3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2011; 2:317-323. doi:10.7150/jca.2.317 This volume Cite
Review
Immune Effects of Trastuzumab
“Sapienza” University of Rome, Italy
Received 2011-4-23; Accepted 2011-5-24; Published 2011-5-25
Abstract
Trastuzumab's targeted therapy has become a stronghold for human epidermal growth factor receptor 2 positive breast cancer patients. This humanized monoclonal antibody binds to the extracellular juxta-membrane domain of HER2 and inhibits the proliferation and survival of HER2 dependent cancer cells. The ways by which this molecule exerts its action have been partially elucidated but several new mechanisms are being constantly identified. Several new agents are being introduced that interfere with HER2. Several new immunotherapy strategies are being introduced in order to direct the immune system against cells and tissues that aberrantly overexpressed HER2. We review the strategies currently adopted and those suggested against HER2 expressing tumors.
Keywords: autologous cells vaccines, Trastuzumab, HER2, Lapatinib, allo-vesicles
INTRODUCTION
HER2 (Human Epidermal growth factor Receptor 2) is a tyrosine kinase receptor of the family that includes HER1 (EGFR), HER3 and HER4. This receptor mediates cell differentiation and proliferation in both embryonic and adult tissues. More than 30 ligands have been identified that bind epidermal growth factor receptor with the exception of HER2, which is the only deaf member. HER2 over-expression can therefore induce transformation in a ligand independent way [1].
HER2 RECEPTOR
Prognostic effects of HER2 over-expression in breast cancer patients
The HER2, also known as neu or c-erbB-2 [2], is a proto-oncogene located on the long arm of human chromosome 17 (17 q21-q22) that encodes a protein of 185 kDa [1, 2].
HER2 activation induces cell proliferation, migration and differentiation. Furthermore HER2 is involved in an anti-apoptotic mechanism and in the production of vascular epidermal growth factor (VEGF). All these activities are mediated by two intracellular pathways which include PI3K and MAP-kinase [3].
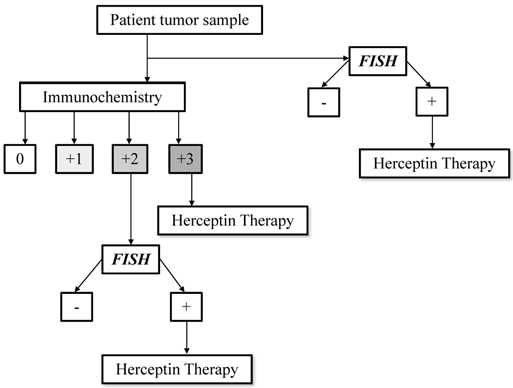
In human tissues HER2 gene amplification level and protein overexpression can be tested by Fluorescence In Situ Hybridization (FISH) and/or immunohystochemistry (IHC) respectively, according to the guidelines of the American Society of Clinical Oncology (ASCO) and College of American Pathologist (CAP) (Figure 1) [4].
Recently some authors advocated the use of mRNA dosage as a more adequate diagnostic tool [5]. The incidence of HER2 receptor expression, evaluated by IHC in different neoplasms is reported in Table 1.
A significant correlation between HER2 gene overexpression and lymph nodal metastasis has been identified in breast cancer. Furthermore c-erbB2 is associated with drug resistance [12].
In the absence of specific targeted therapies HER2 gene amplification represents one of the most important negative prognostic factors in early and advanced breast cancer [6].
TRASTUZUMAB
Trastuzumab and clinical outcome
Standard treatment for breast cancer patients includes surgery, chemotherapy, radiotherapy and hormonal therapy that are administered according to clinical, pathological and biological characteristics [13, 14]. Since 1998 the FDA has approved Trastuzumab for the treatment of recurrent HER2 positive BC patients. Emerging evidence from large phase III randomized trials have extended the indications of Trastuzumab to the vast majority of IHC triple positive or FISH positive HER2 patients. Briefly the results of the most relevant clinical trials are summarized in Table 2.
HER2 testing algorithm. Modified from Hanna MW, Mod Pathol 2006.
