3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2011; 2:94-106. doi:10.7150/jca.2.94 This volume Cite
Research Paper
Pathogenesis of Ovarian Clear Cell Adenofibroma, Atypical Proliferative (Borderline) Tumor, and Carcinoma: Clinicopathologic Features of Tumors with Endometriosis or Adenofibromatous Components Support Two Related Pathways of Tumor Development
1. Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA, USA;
2. Department of Gynecologic & Breast Pathology, Armed Forces Institute of Pathology, Washington, DC, USA;
3. Department of International Health, School of Public Health, The Johns Hopkins University, Baltimore, MD, USA;
4. Current affiliation: Department of Pathology, Walter Reed Army Medical Center, Washington, DC, USA
Received 2011-2-5; Accepted 2011-2-21; Published 2011-2-21
Abstract
The clinicopathologic features of 472 ovarian epithelial clear cell neoplasms (4 adenofibromas [AFs], 41 atypical proliferative [borderline] tumors [APTs], and 427 carcinomas [CAs]) were studied in order to elucidate the morphologic steps involved in the pathogenesis of these tumors and determine whether clear cell CA is a type I or type II tumor in the dualistic model of ovarian carcinogenesis. Thirty-three percent of the CAs had an adenofibromatous background [CA(AF+)], and 67% did not [CA(AF-)]. Endometriosis was found in all types of tumors, but tumors arising in endometriotic cysts were more frequent with CA(AF-)s (p<0.0001). The subset of women with CA(AF-)s with endometriosis were younger (p<0.0001), their tumors were more frequently cystic (p<0.0001), they more commonly had a mixed carcinoma component of non-clear cell type (p=0.006), and they were more frequently oxyphilic (p=0.015) compared with CA(AF+)s. The architecture of the former tumors was more commonly papillary compared to tubulocystic in the latter (p=0.0006). Atypical endometriosis was more common in CA(AF-)s than in AFs, APTs, and CC(AF+)s [p=0.004]. The subset of CA(AF-)s without endometriosis presented more frequently in advanced stage (>I) and were higher grade compared to CA(AF+)s or CA(AF-) with endometriosis (p-values, <0.0001 to 0.0071). All AFs and APTs were stage I compared to 79% of CA(AF+)s. An increase in mean tumor size correlated with each respective tumor category from AF (6.8 cm) to CA(AF+) [12.9 cm]. Notable nuclear atypia was absent in all AFs but was focally present in 27% of APTs and in the adenofibromatous background of 24% of the CA(AF+)s. An increase in the proportion of carcinoma in the CA(AF+)s correlated with an increase in grade and advanced stage. In summary, ovarian clear cell CA appears to develop along two pathways, both of which are related to endometriosis. We speculate that, in one, epithelial atypia arises in an endometriotic cyst and then evolves into clear cell CA, and, in the other, non-cystic endometriosis induces a fibromatous reaction resulting in the formation of AF, which then develops into APT and subsequently a clear cell CA. The absence of endometriosis or adenofibromatous components in CC(AF-)s may be due to overgrowth and obliteration by the invasive carcinoma. Finally, the findings in this study support the view that both types of clear cell CA [CC(AF+) and CC(AF-)] are more closely related to type I tumors.
Keywords: Ovary, clear cell carcinoma, adenofibromatous, endometriosis, pathogenesis.
INTRODUCTION
The dualistic model of ovarian carcinogenesis divides epithelial tumors into two groups designated type I and type II.40,41,59 Type I tumors are generally low-grade and include low-grade serous carcinoma, endometrioid carcinoma, mucinous carcinoma, and malignant Brenner tumor. They present in low stage, behave in an indolent fashion, are characterized by mutations of KRAS, BRAF, PTEN, and/or CTNNB1 and/or microsatellite instability, and display a relatively low level of chromosomal instability. They develop in a slow step-wise fashion from well-established precursor lesions such as cystadenofibroma and borderline tumors. In contrast, the type II tumors are high-grade and include high-grade serous carcinoma, transitional cell carcinoma, malignant mesodermal mixed tumor, and undifferentiated carcinoma. They are highly aggressive, present in advanced stage, have a very high frequency of TP53 mutations, and display a high level of chromosomal instability. They are not associated with the usual precursor lesions, and a proportion develop from intraepithelial carcinomas in the fallopian tube.
The pathogenesis of clear cell carcinoma (CA), however, is enigmatic and does not perfectly fit into this model because it has features shared with both type I and type II tumors. For example, like some type I tumors, clear cell CA is associated with endometriosis, adenofibromas (AFs)/atypical proliferative [borderline] tumors (APTs); low-stage disease; variable frequency of mutations of PIK3CA, KRAS, and PTEN and microsatellite instability; low frequency of p53 mutations; and low to moderate level of chromosomal instability.8,9,14,19,25,32,37,38,40,56,59,65,70,71,75 However, similar to type II tumors, clear cell CA is high-grade and associated with poor behavior when it is of high-stage.8,20,24,25,44,58,64,66
Although numerous epidemiologic, clinical/radiologic, histologic, immunohistochemical, and molecular studies have provided compelling evidence that endometriosis/endometriotic cysts are precursors of clear cell CA,2,3,5-7,10,12,15,17,20,21,23,26-36,39,42,43,45-50,53,54,57,63,67,69,70,72,75,76 the exact pathogenic relationship of clear cell AF/APT with CA is ambiguous. Reports of small clear cell CAs/microinvasion arising in APT, the presence of an adenofibromatous background within clear cell CA, and molecular data showing (a) a clonal relationship between clear cell AF, APT, and CA and (b) tumor progression of AF/APT all suggest an adenofibroma-carcinoma sequence.4,30,55,70,74,75,77 Some studies have demonstrated clinicopathologic and molecular differences between endometriosis-associated clear cell CAs, which are commonly cystic, and those containing an adenofibromatous background and proposed that each may have a different pathogenesis.70,73,75 However, the numbers of adenofibromatous CAs in these studies are relatively small, and this model implies that both pathways are independent of one another although some adenofibromatous tumors can be associated with endometriosis.4,46,55,70,75,77 Thus, the relationship between endometriosis-associated and adenofibromatous pathways of development of clear cell CA, as well as the pathogenesis of clear cell AF or APT, are unclear, and the clear cell adenofibroma-carcinoma sequence is poorly understood.
In order to better elucidate the morphologic steps involved in the pathogenesis of ovarian epithelial clear cell tumors and determine whether clear cell CA is a type I or type II tumor, we studied the clinicopathologic features of 472 ovarian clear cell AFs, APTs, and CAs, the largest investigation of this kind to date.
