3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2012; 3:246-256. doi:10.7150/jca.3042 This volume Cite
Research Paper
Incidence of Malignancy after Living Kidney Transplantation: A Multicenter Study from Iran
1. Nephrology and Urology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
2. Departement of Nephrology, Tehran University of Medical Sciences, Tehran, Iran.
3. Departement of kidney transplantation, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4. Departement of Nephrology, Urmia University of Medical Sciences, Urmia, Iran.
5. Departement of Nephrology, Tabriz University of Medical Sciences, Tabriz, Iran.
6. Departement of Nephrology, Babol University of Medical Sciences, Babol, Iran.
7. Departement of Nephrology, Isfahan University of Medical Sciences, Isfahan, Iran.
8. Departement of Nephrology, Sari University of Medical Sciences, Sari, Iran.
9. Departement of Nephrology, Kerman University of Medical Sciences, Kerman, Iran.
10. Departement of Nephrology, Bandar Abbas University of Medical Sciences, Bandar Abbas, Iran.
11. Departement of Nephrology, Ahvaz University of Medical Sciences, Ahvaz, Iran.
12. Departement of Nephrology, Mashhad University of Medical Sciences, Mashhad, Iran.
13. Departement of Nephrology, Rasht University of Medical Sciences, Rasht, Iran.
14. Departement of Nephrology, Ghom University of Medical Sciences, Ghom, Iran.
15. Departement of Nephrology, Artesh University of Medical Sciences, Tehran, Iran.
16. Departement of Nephrology, ZanjanUniversity of Medical Sciences, Zanjan, Iran.
Received 2011-5-21; Accepted 2011-8-9; Published 2012-6-5
Abstract
Malignancy is a common complication after renal transplantation. However, limited data are available on post-transplant malignancy in living kidney transplantation. Therefore, we made a plan to evaluate the incidence and types of malignancies, association with the main risk factors and patient survival in a large population of living kidney transplantation. We conducted a large retrospective multicenter study on 12525 renal recipients, accounting for up to 59% of all kidney transplantation in Iran during 22 years follow up period. All information was collected from observation of individual notes or computerized records for transplant patients. Two hundred and sixty-six biopsy-proven malignancies were collected from 16 Transplant Centers in Iran; 26 different type of malignancy categorized in 5 groups were detected. The mean age of patients was 46.2±12.9 years, mean age at tumor diagnosis was 50.8±13.2 years and average time between transplantation and detection of malignancy was 50.0±48.4 months. Overall tumor incidence in recipients was 2%. Kaposis' sarcoma was the most common type of tumor. The overall mean survival time was 117.1 months (95% CI: 104.9-129.3). In multivariate analysis, the only independent risk factor associated with mortality was type of malignancy. This study revealed the lowest malignancy incidence in living unrelated kidney transplantation.
Keywords: malignancy, kidney transplantation, incidence.
INTRODUCTION
Kidney transplantation is generally accepted as the best treatment for patients with end stage renal disease (ESRD) requiring renal replacement therapy which improves both the quality of life and life span of patients (1-5). Although the new and potent immunosuppressive agents have successfully reduced the risk of rejection after kidney transplantation; however, cardiovascular disease, infectious and neoplastic complications are increasing (3, 6). Cancer is the second cause of death in renal transplant recipients (2) and it is expected that the mortality due to cancer will be moved to become the first cause of death within the next two decades (7). The overall reported post-transplant malignancy incidence varies from 2% to 31%; however, it happen in a percentage as high as 34-50% among renal transplant recipients (RTRs) followed for more than 20 years (7). Many studies have demonstrated an increased risk of cancer among RTRs when compared with an age- and gender-matched general population or in patients undergoing dialysis (1-4). In general, the risk of developing malignancy in organ transplants is 3-4 times greater than general population and the risk of certain types of cancer is as high as 20-500 folds (3, 8, 9).
The majority of the literature on malignancy in kidney transplantation is drawn from deceased transplants and limited data are available on post-transplant malignancy in living kidney transplantation, especially living unrelated renal transplantation (LURT). Although Kasiske et al (2004) (10) reported the largest study (n = 35765) about the incidence rates of malignancies among first-time kidney recipients, however, living donor transplantation only accounted 24% all of them (i.e., n=8584). Furthermore, the other huge studies (11-16) only focused on deceased kidney transplantation.
Therefore, we made a plan to evaluate the prevalence, incidence, characteristics, potential predictors of death, relationship to immunosuppressive drugs, common cancers, current opinions on management, patient survival and the association with the main risk factors of prognosis of malignancy following renal transplantation, particularly in a large population of LURT (10960 cases out of all 12525 RTRs) (17, 18).
PATIENTS AND METHODS
Recipient population
We conducted a large retrospective multicenter study on 12525 RTRs, accounting for up to 59% of all kidney transplantation in Iran (19), to determine the incidence and types of malignancies occurring after renal transplantation and their impact on patient and graft survival between Oct 1984 and Dec 2008. The majority of our patients received a kidney from a living unrelated donor (87.5%), following 9.8% living related and 2.7% deceased donor transplantation. The mean age of RTRs was 37.7±15.2 years (range: 3 to 86 years), 7885 (63%) male and 4640 (37%) female. The duration of study was 24 years and patients were followed-up until graft loss, patient death or the date of last visit subsequently 266 biopsy-proven malignancies were collected from 16 Transplant Centers in Iran. The patient characteristics are shown in Table 1. This study was approved by the local Ethics Committee of Baqiyatallah University of Medical Sciences.
Patients with other organ transplants, history of previous malignancy and transplantation from deceased donors with past history of malignancy were excluded.
Definition
1- The definition of acute rejection was based on the conventional pathologic criteria, Banff classification, and clinical criteria of the reporting centers.
2- Treatment modalities were considered according to the type of cancer, staging of disease, and involved organs. Management included a combination of reduction, withdrawal or changing of the immunosuppressive agents, chemotherapy, radiotherapy, hormone therapy and surgical resection.
3- Non Kaposi's sarcoma tumors (non-KS) included SCC (squamouse cell carcinoma), BCC (basal cell carcinoma) and melanoma.
