Impact Factor
ISSN: 1837-9664
J Cancer 2013; 4(7):577-584. doi:10.7150/jca.7028 This issue Cite
Research Paper
Survival Advantage in Patients with Metastatic Breast Cancer Receiving Endocrine Therapy plus Sialyl Tn-KLH Vaccine: Post Hoc Analysis of a Large Randomized Trial
1. Departments of Breast Medical Oncology (NKI, JLM), Melanoma Medical Oncology (DZ), and Surgical Oncology (EAM), The University of Texas MD Anderson Cancer Center, Houston, TX;
2. Research and Development, Biomira Inc., Edmonton, AB, Canada (DS, MT);
3. University of Alberta, Edmonton, AB, Canada (DS);
4. Global IQ Inc., Edmonton, AB, Canada (MT);
5. Mount Vernon Cancer Center, Northwood, Middlesex, UK (DM).
Received 2013-6-27; Accepted 2013-8-7; Published 2013-8-22
Abstract
Background: A multicenter, double blinded, randomized phase III trial of the therapeutic cancer vaccine sialy1-Tn (STn) conjugated to keyhole-limpet Hemocyanin (KLH) was completed in an international cohort of 1,028 women with metastatic breast cancer who had nonprogressive disease or no evidence of disease after first-line chemotherapy (ClinicalTrials.gov, (NCT00003638). STn-KLH was safe and relatively well tolerated but did not affect time to progression (TTP) or overall survival (OS) duration. The purpose of this post hoc analysis was to explore whether patients who received concurrent endocrine therapy and STn-KLH had a TTP or OS benefit.
Methods: A retrospective, blinded review of the data from the phase III trial of STn-KLH was performed to ensure that strata assignments were appropriate. We then studied the effect of concomitant endocrine therapy and STn-KLH or KLH on TTP and OS in the cohort described above. We also assessed the TTP and OS by antibody responses in patients who received endocrine therapy.
Results: The women treated with concomitant endocrine therapy, a pre-stratified subset comprising approximately one-third of the original study population, and STn-KLH had longer TTP and OS than the control group of women who received KLH alone. Moreover, of the women who received endocrine therapy, those who had a median or greater antibody response (titer >1:320 toward ovine sub maxillary mucin) to the STn-KLH vaccine had significantly longer median OS than those who had a below-median antibody response.
Conclusion: Adding STn-KLH to endocrine therapy may improve clinical outcomes with few adverse effects for women with metastatic breast cancer.
Keywords: sialy1-Tn, keyhole-limpet Hemocyanin, metastatic breast cancer
INTRODUCTION
Therapeutic cancer vaccines are tumor antigen-like molecules designed to stimulate humoral and/or cell-mediated immunity and to recognize and selectively kill cancer cells. Sialyl-Tn (STn) is a naturally occurring carbohydrate epitope found on a variety of glycoproteins, including mucin 1 (MUC 1), expressed by many types of tumor cells and is believed to have functional significance in tumor growth and metastasis (1). Increased expression of STn has been associated with tumor aggression and poor prognosis of breast cancer, in addition to various other epithelial cancers (2-5). Furthermore, preclinical data suggest that there is crosstalk between the estrogen receptor (ER) and the MUC1 pathways (6), and it has been shown that MUC1 stimulates ER-α-mediated transcription and contributes to estrogen-mediated growth and survival of breast cancer cells. Thus, MUC1 has been proposed to be an oncoprotein that activates ER-α function (7).
Theratope (Biomira Inc., Edmonton, AB, Canada), a therapeutic cancer vaccine, utilizes a synthetic antigen that mimics the STn antigen and is conjugated to the high-molecular-weight protein carrier keyhole limpet hemocyanin (KLH) and administered with an adjuvant, Detox B stable emulsion (later renamed after reformulation as Enhanzyn, Corixa Corp., Hamilton, MA). The vaccine has encouraging safety and immunogenicity profiles in breast cancer patients, as demonstrated in phase I, II, and III clinical trials (8-15). The importance of specific anticancer antigen humoral immune responses in metastatic cancer has been established (16-18). A study of patients with metastatic breast cancer showed that the baseline antibody titers to ovine sub maxillary mucin (OSM), a natural mucin that is known to present “multimers” or “clusters” of the STn epitope similar to that of the STn antigen (19), were negligible. With vaccination with STn-KLH, however, patients who showed high levels of anti-mucin IgG and CD69+CD4+ T cells (activated helper T cells) and low levels of CD20-HLA-DR+ cells (a suppressive T-cell phenotype), were correlated with prolonged survival (20). In another study, metastatic adenocarcinoma patients with an anti-OSM IgG antibody titer greater than the overall median survived longer than those with a titer less than or equal to the overall median titer (9). In addition, a multicenter bridging trial of the current formulation of STn-KLH plus Enhanzyn (Corixa Corp., Hamilton, MA) demonstrated the vaccine's safety and immunogenicity (Biomira Inc., unpublished data).