MATERIALS AND METHODS
The study was performed with approval of the Institutional Review Board at the Armed Forces Institute of Pathology (AFIP). All ovarian tumors originally diagnosed as clear cell AF, APT/borderline tumor /tumor of low malignant potential, and clear cell CA from 1971 to 2004 were retrieved from the files of the AFIP in Washington, D.C. (n=495), and all available H&E slides were re-reviewed. Tumors were subclassified as AF, APT, and CA. Diagnostic criteria for AF and APT were based on those used by Bell and Scully and Roth et al.4,55 Although criteria for the distinction of clear cell AF/APT from CA have not been firmly established, cases showing haphazard infiltrative growth or single cells within stroma combined with desmoplastic, myxoid/edematous, or inflammatory stromal alterations were classified as CA. For cases not exhibiting classic forms of destructive stromal invasion, confluent architecture in solid tumors or complex growth in intra-cystic tumors was also considered a pattern of CA. Furthermore, tumors with AF-like architecture but lacking typical patterns of stromal invasion were classified as CA if the histologic features significantly exceeded that of the conventional appearance of clear cell AF or APT. Tumors not qualifying as an epithelial clear cell tumor (AF, APT, or CA) after re-review were excluded. Also, CAs with clear cytoplasm that did not contain any classic architectural and cytologic features of ovarian clear cell CA61 were not included. After re-review, 23 cases were excluded; thus, 472 cases were the subject of this study.
Clinical data and gross information were extracted from operative notes and the original pathology reports, respectively. FIGO stage was based on a combination of clinical data, information from the original pathology report, and review of H&E slides of non-ovarian tissues. A FIGO stage was assigned only for cases in which there were sufficient non-ovarian tissues available for histologic review, which included variable combinations of slides from salpingo-oophorectomies, hysterectomies, omentectomies, lymph node dissections, and staging biopsies. Clinical follow-up for a sufficient number of cases was not available. Requests were sent to clinicians, but too few responses were received; thus, meaningful statistical analysis for follow-up was not possible. However, the staging data were compared between tumor categories.
The frequency of endometriosis, which included both endometriotic implants and endometriotic cysts (endometriomas), its location, and presence or absence of cytologic atypia were recorded. The designation “endometriosis anywhere” included endometriosis within the ovarian tumor, residual ipsilateral normal ovarian tissue, the contralateral normal ovary, or extra-ovarian sites; however, the frequency of endometriosis was based only on cases in which tissues other than the ovarian tumor were removed. Endometriosis in the ovarian tumor was designated as such if it was present either within the tumor itself or in adjacent residual ipsilateral normal ovarian tissue. As definitive criteria for the distinction of atypical endometriosis from either benign degenerative/metaplastic changes or intraepithelial clear cell CA in endometriosis have not been validated, as well as that this morphologic distinction is unlikely to be reproducible, notable epithelial atypia in endometriosis was not further classified in this study.
Epithelial atypia within AFs or APTs was assessed. As intraepithelial CA in clear cell tumors has not been stringently defined, that terminology was not used, and no attempt was made to determine which atypical foci represented intraepithelial CA. Thus, notable atypia, which was not unequivocally clear cell CA, was simply recorded as present or absent.
CAs were assessed for various histologic features, including grade and an adenofibromatous background. The type of architectural pattern (tubulocystic, papillary, glandular, solid) was recorded and categorized as pure if it composed >90% of the tumor and mixed if ≥10% of a second type was present. The presence of other tumor types was noted and designated “mixed carcinoma” if the non-clear cell component accounted for ≥10% of the tumor.
CAs were assigned a histologic grade using a modification of the universal grading system/“Silverberg grade”60,62 as follows: The architectural score was based on the proportion of solid growth- 1 point, ≤5%; 2 points, 6-50%; and 3 points, >50%. The predominating nuclear score was determined- 1 point, uniform nuclei with smooth nuclear contours and inconspicuous nucleoli; 2 points, enlarged nuclei with hyperchromasia, increased nuclear-to-cytoplasmic ratios, and nucleoli visible under high-power (40x objective) magnification; and 3 points, marked cytologic atypia, large nuclei, and usually vesicular chromatin with prominent nucleoli. The mitotic score in the most mitotically active area of the tumor was based on the number of mitotic figures (MFs) per 10 high-power fields (HPFs) [40x objective field area, 0.237 mm2) per the highest count method- 1 point, 0-2 MFs/10 HPFs; 2 points, 3-7 MFs/10 HPFs; and 3 points, ≥8 MFs/10 HPFs. Points for each score were summed, and the final grade was determined as follows: Grade 1, 3-5 points; grade 2, 6-7 points; and grade 3, 8-9 points. These grading criteria depart from the original scheme60,62 (different architectural criteria and lower mitotic index cut-points) in order to allow for simplistic architectural grading (analogous to that for endometrial carcinoma) and because ovarian clear cell CAs typically have low mitotic indices.
CAs were subclassified as those with and without an adenofibromatous background [CA(AF+) and CA(AF-), respectively], and their clinicopathologic features were compared. The background of the CA was considered “adenofibromatous” if it had histologic features of an AF or APT. In addition to grading the CA, the presence of atypia in the adenofibromatous component of the tumor was recorded in the same fashion as for AFs and APTs.
All statistical analyses were performed in SAS version 9.2 (SAS institute, Inc.; Cary, NC, USA), and the significance level was set at 0.05. Ranges and frequency distributions of all continuous and categorical variables were examined. The t-test was used to compare the mean differences in age and tumor size. Chi-square or Fisher exact tests were applied for comparison of stage, bilaterality, gross appearance, grade, architectural pattern, mixed carcinoma component, presence of oxyphilic features, and categories of endometriosis.
RESULTS
Clear Cell Adenofibroma (n=4)
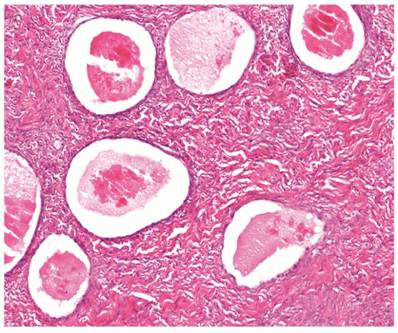
The clinicopathologic features with respect to age, stage, bilaterality, tumor size, and gross appearance are shown in Table 1. Histologically, the tumors were composed of glands separated by abundant fibromatous stroma (Fig. 1). The glands were typically small to medium in size, mostly round, uniform in size and shape, and occasionally filled with eosinophilic secretions. They were lined by 1 or 2 layers of flat to low-cuboidal cells with scant to moderate pale or clear cytoplasm. The nuclei were usually small, uniform, and flat to round. The nuclei exhibited either no or mild atypia, but notable nuclear atypia was not present in any case. Mitotic figures were not identified. Associations with endometriosis are shown in Tables 2 and 3.
Clear cell adenofibroma. Simple glands lined by non-atypical flat cells are separated by abundant fibromatous stroma. The glands are cystically dilated and filled with eosinophilic secretions.