HER2 overexpression in different neoplasms
| AUTHORS | TYPE OF NEOPLASMS | IHC HER2-OVEREXPRESSION (%) |
|---|---|---|
| Slamon et al. [6] | Breast cancer | 30% |
| Slamon et al. [7] | Ovarian cancer | 25-30% |
| Hofmann M et al. [8] | Gastric cancer | 7-34% |
| Santin et al. [9] | Endometrial cancer | 10% |
| Liu et al. [10] | Lung cancer | 9% |
| Langner et al. [11]. | Urinary tract transitional cells carcinoma | 0-10% |
Results of clinical trials
| CLINICAL TRIALS | ||||
|---|---|---|---|---|
| Slamon DJ et al. [15] | METASTATIC BREAST CANCER | CT alone | 20.1 median OS | p=0,046 |
| CT plus Trastuzumab | 25.1 median OS | |||
| Romond EH et al. [16] | EARLY STAGES LOCALLY INVASIVE BREAST CANCER | CT alone | 86.6% OS at 4 years | p=0,015 |
| CT plus Trastuzumab | 91.4% OS at 4 years | |||
| Piccart-Gebhart MJ et al. [17] | CT alone | 95.1% OS at 2 years | p=0,26 | |
| CT plus Trastuzumab | 96% OS at 2 years | |||
| Smith I et al. [18] | CT alone | 89.4% OS at 2 years | p=0,005 | |
| CT plus Trastuzumab | 92.4% at 2 years | |||
OS=overall survival
Trastuzumab Mechanisms of Action
The ways through which Trastuzumab exerts its antitumor activity have not yet been completely unrevealed, however certain extracellular and intracellular mechanisms have been identified.
Intracellular action
By blocking dimerization, Trastuzumab interferes with the activity of the intracellular signaling activated by HER2 in healthy and mostly neoplastic tissues. This effect plays its role by inhibiting DNA damage repair, inducing cell cycle arrest and inhibiting tumor angiogenesis.
Trastuzumab decreases expression of p21/WAF1 and promotes early escape from G1 phase inducing accumulation of DNA damage and apoptosis [19]. This MoAb modulates the phosphorylation of p27Kip1 [20], protein involved in cell-cycle arrest in G1 phase [21]. It decreases survivin levels with consequently reduction in apoptosis resistance [22]. In addition Trastuzumab reduces VEGF secretion and therefore angiogenesis in neoplastic tissues.
Extracellular action
Trastuzumab plays its activity also through its antibody characteristics which affect the immunological control of neoplastic cells, islet and tissues. In recent years abundant evidences have demonstrated the pivotal role of the immune system on tumorogenesis and tumor progression in several different neoplasms [23-26].
Anti-HER2 MoAb activates both the innate and the adaptive immune system. The effect on the innate immune system, in particular Natural Killer (NK) cells, is mediated by Fc portion. In vitro and in vivo evidences have clearly demonstrated that this antibody is able to induce granzyme release with consequent tumor cells killing [27]. In addiction the effect of IFNγ released by activated NK cells is potentiated by the intracellular anti-apoptotic effect mediated by STAT1 [28].
A less intuitive but possibly important mechanisms are mediated by adaptive immune system. Trastuzumab-HER2 complexes are most rapidly internalized, thus HER2 undergoes to an increased intracellular degradation with a consequent production of HER2 epitopes. HLA class I molecules can be loaded with these HER2 fragment, allowing lysis of tumor cells by specific CTLs [29].
So far, it has been reported that Trastuzumab is able to induce a long lasting immune response [30], reduce circulating T regulatory cells (Tregs) [31] and alter the balance between Tregs and Th17 [32].
Lastly, apoptosis induced by NK cells generates apoptotic bodies which are easily fagocitated by APCs thereby increasing the number of specific antitumor circulating CTLs [33]. It is therefore evident how Trastuzumab not only activates the adaptive immune system against HER2 but represents an effective tool that determines also epitope spreading.
Resistance to Trastuzumab
Mechanisms involved to Trastuzumab resistance are HER2 mutation, cross-talk among the other extracellular HER proteins that induces incomplete inhibition of target receptor [34], masking of HER2 proteins mediated by MUC1/MUC4 glycoprotein (MUC1/MUC4) [35], inhibition of insulin-like growth factor [36] and tensin homologue (PTEN) deficiency [37].