A large phase III randomized trial utilizing STn-KLH (13), showed that the vaccine is well tolerated, in patients with metastatic breast cancer, however, no overall benefit in TTP or survival was observed. In an exploratory analysis of the survival data patients who received hormonal therapy with the STn-KLH vaccine and had anti-OSM IgG titers higher than the median of 1:320 had a longer median OS compared with the KLH-control group (14). To confirm this observation, we undertook a post hoc analysis of the TTP and OS data of the patients who were concurrently using an antiestrogen, most often a SERM or AI; this subgroup constituted approximately one third of the trial population (13). The goal of this study was to determine whether the STn-KLH vaccine combined with endocrine therapy provided a clinical benefit and to explore any differential effects between the two classes of endocrine therapy when used with the STn-KLH vaccine.
METHODS
Patients and Treatment
All patient data used in this study were obtained as part of an institutional review board-approved randomized phase III clinical trial testing the use of STn-KLH versus KLH (the control arm) in 1,028 women with metastatic breast adenocarcinoma that was nonprogressive after first-line chemotherapy. An original report of the trial and its findings has been published (13). Briefly, patients were evaluated for eligibility and signed an informed consent form before enrollment. Patients were randomized to receive the STn-KLH vaccine or KLH after 1 intravenous dose of cyclophosphamide, given to decrease numbers of circulating T-regulatory cells (10). At study entry, participants were stratified into one of two groups: those who had no evidence of breast cancer and those who had non progressive disease. After approximately 150 participants had been enrolled in the study, an institutional review board-approved protocol amendment was introduced to allow women to enter the study while receiving concomitant endocrine therapy, thereby to increase the enrollment rate. To minimize the potential for bias, participants were stratified by whether they did or did not receive concomitant endocrine therapy at study entry.
Patient Data Adjustment for Exploratory Analyses
We undertook a post hoc, blinded, retrospective review of all study participants to ensure that their inclusion in the study and their stratum assignments were appropriate. This review was undertaken by a group of physicians commissioned by the study sponsors (Biomira Inc. and Merck KGaA, Darmstadt, Germany). All reviewers were blinded to the participants' treatment arms, original stratification assignments, and outcomes. The endocrine therapy data set defined by these reviewers, which included only participants who received concurrent endocrine therapy, was then used to analyze the primary endpoints of TTP (number of months, from first vaccination date until disease progression) and OS (months from first vaccination to death or last follow-up), as well as TTP and OS time in relation to the magnitude of the antibody response. OS data were collected for additional 12 months following the end of the study.
Levels of three types of IgG antibodies (anti-OSM IgG, anti-STn IgG, and anti-KLH IgG) were evaluated and assessed for associations with TTP and OS in the STn-KLH group. For the group that received KLH carrier with the endocrine therapy, the anti-KLH IgG was relevant and therefore was measured.
Statistical Methods
OS by specific serum antibody responses of participants in the sponsor-defined dataset were appraised by applying Cox proportional hazard regression models and Kaplan-Meier survival curves. Cox P-values were calculated using response to first-line chemotherapy and concurrent endocrine therapy use, with the inclusion of the prognostic variable of time from initial diagnosis to first metastasis. Data were analyzed using SAS version 9 software (SAS Institute, Inc., Cary, N.C.). The results were explored for evidence, or lack of, benefit from the combination of endocrine therapy with STn-KLH; notable outcomes and P‑values less than.05 were considered statistically meaningful. All analyses using the sponsor-defined dataset were undertaken post hoc, and no adjustments for multiplicity of statistical tests were foreseen.
Role of the Funding Sources
Merck KGaA, after partnering with Biomira Inc. had full ownership of the data. Merck KGaA funded Biomira Inc.'s project manager and statistician to assist in the completion of this analysis.