Clinicopathologic features of clear cell adenofibroma and atypical proliferative (borderline) clear cell tumor*
| Clinicopathologic feature | Adenofibroma (n=4) | Atypical proliferative (borderline) tumor (n=41) |
|---|---|---|
| Mean patient age (years) [range] | 61 (50-75) | 60 (30-85) |
| Stage I | 2/2 (100%) | 15/15 (100%) |
| Stage >I | 0/2 (0%) | 0/15 (0%) |
| Bilateral | 0% | 1 (2%) |
| Mean tumor size (cm) [range] | 6.8 (4-9) | 9.0 (2-20) |
| Cystic† | 0% | 4 (10%) |
| Mixed cystic and solid† | 1 (25%) | 31 (76%) |
| Solid† | 3 (75%) | 6 (15%) |
*, The denominator for all calculations is based on the total number in that patient group unless specified otherwise
†, Predominant gross appearance
Endometriosis associated with ovarian clear cell neoplasms
| Location of endometriosis | Adenofibroma (n=4) | Atypical proliferative (borderline) tumor (n=41) | Carcinoma with adenofibromatous background (n=141) | Carcinoma without adenofibromatous background (n=286) | p-value* |
|---|---|---|---|---|---|
| Endometriosis anywhere | 1/2 (50%) | 7/35 (20%) | 33/130 (25%) | 87/268 (32%) | 0.078 |
| Endometriosis in ovarian tumor | 0% | 6 (15%) | 16 (11%) | 57 (20%) | 0.02 |
| Tumor arising directly within endometriotic cyst | 0% | 1 (2%) | 6 (4%) | 46 (16%) | <0.0001 |
*, All adenofibromatous tumors combined (adenofibroma + atypical proliferative [borderline] tumor + carcinoma with adenofibromatous background) vs. carcinoma without adenofibromatous background
Atypical endometriosis in ovarian clear cell neoplasms*
| Adenofibroma | Atypical proliferative (borderline) tumor (n=6) | Carcinoma with adenofibromatous background (n=16) | Carcinoma without adenofibromatous background (n=57) | p-value† |
|---|---|---|---|---|
| - | 1 (17%) | 2 (13%) | 25 (44%) | 0.004 |
*, The denominator for all calculations is based on the number of patients in that group with typical or atypical endometriosis in the ovarian tumor
†, All adenofibromatous tumors combined (atypical proliferative [borderline] tumor + carcinoma with adenofibromatous background) vs. carcinoma without adenofibromatous background
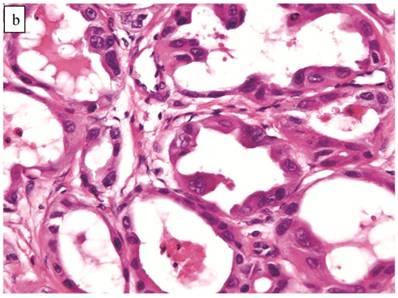
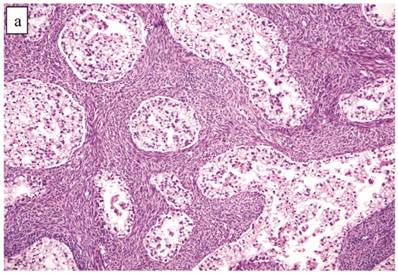
Atypical Proliferative (Borderline) Clear Cell Tumor (n=41)
The clinicopathologic features with respect to age, stage, bilaterality, tumor size, and gross appearance are shown in Table 1. Histologically, the tumors contained a background of AF and exhibited greater glandular crowding as well as more variation in size and shape of glands, including cystic dilatation, compared with AFs (Fig. 2A). Occasional glands showed slightly more stratification of the epithelium (2-4 layers) but generally did not have substantial architectural complexity, such as papillary, solid, or cribriform patterns. The nuclei were mostly small, uniform, and flat to round but occasionally had mild nuclear enlargement and slight hyperchromasia; nucleoli, if present, were usually small (Fig. 2B).
Atypical proliferative (borderline) clear cell tumor. (A) The glands show a greater degree of crowding and variation in size and shape compared with clear cell adenofibroma. (B) The glands in many areas are lined by flat non-atypical cells. (C) Focal notable nuclear atypia is present in the glandular epithelium.
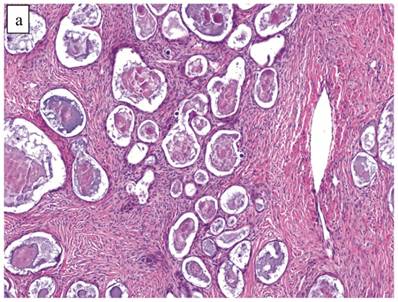
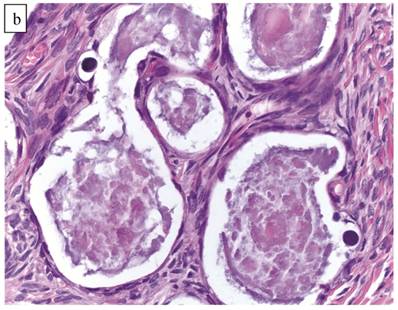
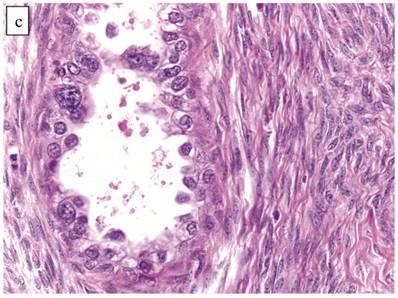
Only one case had mitotic activity (1 MF/10 HPFs). Notable nuclear atypia was focally present in 11 (27%) cases (Fig. 2C); however, such foci lacked associated stromal alterations and recognizable forms of invasion, such as an haphazard infiltration of glands, glandular confluence, mitotic activity within glands, or the conventional appearance of clear cell CA.
Associations with endometriosis are shown in Tables 2 and 3. Foci of atypia in endometriosis in the ovarian tumor were characterized by nuclear enlargement, hyperchromasia, and occasional hobnail morphology of the endometriotic epithelium, but architectural features of CA in such foci were absent.
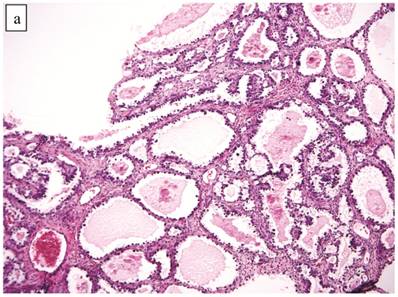
Clear Cell Carcinoma with Adenofibromatous Background (n=141)
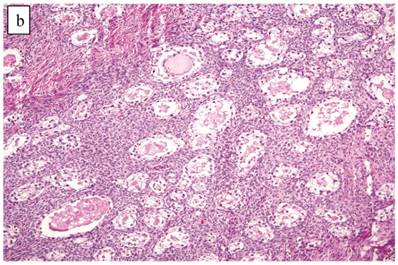
The clinicopathologic features with respect to age, stage, bilaterality, tumor size, gross appearance, and distributions of grade and architectural pattern are shown in Table 4. Between cases, the CAs histologically displayed combinations of tubulocystic (Fig. 3A), papillary, and solid architectural patterns; hobnail, polygonal, and flat cell shapes (Fig. 3B); clear or oxyphilic cytoplasm; a spectrum of atypia ranging from mild to severe; generally low mitotic indices; and hyalinized stroma, similar to the features seen in ovarian clear cell CAs described in detail elsewhere.13,47,70,75,77
The background of the tumor contained variable amounts of adenofibromatous components (Fig. 3C). The proportion of the clear cell CA component in the tumors ranged from focal to diffuse, but cases with focal CA components exceeded conventional definitions for microinvasion (>3 mm in greatest dimension). Notable nuclear atypia was focally present in the adenofibromatous background in 34 (24%) cases, and the histologic appearance of such foci was similar to those with notable atypia in APTs described above. A comparison with non-adenofibromatous clear cell CAs is shown in Table 4.