4- Tumors of breast, ovary and uterine in female, prostate and seminoma in male and renal cell carcinoma (RCC) and transitional cell carcinoma (TCC) of bladder in both gender were considered as genitourinary and reproductive system (GU & RS) neoplasms.
5- The term of solid tumors was used for all the malignancies except for skin tumors, PTLD (post transplantation lymphoproliferative disorder) and GU & RS cancers.
6- Patients with tumor were categorized into 5 groups according to their type of neoplasm: Non-KS, KS, PTLD, GU & RS tumors and solid tumors.
7- Monoclonal antibody (ATG/ALG) can be required for induction therapy and for acute steroid-resistant rejection episodes during the first three months following kidney transplantation. Induction therapy with ATG/ALG was used for highly sensitized patients, those receiving kidneys from deceased donors with delayed graft function, patients with poorly matching living donors, and patients with the second or more transplants. None of the patients took OKT3.
Main demographic and clinical characteristics of 266 post-transplant malignancies in living kidney transplant recipients.
| Variables | Number (%) | Cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| KS | Non-KS | PTLD | GU & RS | Solid | p | |||
| Total | 84 (31.6) | 57 (21.1) | 72 (27.1) | 25 (9.3) | 28 (10.4) | |||
| Gender | Male | 180 (67.7) | 54 (30) | 48 (26.7) | 43 (23.9) | 14 (7.8) | 21 (11.7) | 0.01 |
| Female | 86 (32.3) | 30 (35.3) | 9 (9.4) | 29 (34.1) | 11 (12.9) | 7 (8.2) | ||
| Age | ≤30 | 33 (12.9) | 8 (24.2) | 3 (9.1) | 18 (54.5) | 2 (6.1) | 2 (6.1) | 0.007 |
| 31-50 | 117 (45.9) | 35 (29.9) | 17 (14.5) | 36 (30.8) | 13 (11.1) | 16 (13.7) | ||
| >50 | 105 (41.2) | 36 (34.3) | 36 (34.3) | 15 (14.3) | 8 (7.6) | 10 (9.5) | ||
| Graft status | Active | 232 (89.6) | 73 (31.5) | 53 (22.8) | 64 (27.6) | 20 (8.6) | 22 (9.5) | 0.02 |
| loss | 27 (10.4) | 10 (37) | 1 (37) | 7 (25.9) | 4 (14.8) | 5 (18.5) | ||
| Patient status | Died | 67 (25.4) | 12 (17.9) | 1 (1.5) | 28 (41.8) | 9 (13.4) | 17 (25.4) | 0.0001 |
| Alive | 197 (74.6) | 72 (36.5) | 56 (28.4) | 43 (21.8) | 15 (7.6) | 11 (5.6) | ||
| ALG/ATG | No | 183 (83.6) | 55 (30.1) | 46 (25.1) | 49 (26.8) | 16 (8.7) | 17 (9.3) | 0.06 |
| Yes | 36 (16.4) | 12 (33.3) | 2 (5.6) | 13 (36.1) | 5 (13.9) | 4 (11.1) | ||
| Treatment modality | Discontinue | 80 (38.6) | 23 (28.8) | 11 (13.8) | 29 (36.3) | 6 (7.5) | 11 (13.8) | 0.002 |
| Decrease | 74 (27.8) | 36 (48.6) | 17 (23) | 9 (12.2) | 5 (6.8) | 7 (9.5) | ||
| Change | 28 (10.5) | 8 (28.6) | 6 (21.4) | 11 (39.3) | 1 (3.6) | 2 (7.1) | ||
| unmodified | 25 (12.1) | 1 (4) | 9 (36) | 8 (32) | 4 (16) | 3 (12) | ||
| Response to treat | No | 56 (21.1) | 6 (10.7) | 2 (3.6) | 27 (48.2) | 6 (10.7) | 15 (26.8) | 0.0001 f |
| Yes | 119 (44.7) | 49 (41.2) | 34 (28.6) | 26 (21.8) | 7 (5.9) | 3 (2.5) | ||
| Relapse | No | 18 (85.7) | 4 (22.2) | 3 (16.7) | 7 (38.9) | 2 (11.1) | 2 (11.1) | 0.3 f |
| Yes | 3 (14.3) | 1 (33.3) | 2 (66.7) | 0 | 0 | 0 | ||
| Metastasis | No | 156 (75.7) | 72 (46.2) | 53 (34) | 19(12.2) | 6(3.8) | 6 (3.8) | 0.0001 f |
| Yes | 50 (24.3) | 11 (22) | 3 (6) | 14(28) | 6(12) | 16 (32) | ||
| CMV infection after cancer | No | 43 (78.2) | 15 (34.9) | 5 (11.6) | 13(30.2) | 7(16.3) | 3 (7) | 0.8 f |
| Yes | 12 (21.8) | 6 (50) | 2 (16.7) | 3(25) | 1(8.3) | 0 | ||
| Rejection | No | 148 (79.1) | 50 (33.9) | 35 (23.6) | 40(27) | 8(5.4) | 15 (10.1) | 0.06 f |
| Yes | 39 (20.9) | 12 (30.8) | 4 (10.3) | 12(30.8) | 7(17.9) | 4 (10.3) | ||
| Immunossuppresive | MMF | 96 (38.7) | 41 (42.7) | 16 (16.7) | 21(21.9) | 7(7.3) | 11 (11.5) | 0.01 |
| AZA | 152 (61.3) | 34 (22.4) | 39 (25.7) | 47(30.9) | 15 (9.9) | 17 (11.2) | ||
f: Fisher, CMV: cytomegalovirus, MMF: mycophenolatemofetil, AZA: azathioprine, ALG/ATG: antilymphocyte/antithymocyte globulin, Non-KS: Non Kaposi's sarcoma, KS: Kaposi's sarcoma, PTLD: post transplantation lymphoproliferative disorder, GU & RS: genitourinary and reproductive system.