RESULTS
Patient's' Characteristics
After study strata (no evidence of disease/nonprogressive disease, endocrine therapy use/no endocrine therapy use) were reassessed post hoc, 31 participants (9%) were reassigned to a more appropriate treatment stratum: if a patient was ER negative but receiving endocrine therapy according to the reviewer, she will be removed from the post-hoc analysis; on the other hand, if a patient was initially considered ER negative but on review of data was found to be ER positive, that patient will be grouped with the eligible patients for post-hoc analysis. The sponsor-defined endocrine dataset, confirmed by the review team, had 350 patients: 180 in the STn-KLH treatment group and 170 in the control group. These two groups were well balanced (Table 1).
Baseline characteristics of participants who received endocrine therapy (sponsor-defined subset)
| Participant characteristics | Treatment | Total (n = 350) | |||
|---|---|---|---|---|---|
| KLH (n = 170) | STn-KLH (n = 180) | P | |||
| Median age, y | 54.0 | 53.0 | 53.0 | ||
| Estrogen receptor status of tumor, no. (%) | Positive | 133(78.2) | 142(78.9) | 275(78.6) | |
| Negative | 17(10.0) | 16(8.9) | 33(9.4) | .94 | |
| Not available | 20(11.8) | 22(12.2) | 42(12.0) | ||
| Progesterone receptor status of tumor, no. (%) | Positive | 95(55.9) | 114(63.3) | 209(59.7) | |
| Negative | 28(16.5) | 27(15.0) | 55(15.7) | .33 | |
| Not available | 47(27.6) | 39(21.7) | 86(24.6) | ||
| HER2/neu receptor over expression, no. (%) | Yes | 24(14.1) | 22(12.2) | 46(13.1) | |
| No | 57(33.5) | 68(37.8) | 125(35.7) | 0.68 | |
| Unknown | 89(52.4) | 90(50.0) | 179(51.2) | ||
| Type of metastatic disease, no. (%) | Visceral | 132(77.6) | 131(72.7) | 263(75.1) | .65 |
| Superficial | 14(8.2) | 17(9.4) | 31(8.9) | ||
| Bone only | 24(14.2) | 32(17.8) | 56(16.0) | ||
| Time from primary diagnosis to first metastasis, median (interquartile range), mos. | 33.4 | 37.0 | 36.2 | .67 | |
| (6.5), (66.2) | (11.3), (71.9) | (9), (68) | |||
| Time from first metastasis to first injection of vaccine, median (interquartile range), mos. | Median | 7.8 | 7.8 | 7.8 | .49 |
| 25th, 75th percentile | 6.1,10.0 | 6.4,9.9 | 6,10 | ||
| Type of endocrine therapy, no. (%) | SERM | 67(39.4) | 78(43.3) | 145(41.4) | .63 |
| AI | 88(51.8) | 84(46.7) | 172(49.1) | ||
| Other | 15(8.8) | 18(10.0) | 33(9.5) | ||
AI = aromatase inhibitor; KLH = keyhole-limpet Hemocyanin; SERM = selective estrogen receptor modulator; STn = Sialyl-Tn.
Survival at time of final analysis of study end points by Anti-mucin Antibody Response
A statistically meaningful OS difference was seen in patients who received STn-KLH plus endocrine therapy compared to KLH group, at 1 year of follow-up (Table 2). Among the patients treated with STn-KLH plus endocrine therapy, those who had an anti-OSM IgG titer greater than or equal to the median (1:320) had a significantly longer median OS than those with a titer lower than the median (median OS times at end of study, 39.6 months and 25.4 months, respectively; Cox P =.005). In the same treatment group the former also tended to have a longer TTP (median TTPs, 10.6 months and 6.3 months, respectively; Cox P =. 078). On the other hand, among the participants who received unconjugated KLH plus endocrine therapy (control group), those who had an anti-KLH IgG titer less than the median (1:81920) survived longer than those whose titer was greater than or equal to the median; this difference in median OS time was statistically meaningful when analyzed at the planned end of the study per the protocol (Cox P =. 0072) but was not significant in analyses performed after subsequent follow-up of 12 months (Table 2).
