3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(1):41-50. doi:10.7150/jca.32724 This issue Cite
Research Paper
Risk of immune-related diarrhea with PD-1/PD-L1 inhibitors in different cancer types and treatment regimens
Cancer center, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China
Received 2019-1-1; Accepted 2019-6-25; Published 2020-1-1
Abstract
Objective: To compare the incidence and severity of diarrhea among different tumor types and treatment regimens, and also compared with CTLA-4 inhibitors in randomized controlled trials.
Methods: MEDLINE, PMC database and EMBASE were retrieved until December 2018. Studies were eligible if they were randomized controlled trials and included participants undergoing PD-1/PD-L1 inhibitors for cancer, measured a treatment side effect of diarrhea, and reported quantitative data. The risks of diarrhea in PD-1/PD-L1 inhibitors were compared among different treatment regimens.
Results: Totally 21 studies involving 11554 patients were included for meta-analysis. For all-grade diarrhea, the risk after the PD-1/PD-L1 inhibitors plus CTLA-4 inhibitor combination was 1.90 times significantly higher than that of monotherapy, and the risk was 0.69 and 0.60 times significantly lower than that of monotherapy compared with chemotherapy and ipilimumab. The incidence of diarrhea was not significantly different between PD-1/PD-L1 inhibitor monotherapy versus placebo or between low-doses versus high-doses. For high-grade (grade ≥ 3) diarrhea, the risk after the PD-1/PD-L1 inhibitors plus CTLA-4 inhibitor combination was 3.29 times significantly higher than that of monotherapy, the risk in PD-1/PD-L1 inhibitors monotherapy was 0.50 and 0.38 times significantly lower than chemotherapy and ipilimumab respectively. No significant difference was found in the incidence of diarrhea between PD-1/PD-L1 inhibitor monotherapy versus placebo or between low-doses versus high-doses whether in all-grade or high-grade group.
Conclusions: PD-1/PD-L1 inhibitors have a lower risk of developing diarrhea than chemotherapy and CTLA-4 inhibitor. There is no direct relationship between the dose of PD-1/PD-L1 inhibitors and the risk of developing diarrhea.
Keywords: Cancer, Diarrhea, Randomized controlled trials, PD-1/PD-L1 inhibitors, CTLA-4 inhibitor, Chemotherapy
Introduction
Increasing evidence proves the significant efficacy of immune checkpoint inhibitors (ICIs) in treatment of advanced cancers [1-4]. ICIs targeting the programmed cell death protein 1/programmed death ligand 1 (PD-1/PD-L1) pathway significantly improve the progression-free survival and overall survival compared with standard chemotherapy, so PD-1/PDL1 antibodies are currently approved for treatment of various malignancies [5-11]. Since the anti-PD-1 antibody pembrolizumab was approved in September 2014 for treatment of advanced melanoma, the clinical development of PD-1/PD-L1 inhibitors as anticancer drugs has been widely expanded. Currently, the Food and Drug Administration has approved PD-1/PD-L1 inhibitors for treatment of 9 types of cancers. For instance, pembrolizumab can be used to treat melanoma [2, 12-14], non-small cell lung cancer (NSCLC) [7, 15-19], head and neck squamous cell carcinoma (HNSCC) [20], Hodgkin's lymphoma [21], urothelial cancer [22, 23] and gastric cancer [24]. Anti-PD-1 antibody nivolumab is recommended for treating melanoma [11, 25], renal cell carcinoma (RCC)[26], Hodgkin's lymphoma [27, 28], urine epidermal cancer [29], MSI-H colon cancer [30] and hepatocellular carcinoma [31]. Anti-PD-L1 antibody atezolizumab is suggested for treatment of urothelial cancer [22, 32] and NSCLC [6, 33], and anti-PD-L1 antibodies avelumab and durvalumab can be used to treat urothelial cancer[34, 35]. Compared with cytotoxic chemotherapy, ICIs have unique toxicity based on their action mechanism, which is considered to be immune-related adverse event (IRAE) [36-39]. Inhibiting the PD-1/PD-L1 pathway may lead to autoimmune toxicity, some of which may be severe or even life- threatening [36, 40].