Immunosuppression protocols
The immunosuppressive therapy was based on cyclosporine/sirolimus, mycophenolate mofetil (MMF)/azathioprine (AZA) and steroids. Before 2000, patients received dual maintenance immunosuppression with prednisone and cyclosporine/AZA or triple therapy with cyclosporine, prednisone, and AZA. Afterwards, the majority of patients received cyclosporine, prednisone, and MMF as well (20).
STATISTICS
Data were analyzed with SPSS version 17.0. Quantitative variables were expressed as mean ± standard deviation, whereas qualitative variables were shown as number and percentage. Continuous data were compared by Student's t-test, and categorized data were analyzed using the Chi-square or Fisher's exact test.
Cancer-free patient survival rate was defined as the time from diagnosis of the tumor to death. The Kaplan-Meier method was used to calculate actuarial survival curves, and univariate comparison between groups was carried out by using the log-rank test. Cox proportional hazards survival regression model was used to evaluate the effect of risk factors on patient survivals. The significance level was set at P <0.05.
RESULTS
Patient population
The patients with malignancy were followed after diagnosis of cancer for a median follow up time of 22 months (min 1 month and max 168 months). The male/female ratio was 2.1:1. The mean age of patients was 46.2±12.9 years (range 12-72 years); on average, men were older than women (47.1±13 years vs. 44.75±12.4 years; p=0.1). In addition, t-test analysis revealed that the risk of malignancy increased with age compared to those with no cancer (46.2±12.9 years vs 37.7±15.2 years, p<0.001). Mean age at tumor diagnosis was 50.8±13.2 years (range 15.5-82 years), and the average time between transplantation and detection of malignancy was 50.0±48.4 months (median 36, range 1-284 months). The lowest and highest median times to development of cancer were observed in KS and GU & RS malignancy (13 months, range 2-143 months vs 72 months, 8-240 months), respectively (table 2).
Median time for diagnosis and follow up after transplantation.
| Median time (month) | KS | Non-KS | PTLD | GU & RS | Solid tumor |
|---|---|---|---|---|---|
| Transplantation until diagnosis (month) | 13 | 60 | 46 | 72 | 36 |
| follow up period (month) | 23 | 34 | 12 | 12 | 9 |
Non-KS: Non Kaposi's sarcoma, KS: Kaposi's sarcoma, PTLD: post transplantation lymphoproliferative disorder, GU & RS: genitourinary and reproductive system.
Tumor incidence
Overall, tumor incidence of cancer in renal transplants was 2% and cumulative incidences of any detected malignancy after kidney transplantation during first 3 years are summarized in table 3. PTLD was the most common type of malignancy in age below 30 years as well as age between 30-50 years (18 cases; 54.5% and 36 cases; 30%, respectively). However, KS was the most common type of tumor (26 patients; 24.8%) in above 50 years old patients. The majority of patients with KS had limited skin and/or mucosal disease (87% of recipients), while 13% of them had visceral involvement. The skin cancer (141 recipients; 52.9%) was the most frequently observed malignancy after renal transplantation including KS, SCC, BCC and melanoma, followed by PTLD (72 cases; 27%); whereas GU & RS tumors (25 cases; 9.4%) was the most common malignancy among the other visceral tumors. The most frequent tumor in men and women was KS (53 patients; 61.9% and 31 cases; 38.1%, respectively), which had low mortality (n=7; 11.1% and n=5; 15.6%, respectively) (table 4).
RCC was seen in 6 patients of which 2 (33.3%) suffered from native RCC. We also observed a case of breast cancer in a male patient.
AZA-based regimens was used in approximately one third of recipients (4771 cases; 38.1%), while the rest was on MMF-based therapy (7736 patients; 61.9%). In addition, about one third of female gender (1703 cases; 35.7%) was treated with AZA. In this study, the incidence of cancer in men was significantly greater than in women (67.8% vs 32.2%, p=0.009) and recipients who were on AZA-based regimens were significantly associated with higher rate of cancer in comparison with those who were on MMF-based immunosuppression (61.3% vs 38.7%, p<0.001). The most common malignancy with AZA-based protocol was PTLD, while KS was the most frequently observed tumor in MMF-based regimens (table 1). On the other hand, AZA-based immunosuppression was associated with a higher risk for development of PTLD when compared to MMF therapy (45.3% vs 54.7%, p=0.001).
Tumors developed in 36 (13.5%) of patients treated with ALG/ATG (table 1). In this study, the risk of malignancy was greatest in patients with age older than 30 (222 cases, 87.1%) (p= 0.007). However, no significant association was observed between cancer and patients' gender [177 male (2.2%) vs 84 female (1.8%), p=0.1].
Treatment modalities
Patients were treated with 2 strategies, first of all, standard therapy for malignancy and the second, immunosuppressive modality. Combined surgery, chemotherapy and radiotherapy in 22.95 percent; combined surgery and chemotherapy in 10.7 percent; combined surgery and radiotherapy in 3.8 percent; combined chemotherapy and radiotherapy among 4.6 percent of patients were performed. In addition, single standard treatment with surgery, chemotherapy or radiotherapy was done among 26.7 percent, 29 percent, and 2.3 percent respectively.
Though withdrawal of calcineurin inhibitor and conversion to sirolimus should be considered early after the development of cancer; however, it had no significant benefits when compared to reduction of immunosuppressive therapy (p=1). However, reduction of immunosuppressive agents in patients with tumor was associated with lower rate of graft loss when compared to the withdrawal of immunosuppression (96.7% vs 73.9%, p<0.001). In addition, renal function was preserved when immunosuppression was reduced instead of withdrawn in recipients with cancer (96.7% vs 73.9%, p<0.001).