Long-term Survival with Continued Follow-up
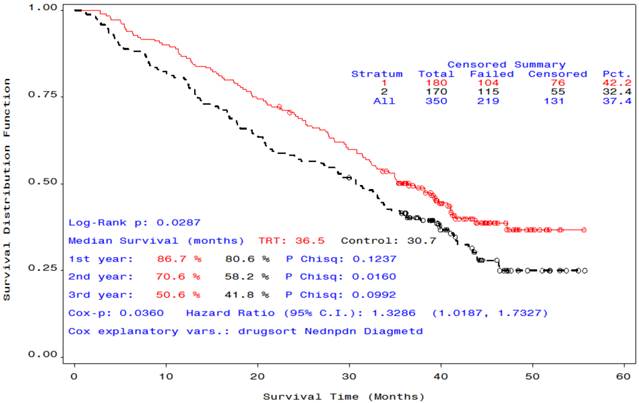
After an additional 12 months of follow-up, the median OS time of the group that received STn-KLH plus endocrine therapy was 36.5 months, while the median OS time of the group that received unconjugated KLH plus endocrine therapy was 30.7 months (Cox P =.036, Log-Rank P=. 029), a statistically meaningful difference (Figure 1).
Median OS (in months) by IgG antibody response at the end of the study, and at 6 and 12 months, thereafter, of the sponsor-defined subsets
| STn-KLH N=142 | KLH N=123 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time of analysis | α-Anti OSM IgG titer relative to median | α-Anti STn IgG titer relative to median | α-Anti KLH IgG titer relative to median | α-Anti KLH IgG titer relative to median | ||||||||
| < (n=45) | ≥ (n=97) | Cox p | < (n=61) | ≥ (n=81) | Cox p | < (n=56) | ≥ (n=86) | Cox p | < (n=48) | ≥ (n=75) | Cox P | |
| At end of study analysis-of all study end points | 25.4 | 39.6 | .0050 | 35.0 | 41.1 | .2183 | 38.2 | 35.0 | .9390 | Not reached | 29.3 | .0072 |
| At 6 months after end of study | 25.4 | 41.1 | .0125 | 35.0 | 41.1 | .3610 | 39.6 | 41.1 | .9118 | 43.0 | 31.6 | .0657 |
| At 1 year | 25.4 | 41.3 | 0.0147 | 35.1 | 41.1 | .3745 | 38.2 | 40.8 | .9346 | 39.5 | 30.8 | .3387 |
IgG = immunoglobulin G; KLH = keyhole-limpet Hemocyanin; OS, overall survival; OSM, ovine submaxillary mucin; STn = Sialyl-Tn.
Overall survival curves of participants who received endocrine therapy plus STn-KLH (red) or KLH (black).
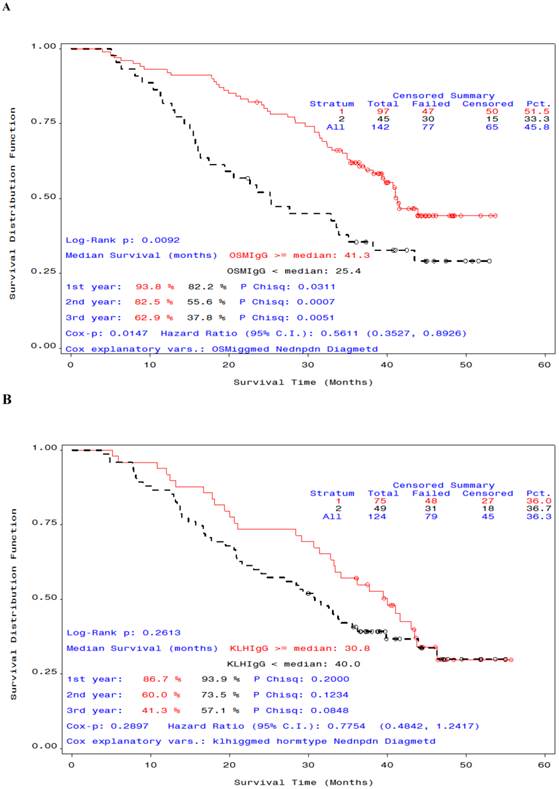
We also examined OS time by antibody response. In patients who received STn-KLH and endocrine therapy, a median or greater anti-OSM IgG titer was associated with a statistically meaningful OS advantage compared with an anti-OSM IgG titer that was lower than the median (median OS at 12-month follow-up analysis, 41.3 months and 25.4 months, respectively; Cox P =.0147, Log-Rank P=.009 (Figure 2,A); however, the difference in OS time by antibody response was not significant for anti-KLH IgG (Figure 2,B). A statistically meaningful median survival of patients who received KLH plus endocrine therapy that was observed at the time of the primary analysis was not maintained with the additional 12 months of follow-up monitoring on study, with a corresponding loss of the statistically meaningful significance (Table 2).