NOVEL STRATEGIES
New anti-HER2 strategies
A detailed description of all tyrosine kinase inhibitors which block the intracellular pathways activated by HER2 is beyond this review's objectives. As follow, this is a brief description of the most important and promising drugs that have been introduced both to increase the effect of Trastuzumab and to bypass the mechanisms of resistance.
Lapatinib, a dual tyrosine kinase inhibitor (EGFR/HER1 and HER2), is the only targeted therapy other Trastuzumab approved by FDA for HER2-positive metastatic breast cancer patients. This small molecule works by competing with ATP for binding sites on intracellular portions of HER1/HER2 and targets the downstream ERK1-2 and PIK3-AKT pathways, which regulate cells proliferation and survival, respectively [38]. The role of Lapatinib in different treatment settings (neoadjuvant, adjuvant and metastatic) is being defined by ongoing trials. Differently from Trastuzumab, Lapatinib stabilizes the HER2 receptor on the cells surface thereby increasing the effect of Trastuzumab and consequent NK activity. On-going analysis on the immunological effect of Lapatinib are currently carried out by our group.
Some new agents such as tyrosine kinase (TKIs), VEGF and mTOR inhibitors, T-DM1, alvespspimycin (HSP90 inhibitor) and poly ADP-ribose polymerase-1 (PARP-1) inhibitors seem to have a theoretical benefit, but results on clinical trials that can define a role in treatment of Trastuzumab-resistant BC, are still unavailable (Table 3, Table 4) [39].
Dual targeting agents in development
| AGENT | TARGET | STAGE OF DEVELOPMENT | IRREVERSIBLE BINDING |
|---|---|---|---|
| Tyrosine kinase inhibitors | |||
| Lapatinib | EGFR/HER1, HER2 | Phase III breast and kidney cancer | No |
| BIBW-2992 | EGFR/HER1, HER2 | Phase II prostate | Yes |
| HKI-272 | EGFR/HER1, HER2 | Phase II breats and NSCLC | Yes |
| CI-1033 | EGFR/HER1, HER2, HER 4 | Phase II breast and NSCLC | Yes |
| Monoclonal antibodies | |||
| Pertuzumab | EGFR/HER1, HER2, HER 4 | Phase II ovarian, NSCLC, breast and prostate | ? |
Agents developed vs HER2-resistant breast cancer
| AGENT | MECHANISM OF ACTION | PHASE OF CLINICAL DEVELOPMENT |
|---|---|---|
| VACCINES | ||
| E75 | Activate cytotoxic T lymphocytes that identify HER2 cancer cells, leading to cell death | 1,2 |
| GP2 | Activate cytotoxic T lymphocytes that identify HER2 cancer cells, leading to cell death | 2 |
| AF37, li-Key | Direct antigenic epitope charging of HLA class II molecules on the cell surface | 2 |
| NEW COMPOUNDS | ||
| T-DM1 | Trastuzumab conjugated with maytansine to improve potency | 1,2,3 |
| KU-0059436 (Ku) | PARP inhibitor | 1,2 |
| Pertuzumab | Inhibits heterodimerization of HER2 and other EGFRs | 1,2,3 |
| Ertumaxomab | Biospecific monoclonal antibody that blcks HER2 and CD3 | 2 |
| Neratinib | Irreversible pan-ERBB inhibitor | 1,2,3 |
| Tanespimycin | HSP90 inhibitor | 1,2 |
| Alvespimycin | HSP90 inhibitor | 1,2 |
| Temsirolimus | mTOR inhibitor | 1,2,3 |
| Everolimus | mTOR inhibitor | 1,2 |
| Pazopanib | Multitargeted inhibitor of VEGFR, PDGFR and c-KIT | 1,2 |
| SIGNAL-TRANSDUCTION INHIBITION | ||
| Anastrozole + trastuzumab | Aromatase inhibitor plus HER2 inhibition | 2,3 |
Adjuvant vaccine immunotherapy
The relationship between cancer, cancer treatments and the immune system is extremely complex. Oncological procedures can activate or modulate an immune response [40, 41]. Furthermore the immune effect of different treatments depends on the timing of administration [25,42].