Frequency and cumulative incidence of malignancy.
| Cancer | Overall Incidence % | Frequency and cumulative incidence | Total frequency | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq1 | Inc1% | Freq2 | Inc2% | Freq3 | Inc3% | frequency | percent | total | |||
| GI | Colon | 0.04 | 2 | 0.75 | 6 | 2.4 | 17 | ||||
| Gastric | 0.03 | 1 | 0.4 | 4 | 1.6 | ||||||
| Rectum | 0.01 | 1 | 0.4 | 2 | 0.8 | ||||||
| pancreases | 0.007 | 1 | 0.4 | 1 | 0.4 | ||||||
| hepatoma | 0.02 | 2 | 0.75 | 3 | 1.2 | ||||||
| esophagus | 0.007 | 1 | 0.4 | 1 | 0.4 | ||||||
| skin | SCC | 0.3 | 5 | 1.9 | 4 | 3.3 | 1 | 3.7 | 40 | 13.5 | 141 |
| BCC | 0.1 | 3 | 1.1 | 2 | 1.9 | 2 | 2.6 | 15 | 6.1 | ||
| Melanoma | 0.01 | 1 | 0.4 | 2 | 0.8 | ||||||
| KS | 0.6 | 40 | 14.3 | 15 | 16.9 | 8 | 20 | 84 | 31 | ||
| SCC+BCC | 0.007 | 1 | 0.4 | ||||||||
| GU & RS | Brest | 0.02 | 1 | 0.4 | 3 | 1.2 | 25 | ||||
| Uterin | 0.01 | 1 | 0.4 | 2 | 0.8 | ||||||
| Ovary | 0.02 | 3 | 1.2 | ||||||||
| Prostat | 0.007 | 1 | 0.4 | 1 | 0.4 | ||||||
| Seminoma | 0.01 | 1 | 0.4 | 2 | 0.8 | ||||||
| RCC | 0.04 | 1 | 0.4 | 1 | 0.75 | 6 | 2.4 | ||||
| TCC | 0.06 | 1 | 0.4 | 8 | 3.3 | ||||||
| Pulmonary | Mesothelioma | 0.007 | 1 | 0.4 | 1 | 0.4 | 3 | ||||
| lung | 0.02 | 1 | 0.4 | 1 | 0.75 | 3 | 1.2 | ||||
| PTLD | 0.5 | 17 | 6.4 | 7 | 3.8 | 7 | 11.6 | 72 | 27.3 | 72 | |
| Thyroid | 0.01 | 1 | 0.4 | 2 | 0.8 | 2 | |||||
| Parathyroid | 0.007 | 1 | 0.4 | 1 | |||||||
| Chondrosarcoma | 0.007 | 1 | 0.4 | 1 | |||||||
| Pelvic sarcoma | 0.007 | 1 | 0.4 | 1 | 0.4 | 1 | |||||
| Brain | 0.02 | 1 | 0.4 | 3 | 1.2 | 3 | |||||
Freq1: frequency 1st year, Freq2: frequency 2nd year, Freq3: frequency 3rd year
Inc1: incidence 1st year, Inc 2: incidence 2nd year, Inc 3: incidence 3rd year
GI: gastrointestinal, GU & RS: genitourinary and reproductive system, PTLD: post transplantation lymphoproliferative disorder, KS: Kaposi's sarcoma, SCC: squamouse cell carcinoma, BCC: basal cell carcinoma, RCC: renal cell carcinoma, TCC: transitional cell carcinoma
Cancer incidence based on age and sex distribution.
| Cancer | Male | Female | Age | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median Tx-dig (mo) | N | % | N | % | ≤30 year | 31-50 year | >50 year | frequency | percent | Total | ||
| GI | Colon | 27 | 6 | 3.4 | 1 (3) | 2 (1.7) | 3 (2.9) | 6 | 2.4 | 17 | ||
| Gastric | 43 | 2 | 1.1 | 2 | 2.4 | 2 (1.7) | 2 (1.9) | 4 | 1.6 | |||
| Rectum | 57 | 2 | 1.1 | 2 (1.9) | 2 | 0.8 | ||||||
| pancreace | 32 | 1 | 0.6 | 1 (0.9) | 1 | 0.4 | ||||||
| hepatoma | 36 | 3 | 1.9 | 3 (2.6) | 3 | 1.2 | ||||||
| esoghagus | 8 | 1 | 0.6 | 1 (1) | 1 | 0.4 | ||||||
| Skin | SCC | 13 | 34 | 19.2 | 5 | 6 | 2 (6.1) | 11 (9.4) | 26 (24.8) | 39 | 13.5 | 141 |
| BCC | 39 | 11 | 6.2 | 3 | 3.6 | 1 (3) | 4 (3.4) | 9 (8.6) | 15 | 6.1 | ||
| Melanoma | 38 | 2 | 1.1 | 1 (0.9) | 1 (1) | 2 | 0.8 | |||||
| KS | 72 | 52 | 29.4 | 30 | 35.7 | 8 (24.2) | 35 (29.9) | 36 (34.3) | 84 | 31 | ||
| SCC+BCC | 76 | 1 | 0.6 | 1 (0.9) | 1 | 0.4 | ||||||
| GU & RS | Brest | 100 | 1 | 1.1 | 2 | 2.4 | 3 (2.6) | 3 | 1.2 | 25 | ||
| Uterin | 57 | 2 | 2.4 | 2 (1.9) | 2 | 0.8 | ||||||
| Ovary | 72 | 3 | 3.6 | 1(3) | 2 (1.7) | 3 | 1.2 | |||||
| Prostat | 22 | 1 | 0.6 | 1 (1) | 1 | 0.4 | ||||||
| Seminoma | 22 | 2 | 1.1 | 1 (0.9) | 2 | 0.8 | ||||||
| RCC | 16 | 5 | 2.8 | 1 | 1.2 | 3 (2.6) | 2 (1.9) | 6 | 2.4 | |||
| TCC | 81 | 5 | 2.8 | 3 | 3.6 | 1(3) | 4 (3.4) | 3 (2.9) | 8 | 3.3 | ||
| Pulmonary | Mesothelioma | 30 | 1 | 0.6 | 3 (2.6) | 1 | 0.4 | 3 | ||||
| Lung | 8 | 2 | 1.1 | 1 | 1.2 | 1 (0.9) | 3 | 1.2 | ||||
| PTLD | 46 | 42 | 23.7 | 28 | 33.3 | 18(54.5) | 36 (30) | 15 (14.3) | 72 | 27.3 | 72 | |
| Thyroid | 24 | 2 | 1.1 | 2 (1.7) | 2 | 0.8 | 2 | |||||
| Parathyroid | 120 | 1 | 1.2 | 1 (0.9) | 1 | 0.4 | 1 | |||||
| Chondrosarcoma | 132 | 1 | 0.6 | 1 (0.9) | 1 | 0.4 | 1 | |||||
| Pelvic sarcoma | 14 | 1 | 1.2 | 1 (1) | 1 | 0.4 | 1 | |||||
| Brain | 62 | 1 | 0.6 | 2 | 2.4 | 1 (3) | 1 (0.9) | 1 (1) | 3 | 1.2 | 3 | |
Tx-dig: transplantation until diagnosis, N: number, mo: month, GI: gastrointestinal, GU & RS: genitourinary and reproductive system, PTLD: post transplantation lymphoproliferative disorder, KS: Kaposi's sarcoma, SCC: squamouse cell carcinoma, BCC: basal cell carcinoma, RCC: renal cell carcinoma, TCC: transitional cell carcinoma
Graft function
Overall death-censored graft loss (graft failure without death) was seen in 11.4% individuals; on the other hand, 50 cases died with a functioning graft. Thus, the main cause of graft loss in our recipients was patient death.