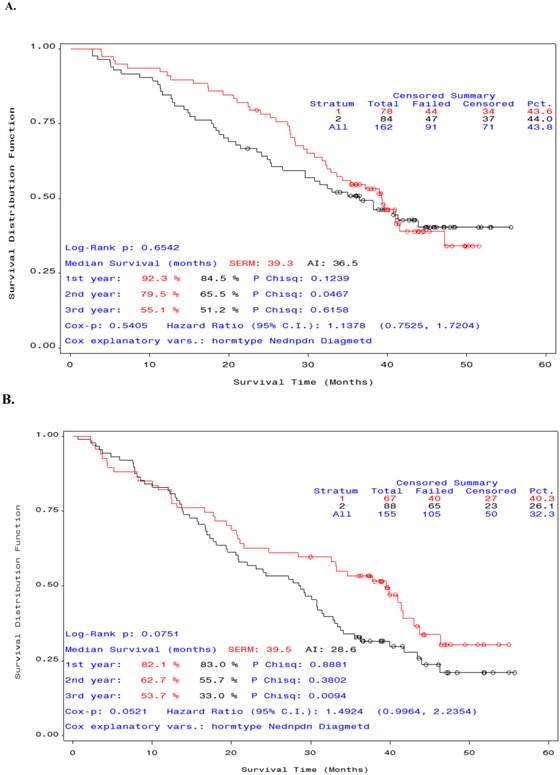
We also analyzed the OS of participants by type of endocrine therapy: SERM or AI. There was no significant difference in median OS between the patients who received SERM versus Al in combination with STn-KLH (Figure 3, A). However, among patients who received unconjugated KLH and endocrine therapy, the median OS was higher in those who received SERM, though not statistically significant (Figure 3, B).
Overall survival curves by antibody response for patients who received endocrine therapy with STn-KLH (A) or with KLH (B) (sponsor-defined subset). A) Patients whose α-anti ovine submaxillary mucin immunoglobulin G titer was equal to or greater than the median (red) survived significantly longer than those whose titer was less than the median (black) P =. 0092); B) For KLH titer, the difference in survival was not significant P =. 26.
Overall survival curves by type of endocrine therapy (SERM or AI) in patients who received STn-KLH or KLH. Patients in the selective estrogen receptor modulator group (red) survived longer than those in the aromatase inhibitor group (black). The difference between the groups that received STn-KLH was not significant (A), but a substantial [but not significant] difference was seen between the groups [A] that received KLH (the control vaccine) (B).
DISCUSSION
This post-hoc study suggests that the combination of STn-KLH vaccine plus endocrine therapy offers a survival advantage to women with metastatic breast cancer. This effect is particularly pronounced in women who have a robust antibody response to the vaccine. These findings, in combination with preclinical data showing a cross talk effect between ER and MUC1 on cell transcription and division, may justify prospective randomized trials for verification.
The number of patients reassigned to a different stratum was small and unlikely to have altered the outcome of the original intent-to-treat analysis. However, the main study (13) was not powered to detect the impact of hormonal therapy in conjunction with the vaccine, and therefore interpretation of the data should be in the context of hypothesis generation.
In the post hoc study reported here, we analyzed OS by anti-OSM, anti-STn, and anti-KLH IgG antibody responses to identify any associations. Intent-to-treat analysis of the subset of participants included in the data sets defined by sponsor-appointed reviewers showed a significantly longer median OS in women who received the STn-KLH vaccine plus endocrine therapy and had anti-OSM IgG serum antibody titers at or above the median value than those who had titers below the median. This phenomenon was upheld over time; the benefit was seen in analyses at the planned end of the study and at 6-month and 12-month follow-up analyses. Among participants who received only the carrier (KLH) with endocrine therapy, those with lower-than-median anti-KLH titers initially had a longer median OS than those with median or higher titers which was not sustained upon longer follow-up. Nevertheless, this prolonged survival suggests that the overall immune function of vaccinated patients, even when challenged by a non- specific (control) antigen, may be responsible for controlling tumor volume.