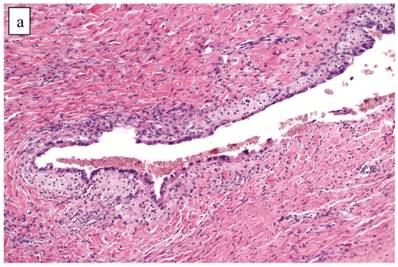
Although the vast majority of tumors displayed overt carcinomatous growth patterns, the architecture of the entire tumor in 7 (5%) cases superficially resembled an AF/APT with fibromatous and unaltered stroma between glands; however, the combined degree of glandular crowding and variation in size and shape of the glands, haphazard arrangement of glands, substantial epithelial stratification, and diffuse marked atypia significantly exceeded the conventional appearance of AF/APT (Fig. 4). In the 6 cases with staging data, 1 (17%) had extra-ovarian disease.
Clinicopathologic features of ovarian clear cell carcinomas*
| Clinicopathologic feature | Carcinoma with adenofibromatous background (n=141) | Carcinoma without adenofibromatous background (n=286) | p-value |
|---|---|---|---|
| Mean patient age (years) [range] | 57 (21-83) | 52 (22-84) | 0.0001 |
| Stage I | 85/107 (79%) | 121/213 (57%) | <0.0001 |
| Stage >I | 22/107 (21%) | 92/213 (43%) | |
| Bilateral | 7 (5%) | 22 (8%) | 0.29 |
| Mean tumor size (cm) [range] | 12.9 (2-30) | 12.3 (2-40) | 0.36 |
| Cystic† | 30/138 (22%) | 120/282 (43%) | 0.0002 |
| Cystic and solid† | 75/138 (54%) | 115/282 (41%) | |
| Solid† | 33/138 (24%) | 47/282 (17%) | |
| Grade 1 | 51 (36%) | 72 (25%) | <0.0001 |
| Grade 2 | 70 (50%) | 91 (32%) | |
| Grade 3 | 20 (14%) | 123 (43%) | |
| Tubulocystic§ | 38 (27%) | 20 (7%) | <0.0001 |
| Glandular§ | 3 (2%) | 5 (2%) | |
| Solid§ | 2 (1%) | 43 (15%) | |
| Papillary§ | 6 (4%) | 27 (9%) | |
| Mixed | 92 (65%) | 191 (67%) | |
| Mixed carcinoma component of non-clear cell type | 6 (4%) | 29 (10%) | 0.04 |
*, The denominator for all calculations is based on the total number in that patient group unless specified otherwise
†, Predominant gross appearance
§, Pure architectural pattern
Carcinoma with adenofibromatous background. The carcinoma component exhibits a (A) tubulocystic pattern with (B) oxyphilic, hobnail, and flat atypical cells. (C) The adenofibromatous component does not show architectural features of clear cell carcinoma or notable atypia.
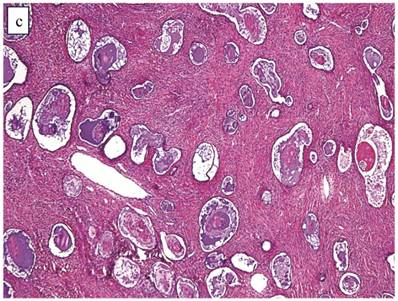
Clear cell carcinoma with adenofibroma-like growth pattern. (A) The glands are separated by fibromatous stroma, but the extent and degree of epithelial proliferation is consistent with clear cell carcinoma despite the lack of an altered stromal response. (B) Separate focus from the same case showing marked crowding of tubules with slightly haphazard arrangement. The size and shape of the tubules are markedly different compared with Fig. 4A.
Associations with endometriosis, including comparison with non-adenofibromatous clear cell CAs, are shown in Tables 2, 3, and 5. Foci of epithelial atypia in endometriosis in the ovarian tumor were similar to those in atypical endometriosis associated with APTs noted above.
Comparison of AFs, APTs, and CA(AF+)s (Table 6) revealed that all AFs and APTs were stage I as were 79% of CA(AF+)s. The tumor categories from AF to CA correlated with a gradual increase in tumor size. Increasing proportions of the CA component in the CA(AF+)s correlated with a slight increase in grade and advanced stage (Table 7).
Association of adenofibromatous background and endometriosis in ovarian clear cell carcinoma (n=427)
| Clear Cell Carcinoma-Associated Components | Frequency |
|---|---|
| Adenofibromatous background (+) Endometriosis (+) | 16 (4%) |
| Adenofibromatous background (+) Endometriosis (-) | 125 (29%) |
| Adenofibromatous background (-) Endometriosis (+) | 57 (13%) |
| Adenofibromatous background (-) Endometriosis (-) | 229 (54%) |
Stage and size of clear cell adenofibromatous tumors*
| Clinicopathologic feature | Adenofibroma (n=4) | Atypical proliferative (borderline) tumor (n=41) | Carcinoma with adenofibromatous background (n=141) |
|---|---|---|---|
| Stage I | 2/2 (100%) | 15/15 (100%) | 85/107 (79%) |
| Stage >I | 0/2 (0%) | 0/15 (0%) | 22/107 (21%) |
| Mean tumor size (cm) [range] | 6.8 (4-9) | 9.0 (2-20) | 12.9 (2-30) |
*, The denominator for all calculations is based on the total number in that patient group unless specified otherwise
Stage and grade of clear cell carcinomas with adenofibromatous background based on proportion of carcinoma component*
| Clinicopathologic feature | Carcinoma with adenofibromatous background: percentage of tumor composed of carcinoma component (n=141) | |||
|---|---|---|---|---|
| <50% (n=42) | 50-75% (n=30) | >75% (n=69) | ||
| Stage I | 32/36 (89%) | 16/20 (80%) | 37/51 (73%) | |
| Stage >I | 4/36 (11%) | 4/20 (20%) | 14/51 (27%) | |
| Grade 1 | 21 (50%) | 9 (30%) | 21 (30%) | |
| Grade 2 | 18 (43%) | 16 (53%) | 36 (52%) | |
| Grade 3 | 3 (7%) | 5 (17%) | 12 (17%) | |
*, The denominator for all calculations is based on the total number in that patient group unless specified otherwise
Clear Cell Carcinoma without Adenofibromatous Background (n=286)
The clinicopathologic features with respect to age, stage, bilaterality, tumor size, gross appearance, and distributions of grade and architectural pattern are shown in Table 4. The histologic appearance was similar to that of the CA components of CA(AF+). Complex intra-cystic growth without destructive invasion of underlying normal ovarian stroma was common (Fig. 5). Twenty-nine tumors (10%) had a mixed carcinoma component of non-clear cell type, 9 of which were associated with endometriosis. A comparison with adenofibromatous clear cell CAs is shown in Table 4.