Mortality rate
At the end of the study, 25% (67) of RTRs died following development of cancer. A higher rate of mortality was seen within the first year after tumor diagnosis (54 cases, 20.5%), while only 4.5% of mortality was observed during the next 5 years. PTLD was the most common cause of death in both genders (20 male and 8 female) and in all age groups (3 in group ≤30 years, 17 in group 31-50 years and 7 in group >50 years).
Patient Survival Data
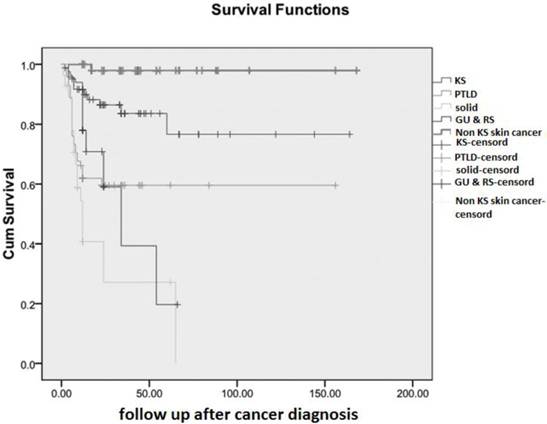
In the current study, overall cumulative patient survival was 74.6% at 14 years after tumor diagnosis and table 5 summarizes the first 5 year cancer free survival rates in 5 groups. In addition, cumulative survival curves are plotted separately for all groups in figure 1. The overall mean survival time was 117.1 months (95% CI: 104.9-129.2) and for all categorized cancer groups, data are shown in table 6. The best overall mean survival rates after treatment of malignancy were observed in patients with SCC or BCC (non-KS group) which was 164.8 months (95% CI: 158.7-170.9), and the worst prognosis was associated with sub-group of solid tumor (mean 25.5 months; 95% CI: 12.7-38.2).
In univariate analysis, the significant risk factors for death included poor graft function (p<0.001), treatment with AZA (P=0.004), acute rejection episode after transplantation (P=0.008), withdrawal of immunosuppressive drugs (p<0.001), non skin tumors (p<0.001), distant metastasis (P<0.001), no response to treatment (p<0.001), and induction therapy with ATG/ALG (p=0.02).
In multivariate analysis, the only independent risk factor associated with mortality was type of malignancy (table 7).
Survival rate.
| cancer | 1st year | 2nd year | 3rdyear | 4 th year | 5 th year |
|---|---|---|---|---|---|
| KS | 77 (91.7) | 74 (88.1) | 73 (86.9) | 73 (86.9) | 72 (85.7) |
| PTLD | 44 (62) | 43 (63.6) | 43 (60.6) | 43 (60.6) | 43 (60.6) |
| Solid | 13 (46.4) | 12 (42.9) | 12 (42.9) | 12 (42.9) | 12 (42.9) |
| GU & RS | 19 (79.2) | 17 (70.8) | 16 (66.7) | 16 (66.7) | 15 (62.5) |
| Non-KS | 57 (100) | 56 (98.2) | 56 (98.2) | 56 (98.2) | 56 (98.2) |
PTLD: post transplantation lymphoproliferative disorder, KS: Kaposi's sarcoma, Non-KS: Non-KS: Kaposi's sarcoma, GU & RS: genitourinary and reproductive system.
Mean survival time.
| cancer | Mean | 95% CI |
|---|---|---|
| KS | 132.26 | 13.4-151.7 |
| PTLD | 96.03 | 78.6-113.4 |
| Solid tumors | 25.49 | 12.7-38.2 |
| GU & RS | 36.23 | 23.4-49.06 |
| Non-KS | 164.8 | 158.7-170.9 |
PTLD: post transplantation lymphoproliferative disorder, KS: Kaposi's sarcoma, Non-KS: Non-KS: Kaposi's sarcoma, GU & RS: genitourinary and reproductive system.
Relative risk of cancers after transplantation compared with skin tumors.
| First year | Second year | Third & fourth year | Fifth year | overall | |
|---|---|---|---|---|---|
| RR (95% CI) P | RR (95% CI) P | RR (95% CI) P | RR (95% CI) P | RR (95% CI) P | |
| Skin | 1 | 1 | 1 | 1 | 1 |
| PTLD | 33.6 (7.4-152.4) 0 | 37.1 (8.1-170.4) 0 | 28.1 (7.6-106.6) 0 | 28.5 (7.6-106.6) 0 | 28.5 (7.5-106.6) 0 |
| Solid | 27.5 (5.6-113.6) 0 | 35.4 (7.3-170.5) 0 | 28.4 (7.04-114.5) 0 | 28.4 (7.04-114.5) 0 | 28.4 (7.05-114.5) 0 |
| GU & RS | 34.6 (4.8-246.8) 0 | 39.9 (5.4-291.6) 0 | 31.9 (5.03-203.3) 0 | 31.9 (5.03-203.2) 0 | 31.9 (5.03-203.3) 0 |
PTLD: post transplantation lymphoproliferative disorder, GU & RS: genitourinary and reproductive system.
cumulative survival of categorized malignancy after kidney transplantation. KS: Kaposi's sarcoma; PTLD: post transplantation lymphoproliferative disorder; Non KS: Non Kaposi's sarcoma tumors; GU & RS: genitourinary & reproductive system.