The mechanisms by which STn-KLH induces an antibody response in breast cancer patients remain unclear. The STn antigen is a disaccharide epitope that is conjugated to a carrier protein, KLH, which was designed to elicit helper T cell responses required for class switching and affinity maturation to form IgG molecules. Such a design often induces the immune system to produce skewed IgG responses toward the carrier protein (KLH). OSM is not a human cancer protein; thus, the IgG response toward OSM in vaccinated patients is probably specific to STn antigens expressed by OSM. However, we cannot definitively exclude the possibility that vaccination also induced antibodies that cross-react with the protein backbone of OSM.
In a review of the literature, Sanchez-Muñoz et al. (21) examined nine randomized trials that evaluated the benefits of maintenance chemotherapy for the treatment of metastatic breast cancer. In studies published between 1987 and 1998, the TTP was 3.2 - 18.7 months. In studies published between 2003 and 2007, some of which included patients receiving concomitant endocrine therapy, TTP was 3.5 - 9.0 months. Sanchez-Muñoz et al. suggested that the benefits of maintenance chemotherapy should be considered in terms of the potential cumulative toxicity of chemotherapy; comparable survival benefits with STn-KLH and an antiestrogen agent, albeit in patients with hormone receptor-positive tumors, were seen without cumulative side effects; the observed median TTP of 10.6 months for women with a robust OSM antibody response and concomitant endocrine therapy was longer than that reported in other studies for patients who received maintenance chemotherapy.
Multiple randomized trials have confirmed that first-line treatment of metastatic breast cancer with an AI yields significantly longer median TTP and OS than treatment with tamoxifen. We examined whether outcome varied depending on whether a SERM or an AI was taken with STn-KLH. Contrary to our expectation, women taking tamoxifen with STn-KLH or KLH survived longer, though not significantly so, than women who took an AI with their immune modulator (Figure 3). This result may have been due to an underpowered sample or to chance. On the other hand, a randomized phase II trial, using a different MUC1 antibody given with an AI as first-line therapy for locally advanced or metastatic hormone-receptor- positive breast cancer did not show any clinical benefit (22) leading us to speculate that anti-MUC1 therapy would be more efficient if used with a SERM rather than an AI. Findings by Wei et al. of a signaling pathway interaction between MUC1 and ERs that result in increased transcription and tumor growth (7) support this clinical observation. Blocking ERs with the use of a SERM appears to be a better strategy for harnessing MUC1 modulation of the ER in post-menopausal women with metastatic breast cancer than blocking the peripheral production of estrogen with A1.
In summary, we found that women with metastatic breast cancer who were given endocrine therapy concurrently with the STn-KLH vaccine experienced a statistically significant OS benefit compared with those treated with unconjugated KLH and endocrine therapy. It is not possible with the current dataset to determine the mechanism of this benefit: It may be related to the burden of metastatic disease, sites of metastasis, or to a currently undescribed interaction between STn-KLH and endocrine therapy, elements that may define tumor with more favorable immune- biology. This study, however, provides justification for combining anti-MUC1 vaccine with a SERM in randomized trials, particularly as adjuvant therapy for patients with a high risk of recurrence or with a high burden of residual disease after receiving neoadjuvant chemotherapy.
Acknowledgements
The authors would like to thank Dr. Sheela Hota-Mitchell of Write On Science (www.writeonscience.com) for assistance with the writing and editing of this manuscript. We also wish to acknowledge the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editorial support and Cynthia Griffin for secretarial assistance. We also wish to acknowledge all the participants in the trial.
Funding
This work was supported by Biomira Inc., Edmonton, AB, Canada, and Merck KGaA. Darmstadt, Germany.
Competing Interests
See funding information in Acknowledgments.