Researchers world-wide are investigating several new families of antigens in female tumors [43-46].
HER2 is one of the most studied targets for active immunotherapy. Several highly immunogenic epitopes have been identified; furthermore some interesting non randomized clinical data have strongly suggested a clinical efficacy when anti-HER2 peptide vaccination is carried out in a particular setting [47].
To be effective, simple peptide vaccination requires to be administered in patients with a competent immunological system. The immune system of women with high tumor burden suffers from an immune suppressed status especially due to a high proportion of regulatory T cells (Tregs) [25].
For this latter group more complex strategies are required in order to guarantee an immune effective response.
Currently some trials have been carried out using E75 and GP2 HER2 peptides. There are strongly suggestive data to demonstrate a synergistic effect between peptide-vaccination and the use of Trastuzumab [48].
More complex methods to induce a immune response include the use of fusion proteins primary constituted by a combination of multiple highly antigenic HER2 epitopes fused to the Fc domain of human IgG1. The rational of this approach is to specifically load the APC expressing the Fc receptors with the HER2 antigen, increasing antigen uptake, processing and epitope presentation.
A third step forward has been developed in a pre-clinical setting using micro-vesicles derived from HER2 transfected cell limphoblastoid cell line carrying HLAI/peptide complexes and co-stimulatory molecules. These vesicles can be utilized to pulse dendritic cells (DCs) and achieve very potent active anti tumor immune responses [49]. Both these approaches can be employed despite the HLA haplotype of the patients, in fact DCs are loaded with long domain of HER protein, thus enabling the DCs to generate a patient tailored repertoire of HER2 epitopes.
Recently DCs therapy have been approved by FDA for the treatment of prostatic cancer [50, 51]. One of the most recent avenues that are being pursued is the use of DCs. Cell therapy remains extremely complex and the quality of cells produce strictly depends on methods and materials adopted [52].
Currently some preliminary experiences on DCs-vaccination pulsed with HER2 peptides are present in literature. Of our interest will be the results of phase II randomized trial which will evaluate the safety and indirectly the immunological efficacy of two DCs vaccination administration strategies.
CONCLUSIONS
HER2 currently represents the most important biological target in the clinical practice for breast cancer patients. Trastuzumab plays its role both through immunological and intracellular mechanisms. Currently new drugs that target this receptor or its intracellular pathways are available and already employed in clinical settings. On the other hand, HER2 targeting strategies exert an important side effect on immunesurveillance, activating anti HER2 specific immune response and enhancing epitope spreading. The integration of immune vaccination strategies within the standard therapeutic framework can be an optimal and an efficacious approach to induce and maintain a long lasting anti-tumor immune response.
Acknowledgements
We would like to thank Ministry of Education, Universities and Research (Italy) and the Italian Association on Cancer Research (AIRC).