DISSCUSION
With the advent of management for infectious and cardiovascular complications after renal transplantation, it is probable that malignancy among RTRs may become an increasingly essential cause of mortality in the future (18). To the best of our knowledge, this is the largest series reporting the results of post-transplant malignancy in LURT patients.
According to some reports, incidence of post-transplant cancer ranges from 2% to 31% (depending on the follow-up period, tumor kind and registry records) and adds up to 34-50% (6). However, in this study the overall tumor incidence in RTRs was 2% which is less than other studies, even from Asian reports (1, 4, 6, 7, 14, 21-24). On the other hand, the relative risk for cancer in post-transplant recipients is different among the various cancers. The reasons for these differences are given below:
1- The available studies are limited, because the main information on cancer in the renal transplanted patients is adopted from the Cincinnati Transplant Tumor Registry, Collaborative Transplant Study, and the Australian and New Zealand Transplant Registry (3), which are in a limited location with a relative homogeneity in medical care, type of donor, geographical location, disease epidemiology, lifestyle, diet, cultural and socioeconomic status. For example regional differences can explain reason of higher skin cancers rate in Australia and New Zealand versus Central Europe (3).
Also, reporting of cancer to registries is often incomplete and probably underestimates the true cancer incidence which potentially leads to difference in overall cancer incidence. On the other hand, single center studies have small sample size therefore are not reliable then using data from different registries, with different population can help to estimate almost true incidence of cancers (25).
2- Several specific malignancies in transplant recipients have been linked with specific viruses, such as Epstein-Barr virus (EBV) which promotes lymphomas as well as hepatitis viruses B and C which are linked with hepatocellular carcinoma and human herpes virus 8 which predisposes to lymphomas and Kaposi's sarcoma. In addition, cervical, penile and vulvar cancers are associated with human papillomaviruses (25). As we know, distribution of viruses is epidemiologically different and so the difference in distribution of viruses induced cancers are expected.
3- Published studies show that geographical factors have also an impact on different forms of post-transplant cancer, since high incidence of gastrointestinal tumors in Japan, urinary tract transitional cell carcinoma in Taiwan and liver cancer in South-East Asia have been reported in kidney transplant recipients (25, 26).
4- Exposure to carcinogens such as total sun exposure and living in a hot climate which are important risk factors for skin cancers, and difference in race as well as genetics can cause difference in skin cancer distribution. For example, studies have shown that Caucasian RTRs living in Queensland, Australia, have the highest global risk of non-melanoma skin cancer (NMSC) (25, 27).
5- Samhan et al revealed that cancer after kidney transplantation was more frequent in deceased donors (15).
6- Wimmer et al showed time and the intensity of immunosuppression augmented the incidence of malignancy (3), therefore the races such as African who need more immunosuppressive drugs after kidney transplantation are more predisposed to cancer (28).
Although in this study following up period was not very long, lower incidence of malignancy may be due to living donors, strict screening, exact immunosuppressive monitoring, lower doses of immunosuppression used in RTRs from living donors, race and lifestyle differences which need further investigation.
Skin cancer
In contrast to Moosa and Gralla study which believes Middle East RTRs rarely have skin malignancies (29): in this study, the skin cancer was the most common form of malignancy among RTRs, a finding that is concordant with other reports (18), but the most common type of skin cancer in our recipients was different from Western countries and similar to Mediterranean, Jewish, Arabic, Caribbean, or African reports which KS is the most frequently observed tumor among our RTRs (3, 7, 10, 20).
SCC and BCC have better prognosis than KS. On the other hand, race and geographic location are the important factors for incidence of KS; therefore, it seems that race and geographic location may be prognostic factors in post-transplant malignancy.
In post-transplant skin cancer, immunosuppressive agents such as azathioprine or cyclosporine not only can directly potentiate damaging effects of UV radiation on skin, but also can augment reduction of local and systemic immune responses during sun exposure. Furthermore, azathioprine sensitizes DNA to UVA radiation, reducing the minimal erythema dose in skin cells of treated patients (30, 31). In one study, a significant association between current use of azathioprine and cutaneous SCC was described (27, 32)
PTLD
In adults, PTLD is the second most common cancer and encompasses up to 12% of post-transplant cancers (18). In children with renal transplant, PTLD is the most common cancer which involves up to 50% of post-transplant cancers (18). It was 4-fold higher in children than adults (33). The frequency of PTLD varies according to the type of organ transplanted, age of recipients (i.e., children versus adults), and the immunosuppressive regimen (33). Furthermore, recipients developing PTLD after organ transplantation have a poor prognosis (25). In the current study, incidence of PTLD was 0.5% and majority of them are presented in the first year (6.4%), and survival rate is lower especially in the first years (Fig 1).
GU & RS tumors
According to Dantal study (2007) (25) about GU & RS cancer in RTRs the most common GU & RS tumors is transitional cell carcinoma and majority of them were diagnosed at early stage and capable of treatment (25, 34). Moreover, Besarani et al. (2006) (34) reported the incidence of urological tumors in the Oxford Transplant Centre among 2100 RTRs within 3 decades and RCC accounted for the most common urological malignancies in these individuals with an incidence of 4.6%. In our study, the majority of GU & RS cancers were late onset with low survival rate and they presented more in middle age and in male gender. Therefore, screening schedules are very helpful for early detection and it is reasonable that all patients after transplantation must be monitored for TCC bladder and for both native and transplanted kidney with a regular ultrasonography.