Diarrhea is a common side effect of cancer treatment that, in severe cases, can lead to death or to patients having to stop lifesaving treatment because often there are no effective therapies to control the diarrhea. Diarrhea in cancer patients can quickly lead to life-threatening consequences such as dehydration, electrolyte imbalance, shock, etc. Compared to chemotherapy-related diarrhea the immunological preparation of PD-1/PD-L1 is prone to cause autoimmune digestive diseases such as ulcerative colitis, and may also cause side effects of diarrhea.
Given the clinical efficacy evidence for a wide spectrum of tumor types, the PD-1 ICI therapy is expected to be increasingly used by oncologists as a monotherapy or in combination with other drugs. Therefore, physicians in cancer immunotherapy must be familiar with the pathogenesis of diarrhea in different tumors and different treatment regimens, and provide useful information to optimize the management of this toxicity. At present, there is no complete description about the clinical experience of anti-PD-1/PD-L1-associated diarrhea patients, or about the management and outcome of this toxicity. Therefore, we conducted a meta-analysis of PD-1 inhibitors in cancer patients and compared the incidence and severity of diarrhea among different tumor types, different treatment regimens.
1. Methods
1.1. Literature selection and data extraction
Two researchers (Lei Zhao and Huihui Li) independently reviewed the databases Medline, PMC database and EMBASE to select potential relevant articles. Any discrepancy between them was resolved by consensus. The following medical subject heading terms were used: PD-1, PDL1, CD274, programmed death receptor 1, programmed death receptor ligand, immune checkpoint inhibitor, nivolumab, BMS936558, pembrolizumab, MK-3475, MPDL3280A, atezolizumab, avelumab, MSB0010718C, durvalumab, and diarrhea. The databases were searched from the inception until December, 2018.
The inclusion criteria were: (a) phase I, II and III trials in cancer patients; (b) random assignment of participants to single PD-1/PD-L1 inhibitor treatment or other control therapy (e.g. ipilimumab, placebo); (c) reporting diarrhea events or event rate and sample size for any all-grade or high-grade (≥3) adverse events;(d) random controlled trial.
The following information was extracted by two independent reviewers (Lei Zhao and Huihui Li) from the included studies: first author, publication year, study name, clinical trial registration number, total number of patients, mean age, trial phase, treatment plan, tumor type, primary inclusion criteria, and numbers of patients with all grades and high-grade treatment-related diarrhea. The treatment regimens were classified as PD-1/PD-L1 inhibitor monotherapy, PD-1/PD-L1 inhibitor plus CTLA-4 inhibitor ipilimumab, chemotherapy, placebo, and ipilimumab. According to the different doses, monotherapy was divided into low-dose group and high-dose group.
1.2. Quality assessment
The two reviewers (Lei Zhao, Huihui Li) used the Jadad scoring method[41] to evaluate the quality of each included study from randomized (0 or 1), double-blind (0, 1 or 2), recorded loss of follow-up and/or exit (0 or 1) and assign hidden (0 or 1). A score ≥ 3 indicates high quality.
1.3. Statistical analysis
Meta-analysis for statistical analysis was performed using Stata12.1 (Stata Corp, College Station, TX, USA). Heterogeneity was analyzed by Q test, and I2<25%, 25%-75%, and >75% indicate mild, moderate and significant heterogeneity, respectively. In case of insignificant heterogeneity between studies indicated as P>0.05, a fixed effect model was used; otherwise a random effect model was used. The incidence of diarrhea was evaluated by relative risk (RR) and 95% confidence interval (CI), and the analysis results were represented by forest maps. Two-tailed p < 0.05 was considered significant. This meta-analysis has been registered on the PROSPERO website (Registration Number: CRD42018111834).
2. Results
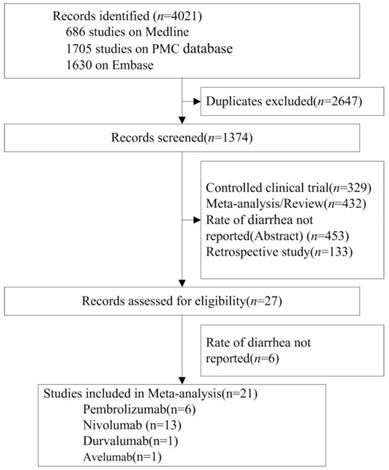
The database search initially returned 4021 studies. After screening and eligibility assessment, a total of 21 randomized controlled trials (RCTs, n=11554 patients) were identified for meta- analysis. ICIs tested in these studies included nivolumab (n=13 studies), pembrolizumab (n=6), avelumab (n=1) and durvalumab (n=1). Tumor types tested included NSCLC (n=7 studies), melanoma (n=10), HNSCC (n=1), Renal Cell Carcinoma (RCC) (n=1), gastric cancer (n=1) and small cell lung cancer (SCLC) (n=1). According to the clinical staging, 15, 5 and 1 of the 21 RCTs were at phase 3, 2 and 1, respectively (Table 1, Figure 1).