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756-760
2. Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U. et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132-1139
3. Hudis CA. Trastuzumab-mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39-51
4. Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009;27:1323-1333
5. Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409-1411
6. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-182
7. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792
8. Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805
9. Santin AD, Bellone S, Van Stedum S, Bushen W, De Las Casas LE, Korourian S, Tian E, Roman JJ, Burnett A, Pecorelli S. Determination of HER2/neu status in uterine serous papillary carcinoma: Comparative analysis of immunohistochemistry and fluorescence in situ hybridization. Gynecol Oncol. 2005;98:24-30
10. Liu L, Shao X, Gao W, Bai J, Wang R, Huang P, Yin Y, Liu P, Shu Y. The role of human epidermal growth factor receptor 2 as a prognostic factor in lung cancer: a meta-analysis of published data. J Thorac Oncol. 2010;5:1922-1932
11. Langner C, Gross C, Rehak P, Ratschek M, Rüschoff J, Zigeuner R. HER2 protein overexpression and gene amplification in upper urinary tract transitional cell carcinoma: systematic analysis applying tissue microarray technique. Urology. 2005;65:176-180
12. Révillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998;34:791-808
13. National Cancer Institute (NCI). http://www.cancer.gov/cancertopics/types/breast
14. Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ; Panel members. Thresholds for therapies: highlights of the St Gallen International Expert. Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319-1329
15. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707-712
16. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CEJr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-1684
17. Piccart-Gebhart MJ, Procter M, Leyland-Jones B. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-1672
18. Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sánchez Rovira P, Piccart-Gebhart MJ; HERA study team. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29-36
19. Pietras RJ, Poen JC, Gallardo D, Wongvipat PN, Lee HJ, Slamon DJ. Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res. 1999;59:1347-1355
20. Le XF, Claret FX, Lammayot A, Tian L, Deshpande D, LaPushin R, Tari AM, Bast RCJr. The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem. 2003;278:23441-23450
21. Le XF, Pruefer F, Bast RC Jr. HER2-targeting antibodies modulate the cyclin-dependent kinase inhibitor p27Kip1 via multiple signaling pathways. Cell Cycle. 2005;4:87-95
22. Asanuma H, Torigoe T, Kamiguchi K, Hirohashi Y, Ohmura T, Hirata K, Sato M, Sato N. Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res. 2005;65:11018-11025
23. Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903-907
24. Bellati F, Visconti V, Napoletano C, Antonilli M, Frati L, Panici PB, Nuti M. Immunology of gynecologic neoplasms: analysis of the prognostic significance of the immune status. Curr Cancer Drug Targets. 2009;9:541-565
25. Napoletano C, Bellati F, Landi R, Pauselli S, Marchetti C, Visconti V, Sale P, Liberati M, Rughetti A, Frati L, Panici PB, Nuti M. Ovarian cancer cytoreduction induces changes in T cell population subsets reducing immunosuppression. J Cell Mol Med. 2010;14:2748-2759
26. Bellati F, Napoletano C, Ruscito I, Pastore M, Pernice M, Antonilli M, Nuti M, Benedetti Panici P. Complete remission of ovarian cancer induced intractable malignant ascites with intraperitoneal bevacizumab. Immunological observations and a literature review. Invest New Drugs. 2010;28:887-894
27. Kahàn Z, Gardi J, Nyàri T, Földesi I, Hajnal-Papp R, Ormàndi K, Làzàr G, Thurzò L, Schally AV. Elevated levels of circulating insulin-like growth factor-I, IGF-binding globulin-3 and testosterone predict hormone-dependent breast cancer in postmenopausal women: a case-control study. Int J Oncol. 2006;29:193-200
28. Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula S, Jones NB, Roda JM, Mani A, Parihar R, Karpa V, Papenfuss TL, LaPerle KM, Biller E, Lehman A, Chaudhury AR, Jarjoura D, Burry RW, Carson WE 3rd. IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-γ production. J Immunol. 2011;186:3401-409
29. Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064-1071
30. Benavides LC, Gates JD, Carmichael MG, Patil R, Holmes JP, Hueman MT, Mittendorf EA, Craig D, Stojadinovic A, Ponniah S, Peoples GE. The impact of HER2/neu expression level on response to the E75 vaccine: from U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2009;15:2895-2904