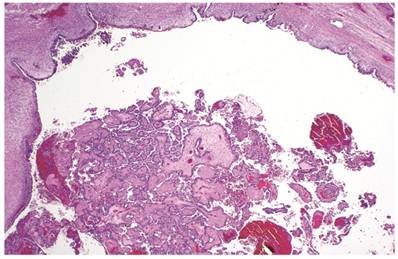
Associations with endometriosis, including comparison with adenofibromatous clear cell CAs, are shown in Tables 2, 3, and 5. The 57 CAs with endometriosis in the ovarian tumor had the following histologic patterns: tubulocystic (n=4), glandular (n=1), solid (n=4), and papillary (n=8). Mixed patterns occurred in 40. Thirty-one of the 46 (67%) cases arising directly within an endometriotic cyst had a predominantly cystic gross appearance. Foci of epithelial atypia in endometriosis in the ovarian tumor were similar to those in endometriosis associated with APTs noted above (Figs. 6 and 7).
Clear cell carcinoma arising within an endometriotic cyst. The tumor is entirely intra-cystic.
Clear cell carcinoma arising within an endometriotic cyst. Typical endometriotic epithelium (bottom) gradually shows greater atypia (middle) as it merges with possible intraepithelial carcinoma (top) and intra-cystic clear cell carcinoma (left).
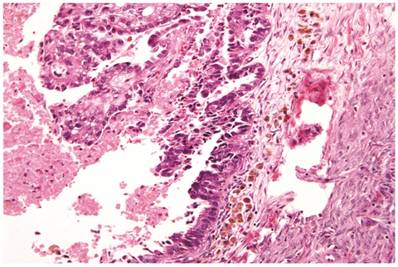
Atypical endometriotic cyst associated with clear cell carcinoma. (A) The endometriotic cyst contains scattered atypical epithelial cells, which were not directly contiguous with the carcinoma (not shown). (B) The atypical cells have round nuclei and abundant eosinophilic cytoplasm. Some atypical nuclei are hyperchromatic whereas others have irregularly distributed chromatin.
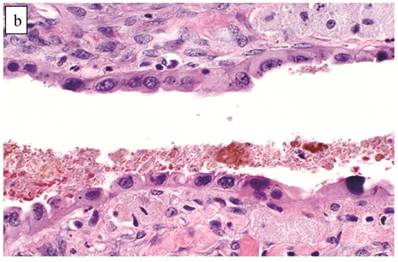
Comparison of Clear Cell Carcinomas (without an Adenofibromatous Background) with Endometriosis vs. Other Tumors
Within the entire category of CC(AF-)s, the subset associated with endometriosis in the ovarian tumor emerged as a distinct subset. Compared with all other clear cell CAs [CC(AF+)s and CC(AF-)s without endometriosis combined], women with CC(AF-)s with endometriosis were younger (p=0.001), and their tumors were more often cystic (p<0.0001) and stage I (p=0.02). Also, they more commonly had a mixed carcinoma component of non-clear cell type (p=0.04), endometriosis in the ovarian tumor (p<0.0001), and tumor arising directly within an endometriotic cyst (p<0.0001). When endometriosis was present in any category of clear cell CA, it was more commonly atypical in CC(AF-) with endometriosis (p=0.004). Differences for other parameters, such as tumor size, grade, architecture, and oxyphilic features, were not statistically significant (p-values, 0.10 to 0.42).
DISCUSSION
This study and others confirm that there are two distinctive forms of clear cell carcinoma based on a number of differing clinicopathologic features- one associated with an adenofibromatous background [CC(AF+)] and the other without an adenofibromatous background [CC(AF-)].70,75 Although endometriosis can be seen in some CC(AF+)s, it is significantly more common in CC(AF-)s. In contrast to all the other types of clear cell CAs, the subset of CC(AF-)s with endometriosis is particularly distinctive since the women with these tumors are younger and their tumors are lower stage and cystic, as they develop within an endometriotic cyst (endometrioma). Previous studies with relatively small numbers of CA(AF+)s have suggested that the pathogenesis of clear cell CA is related to endometriosis in some cases and to AFs in others and that both endometriosis and AFs are precursors. In a study by Yamamoto et al, CA(AF+)s more commonly had a lower grade, tubulocystic architecture, a lower Ki-67 proliferation index, and better overall survival and less frequently were associated with endometriosis compared with CA(AF-)s.75 Cystic clear cell CAs were strongly associated with endometriosis and tended not to have an adenofibromatous background in a study by Veras et al.70 In that study, CA(AF+)s were predominantly tubulocystic in architecture and of higher stage in contrast with cystic CAs, which were more often papillary and had better survival. A study comparing loss of heterozygosity (LOH) between endometriosis-associated clear cell CAs and CA(AF+)s demonstrated significantly higher frequencies of LOH at 3p, 5q, and 11q in the former.73 Indeed, these studies coupled with the findings from our study, which represents the largest morphologic investigation of adenofibromatous clear cell CAs to date, confirm this concept of two pathways of tumor development.
In our study, the three categories of adenofibromatous tumors [AF, APT, and CA(AF+)], as well as the AF, APT, and CA components in CA(AF+)s, represented a continuous morphologic spectrum. Some CA(AF+)s predominantly displayed only mild atypia, and a subset of CA(AF+)s had architectural patterns resembling AFs or APTs. In particular, the adenofibromatous tumors [AF, APT, and CA(AF+)] showed a direct relationship between tumor type and stage, size, and atypia in the adenofibromatous background. Also, the proportion of the CA component in CA(AF+)s correlated with stage and grade. In a study of adenofibromatous clear cell tumors containing these three components by Yamamoto et al, each component displayed progressively higher Ki-67 proliferation indices, respectively.72 In another study by the same investigators, each of the three clear cell components frequently shared LOH on 5q, 10q, and 22q.74 The overall frequency of LOH in each component increased from AF to CA, respectively. LOH on 1p and 13q was rare in the AF/APT components but common in the CA component. Also, the APT components showed a higher frequency of LOH at 1p compared with the AF component. All of these findings are consistent with tumor progression and support a precursor-cancer relationship between clear cell AF and CA with APT as an intermediate step.
The CA(AF-)s with endometriosis, which tended to be cystic, in the present study were significantly associated with atypical endometriosis. In some cases, there was a morphologic continuum within the epithelium of the endometriotic cyst, in which a gradual progressive transition between typical endometriosis, atypical endometriosis, and CA could be seen. It is noteworthy that prior studies have shown a higher frequency of atypia in endometriosis when a co-existent clear cell CA is present.17,42,50,70 Atypical endometriosis also exhibits a Ki-67 proliferation index intermediate between typical endometriosis and clear cell CA.72 A large number of molecular and chromosomal alterations have been identified in typical and atypical endometriosis/endometriotic cysts.2,3,21,26-28,32,36,48,57,67,69 Moreover, an atypical endometriotic cyst recurring as clear cell CA has been reported.46 These observations provide strong evidence that a subset of clear cell CAs arise via the development of atypia in an endometriotic cyst as opposed to non-cystic endometriosis.