Flow Diagram of Study Inclusion.
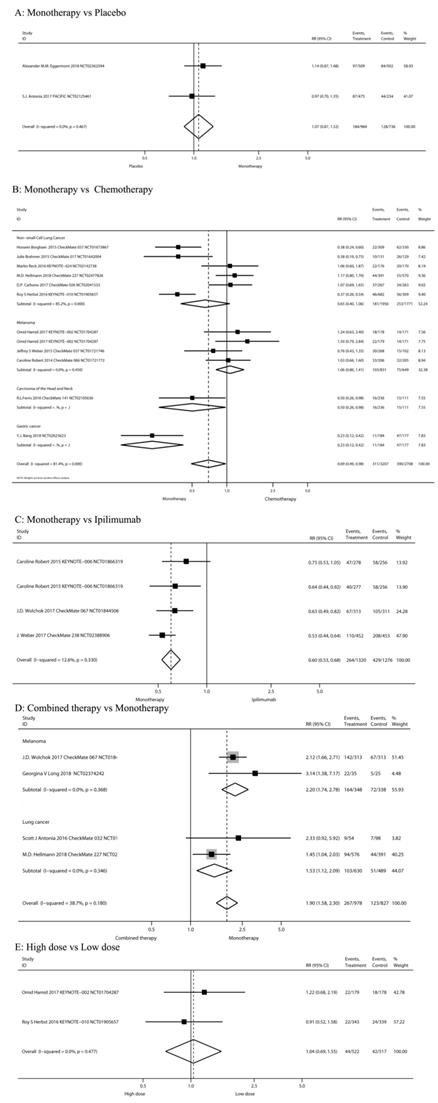
2.1. Risks of diarrhea among different treatment regimens
All-grade diarrhea
The risks of all-grade diarrhea in PD-1/PD-L1 inhibitors were compared among different treatment regimens: PD-1/PD-L1 inhibitor monotherapy versus PD-1/PD-L1 inhibitor plus ipilimumab, versus chemotherapy, versus placebo, versus ipilimumab, and high-dose versus low-dose in the 20 studies (Figure 2).
PD-1/PD-L1 inhibitor monotherapy versus placebo
Two RCTs [42, 43] compared PD-1/PD-L1 inhibitor monotherapy and placebo (n=1,720 patients). Classification based on tumor type included melanoma (n = 1 study) and NSCLC (n = 1). Classification according to the use of ICIs included pembrolizumab (n = 1) and durvalumab (n = 1). The pooled RR of all-grade diarrhea incidence was not significant after PD-1/PD-L1 inhibitor monotherapy (RR 1.07, 95%CI: 0.87-1.32, P=0.516) (Figure 2A).
PD-1/PD-L1 inhibitor monotherapy versus chemotherapy
Eleven RCTs [5, 7, 11, 12, 18, 44-49] compared PD-1/PD-L1 inhibitor monotherapy and conventional chemotherapy (n=5,915 patients). Classification according to tumor type included melanoma (n = 3 study), HNSCC (n=1), Gastric cancer (n=1) and NSCLC (n = 6). Classification according to the use of ICIs included nivolumab (n = 7 study), pembrolizumab (n = 3) and Avelumab (n=1). The risk of all-grade diarrhea after PD-1/PD-L1 inhibitor monotherapy was significantly decreased (RR 0.69, 95%CI: 0.49-0.98, P=0.037; Figure 2B). The risk of all-grade diarrhea after PD-1/PD-L1 inhibitor monotherapy in Carcinoma of the Head and Neck and Gastric cancer patients were significantly decreased (RR 0.50, 95%CI: 0.26-0.98, P=0.043; RR 0.23, 95%CI: 0.12-0.42, P=0.000 Figure 2B).
