate in human breast MCF-7 carcinoma cells. Cell Growth Differ. 1998;9:23-9
19. Castedo M, Perfettini JL, Roumier T, Kroemer G. Cyclin-dependent kinase-1: linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ. 2002;9:1287-93
20. Nakayama S, Torikoshi Y, Takahashi T. et al. Prediction of paclitaxel sensitivity by CDK1 and CDK2 activity in human breast cancer cells. Breast Cancer Res. 2009;11:R12
21. Takahashi T, Yamasaki F, Ueno NT. et al. Cyclin A-associated kinase activity is needed for paclitaxel sensitivity. Mol Cancer Ther. 2005;4:1039-1046
22. Ishihara H, Yoshida T, Kawasaki Y. et al. A new cancer diagnostic system based on a CDK profiling technology. Biochim Biophys Acta. 2005;1741:226-33
23. Van Nes JG, Smit VT, Putter H. et al. Validation study of the prognostic value of cyclin-dependent kinase (CDK)-based risk in Caucasian breast cancer patients. Br J Cancer. 2009;100:494-500
24. Kim SJ, Nakayama S, Miyoshi Y. et al. Determination of the specific activity of CDK1 and CDK2 as a novel prognostic indicator for early breast cancer. Ann Oncol. 2008;19:68-72
25. Symmans WF, Ayers M, Pusztai L. et al. Total RNA yield and microarray gene expression profiles from fine-needle aspiration biopsy and core-needle biopsy samples of breast carcinoma. Cancer. 2003;12:2960-2917
26. Gianni L, Kearns CM, Giani A. et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetics/pharmacodynamic relationships in humans. J Clin Oncol. 1995;13:180-190
27. Aleem E, Kiyokawa H, Kaidis P. Cdc-2-cyclin E complexes regulate the G1/S phase transition. Nat Cell Biol. 2005;7:831-836
28. Pusztai L. Markers predicting clinical benefit in breast cancer from microtubule-targeting agents. Ann Oncol. 2007;18(suppl 12):15-20
29. Hayes DF, Thor AD, Dressler LG. et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496-506
Author contact
![]() Corresponding author: Dr. Naoto T. Ueno. 1515 Holcombe, Unit 1354, Houston, TX 77030. e-mail: nuenoorg.
Corresponding author: Dr. Naoto T. Ueno. 1515 Holcombe, Unit 1354, Houston, TX 77030. e-mail: nuenoorg.

 Global reach, higher impact
Global reach, higher impact