In this study, we found a lower prevalence of breast cancers (male/female: 1/2) in our recipients. However, there is no consensus whether female renal transplant patients rarely got breast cancer. Marcen et al (35) believe prostate cancer in male and breast cancer in female gender which are one of the most common neoplasias in general population, are not (or only slightly) increased among transplant recipients. Nevertheless, Kasiske et al (10) reported a 2-fold increase in breast cancer risk among RTRs compared to the general population (10). Interestingly, breast cancer is known to be a hormonally dependent carcinoma. Many studies have revealed androgen receptor to be often co-expressed with estrogen receptor and progesterone receptor in breast tumors (36).
The main contributing factors of malignancy in kidney transplant patients:
Solid tumors
We found that the worst prognosis was observed in solid tumors patients with high rate of refractoriness to treatment and elevated recurrence rate.
Immunosuppression
One of the most important risk factors for developing malignancies is receiving immunosuppressive agents (25), they can affect host defense and susceptible to malignancy (18). Conversely, some immunosuppressive drugs for instance sirolimus has dual effect on immunosuppression and antitumor effect experimentally and clinically. Published studies have revealed a lesser incidence of new (de novo) malignancies in RTRs whose immunosuppression is mTOR inhibitors than CNIs. One of the important advantage in mTOR inhibitors usage may be the protection of allograft from immunological rejection, while simultaneously preventing from cancer in high-risk group (21).
Although, while considering patient survival until now, any meticulous immunosuppressive protocol has not been confirmed to be better over others, but it seems AZA and calcineurin inhibitors (CNIs) have been more linked with post-transplant malignancies (2). In this study, MMF had less negative impact on the incidence of malignancies than AZA. In the current study, all types of malignancies were associated with AZA based protocol except for KS which was associated with MMF slightly more than AZA based regimen. Data about carcinogenicity of MMF are controversial (25). While MMF was diagnosed as an anti-neoplastic agent, some clinical trials suggested that it was associated with a non-significant tendency towards an increased risk of PTLD in RTRs (37), but PTLD was significantly associated with AZA in our patients. On the other hand, two large studies demonstrated that MMF was not linked with amplification of malignancy (38, 39). Indeed, it seems that there is a trend towards a lower risk of malignancy with MMF (25, 38).
According to other studies in malignant condition, we preferred to change calcineurin inhibitor by sirolimus. However, it was not available until recent years and reduction or withdrawal of immunosuppressive drugs was mostly achieved. Nonetheless, decrease of immunosuppression had significantly better results in terms of response to treatment, prevention of rejection, graft preservation and patient survival compared to immunosuppressive discontinuation. Finally, induction of donor-specific tolerance might be resolved so many complications from long-term immunosuppressive therapies in future (40).
Survival
Despite the high incidence of skin cancers in transplant recipients, these tumors are usually not fatal. Solid organ cancers, although less common, are associated with a far worse prognosis in renal transplant recipients (25). The best survival has been observed in SCC and BCC and the worst in non-hematologic and non-skin tumors. Patient survival is lesser in patients who received monoclonal antibody induction. Thus if monoclonal antibody induction is indicated, consideration to benefit and hazard, in future, is very important.
Although the present results showed no significant difference between gender, age, type of immunosuppression and all type of cancers, we found that the majority of cancers occurred in the first 5 years after transplantation, male gender, patients greater than 40 years, individuals received AZA. Thus, these groups should be considered for screening protocols.
LIMITATION
As this study was retrospective and data has been collected from previous medical records that were completed by several nephrologists without any coordination between them, thus all contributing factors were not accessible and we have some missing data. In addition follow up period was short and we did not follow patients who developed cancer after transplant failure.
Unfortunately, we have no document reports about all kind of malignancy in non-transplant population; subsequently, comparison of cancer rate in transplant patients with the general population was not possible.
CONCLUSION
This study revealed the lowest malignancy incidence in living unrelated kidney transplantation and KS as the most common malignancy in our RTRs. Additionally, immunosuppressive reduction has better outcome than withdrawal. Otherwise, use of immunosuppressive agents must be balanced between the beneficial or hazardous effect of preventing organ rejection or increasing the risk of tumor development.
Acknowledgements
We would like to express our thanks to Dr. Mohsen Reza Heidari for his assistance in editing the English language of this paper.
CONFLICT OF INTEREST
None declared.
References
1. Feng W.W, Wang T.N, Chen H.C, Ho J.C, Ko Y.C. Malignancies after renal transplantation in southern Taiwan: experience in one centre. BJU Int. 2007;99(4):825-9
2. Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69(16):2227-43
3. Wimmer C.D, Rentsch M, Crispin A, Illner W.D, Arbogast H, Graeb C. et al. The janus face of immunosuppression - de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney Int. 2007;71(12):1271-8
4. Navarro M.D, Lopez-Andreu M, Rodriguez-Benot A, Aguera M.L, Castillo DD, Aljama P. Cancer incidence and survival in kidney transplant patients. Transplant Proc. 2008;40(9):2936-40
5. Gandhi M.J, Strong D.M. Donor derived malignancy following transplantation: a review. Cell Tissue Bank. 2007;8(4):267-86