It must be emphasized, however, that occasional APTs and CA(AF+)s in our study also exhibited endometriosis in the ovarian tumor, including atypical endometriosis and tumor arising directly within an endometriotic cyst. Prior series have also described endometriosis specifically within some clear cell AFs, an APT, and CA(AF+)s.4,46,55,70,77 Thus, endometriosis-associated and adenofibromatous pathways of development of clear cell CA are not entirely independent of one another. As the origin of clear cell AF has not been previously proven, particularly as there is no clear cell counterpart in the normal ovary to serve as a precursor, we believe that non-cystic endometriosis (as opposed to an endometriotic cyst) is the precursor of clear cell AF.
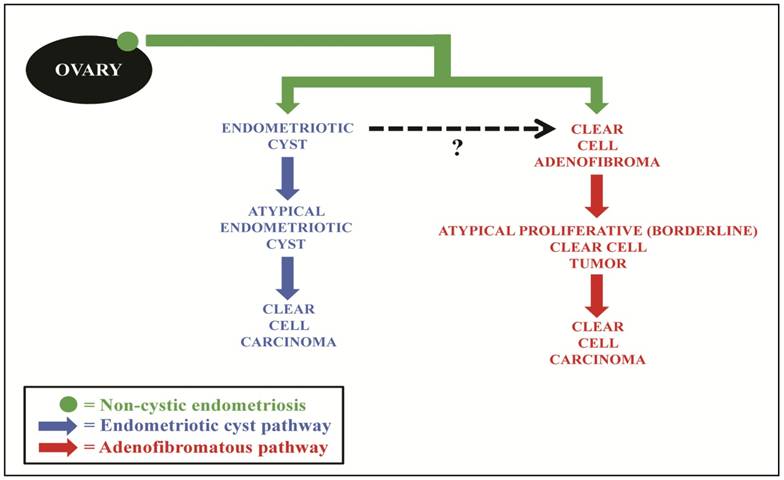
We, therefore, propose that endometriosis is the underlying precursor for both the cystic and the adenofibromatous types of clear cell CA (Fig. 8). The cystic CAs arise from an endometriotic cyst in which atypia develops (i.e., atypical endometriosis, possibly representing intraepithelial CA). In the adenofibromatous pathway, we speculate that non-cystic endometriosis induces a fibromatous stromal reaction resulting in the formation of clear cell AF. With further glandular proliferation, it progresses to an APT which subsequently develops into an invasive carcinoma, possibly through intraepithelial carcinoma. It should be noted that evolution into different forms of clear cell neoplasia based on the type of endometriosis from which it arose (cystic vs. non-cystic endometriosis) is also indirectly supported by the demonstration of differences in the clinical features of women with cystic vs. non-cystic endometriosis of the ovary.68
Proposed model of pathogenesis of ovarian clear cell carcinoma, in which the majority of lesions develop along 1 of 2 pathways. Non-cystic endometriosis (solid green circle) progresses through either an endometriotic cyst pathway (blue) or adenofibromatous pathway (red). In the endometriotic cyst pathway, endometriosis forms an endometriotic cyst and, through epithelial atypia (atypical endometriosis), develops into clear cell carcinoma. In the adenofibromatous pathway, we speculate a fibromatous reaction, which frequently accompanies endometriosis, could result in the development of a clear cell adenofibroma, which subsequently progresses to an atypical proliferative (borderline) clear cell tumor and then to clear cell carcinoma. Black dashed arrow, occasional adenofibromatous tumors may arise from an endometriotic cyst as opposed to non-cystic endometriosis.
As about half of the CAs in this study did not contain endometriosis in the ovarian tumor or adenofibromatous components (features of the type I pathway), we acknowledge that other pathways of tumor development may exist. Similar to the pathogenesis of serous carcinoma, it has been proposed that endometrioid and transitional cell tumors also follow low- and high-grade pathways of pathogenesis.11,18 However, ovarian clear cell CA has p53 mutations only infrequently, and it usually exhibits a low to moderate level of chromosomal instability.1,9,14,16,22,37,38,51,52,71 Thus, it is unlikely that a significant proportion of clear cell CAs evolve through the type II pathway. Also, a small number of clear cell CAs in this study contained a mixed carcinoma component of non-clear cell type. While it is possible that clear cell CA evolved from such mixed non-clear cell components, several of those cases also had either adenofibromatous elements or endometriosis (i.e., precursors of clear cell CA) in the ovarian tumor. The clinicopathologic features in clear cell CAs lacking endometriosis in the ovarian tumor or adenofibromatous components in this study suggest that they are most likely due to tumor progression and overgrowth of the precursor elements.
Because clear cell CA shares features of both type I and type II tumors in the dualistic model of ovarian carcinogenesis, placement of clear cell carcinoma into one of the two groups has been problematic.40,41,59 The step-wise progression sequence of AF, APT, and CA in the adenofibromatous pathway of clear cell CA is analogous to that of other type I tumors. Also, the evolution from an endometriotic cyst is similar to the process that occurs in endometrioid CA, a type I tumor. Thus, from a precursor standpoint, both pathways in the pathogenesis of ovarian clear cell CA more closely resemble a type I rather than type II tumor. This interpretation is also reinforced by clear cell CA's frequent presentation as stage I, its molecular profile, and the relatively low-level of chromosomal instability, similar to other type I tumors.8,9,14,19,25,32,37,38,40,56,59,65,70,71,75 However, this study does not resolve the issue that clear cell CAs are often high-grade and have poor survival when they are of advanced stage (features of type II tumors).8,20,24,25,44,58,64,66
In summary, both types of clear cell carcinomas, adenofibromatous and cystic, appear to be derived from endometriosis. However, the former develop from non-cystic endometriosis and are associated with adenofibromatous components while the latter arise from an endometriotic cyst (endometrioma) and are not associated with an adenofibromatous background. The two pathways are not necessarily mutually exclusive, as there may be overlap in some cases. In any event, both forms of clear cell carcinoma are more closely related to type I tumors from a precursor standpoint.
Acknowledgements
Authors thank Russell Vang, M.D (Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD) for his guidance on this study.