References
1. Julien S, Picco G, Sewell R. et al. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br J Cancer. 2009;100:1746-1754
2. Longenecker BM, Reddish M, Koganty R, MacLean GD. Immune responses of mice and human breast cancer patients following immunization with synthetic sialyl-Tn conjugated to KLH plus Detox adjuvant. Ann N Y Acad Sci. 1993;690:276-291
3. Nakagoe T, Sawai T Tsuji T. et al. Pre-operative serum levels of sialyl Tn antigen predict liver metastasis and poor prognosis in patients with gastric cancer. Eur J Surg Oncol. 2001;27:731-739
4. Kinney AY. Sahin A, Vernon SW, et al. The prognostic significance of sialyl-Tn antigen in women with breast cancer treated with adjuvant chemotherapy. Cancer. 1997;80:2240-2249
5. Holmberg LA, Sandmaier BM. Theratope vaccine (STn-KLH). Expert Opin Biol Ther. 2001;1:881-891
6. van der Vegt B, de Roos MA, Peterse JL. et al. The expression pattern of MUC1 (EMA) is related to tumour characteristics and clinical outcome of invasive ductal breast carcinoma. Histopathology. 2007;51:322-335
7. Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol Cell. 2006;21:295-305
8. MacLean GD, Reddish M, Koganty RR. et al. Immunization of breast cancer patients using a synthetic sialyl-Tn glycoconjugate plus Detox adjuvant. Cancer Immunol Immunother. 1993;36:215-222
9. MacLean GD, Reddish MA, Koganty RR, Longenecker BM. Antibodies against mucin-associated sialyl-Tn epitopes correlate with survival of metastatic adenocarcinoma patients undergoing active specific immunotherapy with synthetic STn vaccine. J Immunother Emphasis Tumor Immunol. 1996;19:59-68
10. Miles DW, Towlson KE, Graham R. et al. A randomized phase II study of sialyl-Tn and DETOX-B adjuvant with or without cyclophosphamide pretreatment for the active specific immunotherapy of breast cancer. Br J Cancer. 1996;74:1292-1296
11. Holmberg LA, Sandmaier BM. Vaccination with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev Vaccines. 2004;3:655-663
12. Miles D, Papazisis K. Rationale for the clinical development of STn-KLH (Theratope) and anti-MUC-1 vaccines in breast cancer. Clin Breast Cancer. 2003;3(Suppl 4):S134-S138
13. Miles D, Roché H, Martin M. et al. Phase III multicenter clinical trial of Sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer patients. Oncologist. 2011;16:1092-1100
14. Ibrahim NK, Murray J, Parker J, Finke L, Miles D. Humoral immune response to naturally occurring STn in metastatic breast cancer patients (MBC pts) treated with STn-KLH vaccine. J Clin Oncol. 2004;22(Suppl):S14 (abstr 2547)
15. Musselli C, Livingston PO Ragupathi G. Keyhole limpet hemocyanin conjugate vaccines against cancer: the Memorial Sloan-Kettering experience. J Cancer Res Clin Oncol. 2001;127(Suppl 2):S20-26
16. Foy TM, Fanger GR, Hand S. et al. Designing HER2 vaccines. Semin Oncol. 2002;29(Suppl 11):S53-S61
17. Cristofanilli M, Hortobagyi GN. New horizons in treating metastatic disease. Clin Breast Cancer. 2001;1:276-287
18. Reilly RT, Emens LA, Jaffee EM. Humoral and cellular immune responses: independent forces or collaborators in the fight against cancer? Curr Opin Investig Drugs. 2001;2:133-135
19. Reddish MA, Jackson L, Koganty RR. et al. Specificities of anti-sialyl-Tn and anti-Tn monoclonal antibodies generated using novel clustered synthetic glycopeptide epitopes. Glycoconj J. 1997;14:549-560
20. Holmberg LA, Oparin DV, Gooley T, Sandmaier BM. The role of cancer vaccines following autologous stem cell rescue in breast and ovarian cancer patients: experience with the STn-KLH vaccine (Theratope). Clin Breast Cancer. 2003;3(Suppl 4):S144-S151
21. Sanchez-Muñoz A, Perez-Ruiz E, Ribelles N. et al. Maintenance treatment in metastatic breast cancer. Expert Rev Anticancer Ther. 2008;8:1907-1912
22. Ibrahim NK, Yariz KO, Bondarenko I. et al. Randomized phase II trial of letrozole plus anti-MUC1 antibody AS1402 in hormone receptor-positive locally advanced or metastatic breast cancer. Clin Cancer Res. 2011;17:6822-6830
Author contact
![]() Corresponding author: Nuhad K. Ibrahim, MD, FACP. Department of Breast Medical Oncology, Unit 1354, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd. Houston, TX 77030. Phone: 713-792-2817; Fax: 713-794-4385; Email: nibrahimorg
Corresponding author: Nuhad K. Ibrahim, MD, FACP. Department of Breast Medical Oncology, Unit 1354, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd. Houston, TX 77030. Phone: 713-792-2817; Fax: 713-794-4385; Email: nibrahimorg

 Global reach, higher impact
Global reach, higher impact