31. Perez SA, Karamouzis MV, Skarlos DV, Ardavanis A, Sotiriadou NN, Iliopoulou EG, Salagianni ML, Orphanos G, Baxevanis CN, Rigatos G, Papamichail M. CD4+CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clin Cancer Res. 2007;13:2714-2721
32. Horlock C, Stott B, Dyson PJ, Morishita M, Coombes RC, Savage P, Stebbing J. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br J Cancer. 2009;100:1061-1067
33. Bellati F, Napoletano C, Ruscito I, Liberati M, Panici PB, Nuti M. Cellular adaptive immune system plays a crucial role in trastuzumab clinical efficacy. J Clin Oncol. 2010;28:369-370
34. Hynes NE, Dey JH. PI3K inhibition overcomes trastuzumab resistance: blockade of ErbB2/ErbB3 is not always enough. Cancer Cell. 2009;15:353-355
35. Nagy P, Friedländer E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473-482
36. Lu Y, Zi X, Pollak M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int J Cancer. 2004;108:334-341
37. Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118-111128
38. Burris HA 3rd, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O'Neil B, Marcom PK, Ellis MJ, Overmoyer B, Jones SF, Harris JL, Smith DA, Koch KM, Stead A, Mangum S, Spector NL. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305-5313
39. Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737-744
40. Zitvogel L, Tesniere A, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of anticancer chemotherapy. Bull Acad Natl Med. 2008;192:1469-1487
41. Napoletano C, Taurino F, Biffoni M, De Majo A, Coscarella G, Bellati F, Rahimi H, Pauselli S, Pellicciotta I, Burchell JM, Gaspari LA, Ercoli L, Rossi P, Rughetti A. RFA strongly modulates the immune system and anti-tumor immune responses in metastatic liver patients. Int J Oncol. 2008;32:481-490
42. Bellati F, Napoletano C, Gasparri ML, Panici PB, Nuti M. Immunologic systemic effect of neoadjuvant chemotherapy requires investigation before tumor-associated lymphocytes can be introduced in breast cancer treatment algorithm. J Clin Oncol. 2010;28:471-472
43. Yakirevich E, Sabo E, Lavie O, Mazareb S, Spagnoli GC, Resnick MB. Expression of the MAGE-A4 and NY-ESO-1 cancer-testis antigens in serous ovarian neoplasms. Clin Cancer Res. 2003;9:6453-6460
44. Resnick MB, Sabo E, Kondratev S, Kerner H, Spagnoli GC, Yakirevich E. Cancer-testis antigen expression in uterine malignancies with an emphasis on carcinosarcomas and papillary serous carcinomas. Int J Cancer. 2002;101:190-195
45. Napoletano C, Bellati F, Tarquini E, Tomao F, Taurino F, Spagnoli G, Rughetti A, Muzii L, Nuti M, Benedetti Panici P. MAGE-A and NY-ESO-1 expression in cervical cancer: prognostic factors and effects of chemotherapy. Am J Obstet Gynecol. 2008;198:99.e1-7
46. Bellati F, Napoletano C, Tarquini E, Palaia I, Landi R, Manci N, Spagnoli G, Rughetti A, Panici PB, Nuti M. Cancer testis antigen expression in primary and recurrent vulvar cancer: association with prognostic factors. Eur J Cancer. 2007;43:2621-2627
47. Peoples GE, Gurney JM, Hueman MT, Woll MM, Ryan GB, Storrer CE, Fisher C, Shriver CD, Ioannides CG, Ponniah S. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23:7536-7545
48. Benavides LC, Sears AK, Gates JD, Clifton GT, Clive KS, Carmichael MG, Holmes JP, Mittendorf EA, Ponniah S, Peoples GE. Comparison of different HER2/neu vaccines in adjuvant breast cancer trials: implications for dosing of peptide vaccines. Expert Rev Vaccines. 2011;10:201-210
49. Napoletano C, Rughetti A, Landi R, Pinto D, Bellati F, Rahimi H, Spinelli GP, Pauselli S, Sale P, Dolo V, De Lorenzo F, Tomao F, Benedetti-Panici P, Frati L, Nuti M. Immunogenicity of allo-vesicle carrying ERBB2 tumor antigen for dendritic cell-based anti-tumor immunotherapy. Int J Immunopathol Pharmacol. 2009;22:647-658
50. Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089-3094
51. Food and Drug Administration (FDA). http://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm210215.htm
52. Napoletano C, Pinto D, Bellati F, Taurino F, Rahimi H, Tomao F, Panici PB, Rughetti A, Frati L, Nuti M. A comparative analysis of serum and serum-free media for generation of clinical grade DCs. J Immunother. 2007;30:567-576
Author contact
![]() Corresponding author: Prof. Marianna Nuti, PhD, Experimental Medicine Department, viale Regina Elena, 324, 00161 Rome Italy. marianna.nutiit
Corresponding author: Prof. Marianna Nuti, PhD, Experimental Medicine Department, viale Regina Elena, 324, 00161 Rome Italy. marianna.nutiit

 Global reach, higher impact
Global reach, higher impact