The opinion and assertions contained herein are the private views of the authors and are not to be construed as official or as representing the views of the Department of the Army or the Department of Defense.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Akahane T, Sekizawa A, Purwosunu Y. et al. The role of p53 mutation in the carcinomas arising from endometriosis. Int J Gynecol Pathol. 2007;26:345-351
2. Ali-Fehmi R, Khalifeh I, Bandyopadhyay S. et al. Patterns of loss of heterozygosity at 10q23.3 and microsatellite instability in endometriosis, atypical endometriosis, and ovarian carcinoma arising in association with endometriosis. Int J Gynecol Pathol. 2006;25:223-229
3. Ballouk F, Ross JS, Wolf BC. Ovarian endometriotic cysts. An analysis of cytologic atypia and DNA ploidy patterns. Am J Clin Pathol. 1994;102:415-419
4. Bell DA, Scully RE. Benign and borderline clear cell adenofibromas of the ovary. Cancer. 1985;56:2922-2931
5. Borgfeldt C, Andolf E. Cancer risk after hospital discharge diagnosis of benign ovarian cysts and endometriosis. Acta Obstet Gynecol Scand. 2004;83:395-400
6. Brescia RJ, Dubin N, Demopoulos RI. Endometrioid and clear cell carcinoma of the ovary. Factors affecting survival. Int J Gynecol Pathol. 1989;8:132-138
7. Brinton LA, Gridley G, Persson I. et al. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176:572-579
8. Chan JK, Teoh D, Hu JM. et al. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109:370-376
9. Cho K, Shih I. Ovarian cancer. Annu Rev Pathol Mech Dis. 2009;4:287-313
10. Crozier MA, Copeland LJ, Silva EG. et al. Clear cell carcinoma of the ovary: a study of 59 cases. Gynecol Oncol. 1989;35:199-203
11. Cuatrecasas M, Catasus L, Palacios J. et al. Transitional cell tumors of the ovary: a comparative clinicopathologic, immunohistochemical, and molecular genetic analysis of Brenner tumors and transitional cell carcinomas. Am J Surg Pathol. 2009;33:556-567
12. Czernobilsky B, Silverman BB, Enterline HT. Clear-cell carcinoma of the ovary. A clinicopathologic analysis of pure and mixed forms and comparison with endometrioid carcinoma. Cancer. 1970;25:762-772
13. Delair D, Oliva E, Kobel M. et al. Morphologic spectrum of immunohistochemically characterized clear cell carcinoma of the ovary: a study of 155 cases. Am J Surg Pathol. 2011;35:36-44
14. Dent J, Hall GD, Wilkinson N. et al. Cytogenetic alterations in ovarian clear cell carcinoma detected by comparative genomic hybridisation. Br J Cancer. 2003;88:1578-1583
15. Erzen M, Rakar S, Klancnik B. et al. Endometriosis-associated ovarian carcinoma (EAOC): an entity distinct from other ovarian carcinomas as suggested by a nested case-control study. Gynecol Oncol. 2001;83:100-108
16. Fujita M, Enomoto T, Inoue M. et al. Alteration of the p53 tumor suppressor gene occurs independently of K-ras activation and more frequently in serous adenocarcinomas than in other common epithelial tumors of the human ovary. Jpn J Cancer Res. 1994;85:1247-1256
17. Fukunaga M, Nomura K, Ishikawa E. et al. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249-255
18. Geyer JT, Lopez-Garcia MA, Sanchez-Estevez C. et al. Pathogenetic pathways in ovarian endometrioid adenocarcinoma: a molecular study of 29 cases. Am J Surg Pathol. 2009;33:1157-1163
19. Gilks CB, Ionescu DN, Kalloger SE. et al. Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum Pathol. 2008;39:1239-1251
20. Goff BA, Sainz de la Cuesta R, Muntz HG. et al. Clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease. Gynecol Oncol. 1996;60:412-417
21. Goumenou AG, Arvanitis DA, Matalliotakis IM. et al. Microsatellite DNA assays reveal an allelic imbalance in p16(Ink4), GALT, p53, and APOA2 loci in patients with endometriosis. Fertil Steril. 2001;75:160-165
22. Ho ES, Lai CR, Hsieh YT. et al. p53 mutation is infrequent in clear cell carcinoma of the ovary. Gynecol Oncol. 2001;80:189-193
23. Horiuchi A, Itoh K, Shimizu M. et al. Toward understanding the natural history of ovarian carcinoma development: a clinicopathological approach. Gynecol Oncol. 2003;88:309-317
24. Itamochi H, Kigawa J, Sugiyama T. et al. Low proliferation activity may be associated with chemoresistance in clear cell carcinoma of the ovary. Obstet Gynecol. 2002;100:281-287
25. Jenison EL, Montag AG, Griffiths CT. et al. Clear cell adenocarcinoma of the ovary: a clinical analysis and comparison with serous carcinoma. Gynecol Oncol. 1989;32:65-71
26. Jiang X, Hitchcock A, Bryan EJ. et al. Microsatellite analysis of endometriosis reveals loss of heterozygosity at candidate ovarian tumor suppressor gene loci. Cancer Res. 1996;56:3534-3539
27. Jiang X, Morland SJ, Hitchcock A. et al. Allelotyping of endometriosis with adjacent ovarian carcinoma reveals evidence of a common lineage. Cancer Res. 1998;58:1707-1712
28. Jimbo H, Hitomi Y, Yoshikawa H. et al. Evidence for monoclonal expansion of epithelial cells in ovarian endometrial cysts. Am J Pathol. 1997;150:1173-1178
29. Jimbo H, Yoshikawa H, Onda T. et al. Prevalence of ovarian endometriosis in epithelial ovarian cancer. Int J Gynaecol Obstet. 1997;59:245-250
30. Kennedy AW, Biscotti CV, Hart WR. et al. Ovarian clear cell adenocarcinoma. Gynecol Oncol. 1989;32:342-349
31. Kita T, Kikuchi Y, Kudoh K. et al. Exploratory study of effective chemotherapy to clear cell carcinoma of the ovary. Oncol Rep. 2000;7:327-331
32. Kobayashi H, Kajiwara H, Kanayama S. et al. Molecular pathogenesis of endometriosis-associated clear cell carcinoma of the ovary (review). Oncol Rep. 2009;22:233-240
33. Kobayashi H, Sumimoto K, Kitanaka T. et al. Ovarian endometrioma--risks factors of ovarian cancer development. Eur J Obstet Gynecol Reprod Biol. 2008;138:187-193
34. Kobayashi H, Yamada Y, Kanayama S. et al. The role of hepatocyte nuclear factor-1beta in the pathogenesis of clear cell carcinoma of the ovary. Int J Gynecol Cancer. 2009;19:471-479
35. Komiyama S, Aoki D, Tominaga E. et al. Prognosis of Japanese patients with ovarian clear cell carcinoma associated with pelvic endometriosis: clinicopathologic evaluation. Gynecol Oncol. 1999;72:342-346
36. Korner M, Burckhardt E, Mazzucchelli L. Higher frequency of chromosomal aberrations in ovarian endometriosis compared to extragonadal endometriosis: A possible link to endometrioid adenocarcinoma. Mod Pathol. 2006;19:1615-1623
37. Kuo KT, Mao TL, Chen X. et al. DNA copy numbers profiles in affinity-purified ovarian clear cell carcinoma. Clin Cancer Res. 2010;16:1997-2008
38. Kuo KT, Mao TL, Jones S. et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174:1597-1601