6. Vegso G, Toth M, Hidvegi M, Toronyi E, Langer R.M, Dinya E. et al. Malignancies after renal transplantation during 33 years at a single center. Pathol Oncol Res. 2007;13(1):63-9
7. Stratta P, Morellini V, Musetti C, Turello E, Palmieri D, Lazzarich E. et al. Malignancy after kidney transplantation: results of 400 patients from a single center. Clin Transplant. 2008;22(4):424-7
8. Hu X.P, Ma L.L, Wang Y, Yin H, Wang W, Yang X.Y. et al. Rapamycin instead of mycophenolate mofetil or azathioprine in treatment of post-renal transplantation urothelial carcinoma. Chin Med J (Engl). 2009;122(1):35-8
9. Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M. et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128-35
10. Kasiske B.L, Snyder J.J, Gilbertson D.T, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4(6):905-13
11. Mazuecos A, Terol JMM, Alvarez TG, Sola E, Benot AR, Dsuna A. et al. Increase in malignancies as cause of death in renal transplant patients. Transplant Proc. 2009;41(6):2159-62
12. Bustami R.T, Ojo A.O, Wolfe R.A, Merion R.M, Bennett W.M, McDiarmid S.V. et al. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant. 2004;4(1):87-93
13. Anaya F, Plaza J, Sanz-Guajardo D, Luque A, Rengel M, Fernandez J. et al. Cancer after renal transplantation. Transplant Proc. 2003;35(2):697-9
14. Pita-Fernandez S, Valdes-Canedo F, Pertega-Diaz S, Seoane-Pillado M, Seijo-Bestilleiro R. Cancer incidence in kidney transplant recipients: a study protocol. BMC Cancer. 2009;9(1):294
15. Samhan M, Al-Mousawi M, Donia F, Fathi T, Nasim J, Nampoory M.R. Malignancy in renal recipients. Transplant Proc. 2005;37(7):3068-70
16. Berardinelli L, Messa P.G, Pozzoli E, Beretta C, Montagnino G. Malignancies in 2,753 kidney recipients transplanted during a 39-year experience. Transplant Proc. 2009;41(4):1231-2
17. Wong G, Chapman J.R. Cancers after renal transplantation. Transplant Rev (Orlando). 2008;22(2):141-9
18. Vasudev B, Hariharan S. Cancer after renal transplantation. Curr Opin Nephrol Hypertens. 2007;16(6):523-8
19. Mahdavi-Mazdeh M, Rouchi AH, Norouzi S, Aghighi M, Rajolani H, Ahrabi S. Renal replacement therapy in Iran. Urol J. 2007;4(2):66-70
20. Einollahi B, Lessan-Pezeshki M, Nourbala M.H, Simforoosh N, Pourfarziani V, Nemati E. et al. Kaposi's sarcoma following living donor kidney transplantation: review of 7,939 recipients. Int Urol Nephrol. 2009;41(3):679-85
21. Kapoor A. Malignancy in kidney transplant recipients. Drugs. 2008;68(Suppl 1):11-9
22. Fraile P, Garcia-Cosmes P, Martin P, Garcia-Bernalt V, Tabernero J.M. Non-skin solid tumors as a cause of morbidity and mortality after liver transplantation. Transplant Proc. 2009;41(6):2433-4
23. Arichi N, Kishikawa H, Nishimura K, Mitsui Y, Namba Y, Tokugawa S. et al. Malignancy following kidney transplantation. Transplant Proc. 2008;40(7):2400-2
24. Chiang Y.J, Chen C.H, Wu C.T, Chu S.H, Chen Y, Liu K.L. et al. De novo cancer occurrence after renal transplantation: a medical center experience in Taiwan. Transplant Proc. 2004;36(7):2150-1
25. Dantal J, Pohanka E. Malignancies in renal transplantation: an unmet medical need. Nephrol Dial Transplant. 2007;22(Suppl 1):i4-10
26. Morath C, Mueller M, Goldschmidt H, Schwenger V, Opelz G, Zeier M. Malignancy in renal transplantation. J Am Soc Nephrol. 2004;15(6):1582-8
27. Ramsay H.M, Fryer A.A, Hawley C.M, Smith A.G, Nicol D.L, Harden P.N. Factors associated with nonmelanoma skin cancer following renal transplantation in Queensland, Australia. J Am Acad Dermatol. 2003;49(3):397-406
28. Harada K.M, Mandia-Sampaio E.L, de Sandes-Freitas T.V, Felipe C.R, Park S.I, Pinheiro-Machado P.G. et al. Risk factors associated with graft loss and patient survival after kidney transplantation. Transplant Proc. 2009;41(9):3667-70
29. Moosa M.R, Gralla J. Skin cancer in renal allograft recipients--experience in different ethnic groups residing in the same geographical region. Clin Transplant. 2005;19(6):735-41
30. O'Donovan P, Perrett C.M, Zhang X, Montaner B, Xu Y.Z, Harwood C.A. et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309(5742):1871-4
31. Perrett C.M, Walker S.L, O'Donovan P, Warwick J, Harwood C.A, Karran P. et al. Azathioprine treatment photosensitizes human skin to ultraviolet A radiation. Br Dermatol J. 2008;159(1):198-204
32. van Leeuwen M.T, Grulich A.E, McDonald S.P, McCredie M.R, Amin J, Stewart J.H. et al. Immunosuppression and other risk factors for lip cancer after kidney transplantation. Cancer Epidemiol Biomarkers Prev. 2009;18(2):561-9
33. Chughtai A, Bastani B. Posttransplant lymphoproliferative disorders. Iran J Kidney Dis. 2008;2(2):57-64
34. Besarani D, Cranston D. Urological malignancy after renal transplantation. BJU Int. 2007;100(3):502-5
35. Marcen R, Galeano C, Fernandez-Rodriguez A, Jimenez-Alvaro S, Teruel J.L, Rivera M. et al. Effects of the new immunosuppressive agents on the occurrence of malignancies after renal transplantation. Transplant Proc. 2010;42(8):3055-7
36. Lari S.A, Kuerer H.M. Biological Markers in DCIS and Risk of Breast Recurrence: A Systematic Review. J Cancer. 2011;2:232-61
37. Gutierrez-Dalmau A, Campistol J.M. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67(8):1167-98
38. Robson R, Cecka J.M, Opelz G, Budde M, Sacks S. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant. 2005;5(12):2954-60
39. Caillard S, Dharnidharka V, Agodoa L, Bohen E, Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80(9):1233-43
40. Andreola G, Chittenden M, Shaffer J, Cosimi A.B, Kawai T, Cotter P. et al. Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. Am J Transplant. 2011;11(6):1236-47
Author contact
![]() Corresponding author: Zohreh Rostami, MD. Nephrology and Urology Research Center, Baqiyatallah University of Medical Sciences, Molla Sadra Ave, Vanak Sq. Tehran, IR. Iran. Email:rostamiir
Corresponding author: Zohreh Rostami, MD. Nephrology and Urology Research Center, Baqiyatallah University of Medical Sciences, Molla Sadra Ave, Vanak Sq. Tehran, IR. Iran. Email:rostamiir

 Global reach, higher impact
Global reach, higher impact