39. Kurman RJ, Craig JM. Endometrioid and clear cell carcinoma of the ovary. Cancer. 1972;29:1653-1664
40. Kurman RJ, Shih I. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151-160
41. Kurman RJ, Shih I. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433-443
42. LaGrenade A, Silverberg SG. Ovarian tumors associated with atypical endometriosis. Hum Pathol. 1988;19:1080-1084
43. Mandai M, Yamaguchi K, Matsumura N. et al. Ovarian cancer in endometriosis: molecular biology, pathology, and clinical management. Int J Clin Oncol. 2009;14:383-391
44. Mizuno M, Kikkawa F, Shibata K. et al. Long-term follow-up and prognostic factor analysis in clear cell adenocarcinoma of the ovary. J Surg Oncol. 2006;94:138-143
45. Modesitt SC, Tortolero-Luna G, Robinson JB. et al. Ovarian and extraovarian endometriosis-associated cancer. Obstet Gynecol. 2002;100:788-795
46. Moll UM, Chumas JC, Chalas E. et al. Ovarian carcinoma arising in atypical endometriosis. Obstet Gynecol. 1990;75:537-539
47. Montag AG, Jenison EL, Griffiths CT. et al. Ovarian clear cell carcinoma. A clinicopathologic analysis of 44 cases. Int J Gynecol Pathol. 1989;8:85-96
48. Nilbert M, Pejovic T, Mandahl N. et al. Monoclonal origin of endometriotic cysts. Int J Gynecol Cancer. 1995;5:61-63
49. Norris HJ, Robinowitz M. Ovarian adenocarcinoma of mesonephric type. Cancer. 1971;28:1074-1081
50. Ogawa S, Kaku T, Amada S. et al. Ovarian endometriosis associated with ovarian carcinoma: a clinicopathological and immunohistochemical study. Gynecol Oncol. 2000;77:298-304
51. Okada S, Tsuda H, Takarabe T. et al. Allelotype analysis of common epithelial ovarian cancers with special reference to comparison between clear cell adenocarcinoma with other histological types. Jpn J Cancer Res. 2002;93:798-806
52. Okuda T, Otsuka J, Sekizawa A. et al. p53 mutations and overexpression affect prognosis of ovarian endometrioid cancer but not clear cell cancer. Gynecol Oncol. 2003;88:318-325
53. Orezzoli JP, Russell AH, Oliva E. et al. Prognostic implication of endometriosis in clear cell carcinoma of the ovary. Gynecol Oncol. 2008;110:336-344
54. Prowse AH, Manek S, Varma R. et al. Molecular genetic evidence that endometriosis is a precursor of ovarian cancer. Int J Cancer. 2006;119:556-562
55. Roth LM, Langley FA, Fox H. et al. Ovarian clear cell adenofibromatous tumors. Benign, of low malignant potential, and associated with invasive clear cell carcinoma. Cancer. 1984;53:1156-1163
56. Ryu SY, Park SI, Nam BH. et al. Prognostic significance of histological grade in clear-cell carcinoma of the ovary: a retrospective study of Korean Gynecologic Oncology Group. Ann Oncol. 2009;20:1032-1036
57. Sato N, Tsunoda H, Nishida M. et al. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052-7056
58. Sato Y, Shimamoto T, Amada S. et al. Prognostic value of histologic grading of ovarian carcinomas. Int J Gynecol Pathol. 2003;22:52-56
59. Shih I, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511-1518
60. Shimizu Y, Kamoi S, Amada S. et al. Toward the development of a universal grading system for ovarian epithelial carcinoma: testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer. 1998;82:893-901
61. Silva EG, Young RH. Endometrioid neoplasms with clear cells: a report of 21 cases in which the alteration is not of typical secretory type. Am J Surg Pathol. 2007;31:1203-1208
62. Silverberg SG. Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol. 2000;19:7-15
63. Stern RC, Dash R, Bentley RC. et al. Malignancy in endometriosis: frequency and comparison of ovarian and extraovarian types. Int J Gynecol Pathol. 2001;20:133-139
64. Sugiyama T, Kamura T, Kigawa J. et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584-2589
65. Takano M, Kikuchi Y, Yaegashi N. et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369-1374
66. Tammela J, Geisler JP, Eskew PNJr. et al. Clear cell carcinoma of the ovary: poor prognosis compared to serous carcinoma. Eur J Gynaecol Oncol. 1998;19:438-440
67. Tamura M, Fukaya T, Murakami T. et al. Analysis of clonality in human endometriotic cysts based on evaluation of X chromosome inactivation in archival formalin-fixed, paraffin-embedded tissue. Lab Invest. 1998;78:213-218
68. Tsukahara Y, Satoh M, Kato J. et al. Clinicopathologic investigation of ovarian endometriosis: a comparative study of cystic and non-cystic types. Nippon Sanka Fujinka Gakkai Zasshi. 1985;37:751-757
69. Varma R, Rollason T, Gupta JK. et al. Endometriosis and the neoplastic process. Reproduction. 2004;127:293-304
70. Veras E, Mao TL, Ayhan A. et al. Cystic and adenofibromatous clear cell carcinomas of the ovary: distinctive tumors that differ in their pathogenesis and behavior: a clinicopathologic analysis of 122 cases. Am J Surg Pathol. 2009;33:844-853
71. Willner J, Wurz K, Allison KH. et al. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007;38:607-613
72. Yamamoto S, Tsuda H, Miyai K. et al. Aberrant expression of p27(Kip1)-interacting cell-cycle regulatory proteins in ovarian clear cell carcinomas and their precursors with special consideration of two distinct multistage clear cell carcinogenetic pathways. Virchows Arch. 2009;455:413-422
73. Yamamoto S, Tsuda H, Suzuki K. et al. An allelotype analysis indicating the presence of two distinct ovarian clear-cell carcinogenic pathways: endometriosis-associated pathway vs. clear-cell adenofibroma-associated pathway. Virchows Arch. 2009;455:261-270
74. Yamamoto S, Tsuda H, Takano M. et al. Clear-cell adenofibroma can be a clonal precursor for clear-cell adenocarcinoma of the ovary: a possible alternative ovarian clear-cell carcinogenic pathway. J Pathol. 2008;216:103-110
75. Yamamoto S, Tsuda H, Yoshikawa T. et al. Clear cell adenocarcinoma associated with clear cell adenofibromatous components: a subgroup of ovarian clear cell adenocarcinoma with distinct clinicopathologic characteristics. Am J Surg Pathol. 2007;31:999-1006
76. Yoonessi M, Weldon D, Satchidand SK. et al. Clear cell ovarian adenocarcinoma. J Surg Oncol. 1984;27:289-297
77. Young RH, Scully RE. Oxyphilic clear cell carcinoma of the ovary. A report of nine cases. Am J Surg Pathol. 1987;11:661-667
Author contact
![]() Corresponding author: Chengquan Zhao, M.D., Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, 300 Halket Street, Pittsburgh, PA 15213; e-mail: zhaocedu
Corresponding author: Chengquan Zhao, M.D., Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, 300 Halket Street, Pittsburgh, PA 15213; e-mail: zhaocedu

 Global reach, higher impact
Global reach, higher impact