PD-1/PD-L1 inhibitor monotherapy versus ipilimumab
Three RCTs [13, 50, 51] compared PD-1/PD-L1 inhibitor monotherapy and ipilimumab (n=2,596 patients). The tumor type was malignant melanoma. Classification according to the use of ICIs was nivolumab (n=2) and pembrolizumab (n=1). The study group of Caroline Robert 2015[13] was divided into two subgroups according to drug intervals and thus can be analyzed as two studies. The pooled RR of all-grade diarrhea incidence after the PD-1/PD-L1 inhibitor monotherapy (nivolumab or pembrolizumab) was significantly decreased (RR 0.60, 95%CI: 0.53-0.68, P=0.000; Figure 2C).
Nivolumab plus ipilimumab compared to nivolumab monotherapy
Four RCTs [42, 48, 50, 52] compared nivolumab plus ipilimumab and nivolumab monotherapy (n= 1,805 patients). Classification of tumor type was SCLC (n=1 study), NSCLC (n=1) and melanoma (n=2). Our meta-analysis reveals that nivolumab plus ipilimumab significantly increased the risk of all-grade diarrhea compared to nivolumab monotherapy (RR 1.90, 95%CI: 1.58-2.30, P=0.000; Figure 2D).
The risks of all-grade diarrhea in PD-1/PD-L1 inhibitors were compared among different treatment regimens.
Characteristics of Relevant Studies
| Analysis Method | Source | Format | Data Set | Tumor Type | Main Inclusion Criterion | Treatment | Sample Size | Age, Median(range),y | Jadad score |
|---|---|---|---|---|---|---|---|---|---|
| Meta-analysis Meta-analysis | R.L.Ferris 2016 CheckMate 141 NCT02105636 | Full text | Randomized, open-label, phase 3 trial | Head and neck | Recurrent squamous-cell carcinoma of the head and neck | 1. Nivolumab 3 mg/kg Q2W 2. Standard therapy | 240 121 | 59(29-83) 61(28-78) | 3 |
| Julie Brahmer 2015 CheckMate 017 NCT01642004 | Full text | Randomized, open-label, international, phase 3 study | NSCLC | Stage IIIB or IV squamous-cell NSCLC | 1. Nivolumab 3 mg/kg Q2W 2. Docetaxel 75 mg/m2 Q3W | 135 137 | 62 (39-85) 64 (42-84) | 3 | |
| Caroline Robert 2015 KEYNOTE-006 NCT01866319 | Full text | Randomized, controlled, phase 3 study | Melanoma | Unresectable stage III or IV melanoma | 1. Pembrolizumab 10 mg/kg Q2W 2. Pembrolizumab 10 mg/kg Q3W 3. Ipilimumab 3 mg/kg Q3W | 279 277 278 | 61 (18-89) 63 (22-89) 62 (18-88) | 3 | |
| Omid Hamid 2017 KEYNOTE-002 NCT01704287 | Full text | Randomised, open-label, phase 2 study | Melanoma | Unresectable stage III or stage IV melanoma not amenable to local therapy | 1. Pembrolizumab 2 mg/kg Q3W 2. Pembrolizumab 10 mg/kg Q3W 3. Chemotherapy(carboplatin , carboplatin plus paclitaxel, dacarbazine, paclitaxel alone or oral temozolomide) | 180 181 179 | 62 (15-87) 60 (27-89) 63 (27-87) | 3 | |
| J.D. Wolchok 2017 CheckMate 067 NCT01844505 | Full text | Double-blind, Randomised, phase 3 trial | Melanoma | Stage III (unresectable) or stage IV melanoma | 1.Nivolumab 1 mg/kg Q3W plus ipilimumab 3 mg /kg Q3W for four doses,followed by nivolumab 3mg/kg Q2W 2.Nivolumab 3 mg/kg Q2W 3.Ipilimumab 3 mg/kg Q3W for four doses | 314 316 315 | 61 (18‒88) 60 (25‒90) 62 (18‒89) | 4 | |
| Michael A. Postow 2015NCT01927419 | Full text | Randomized 2:1 in a double-blinded phase 2 trial | Melanoma | Unresectable, previously-untreated, stage III or IV melanoma with measurable disease | 1. Nivolumab 1mg/kg Q3W plus ipilimumab 3mg/kg first 4 doses,then nivolumab 3mg/kg Q2W 2.Ipilimumab 3mg/kg first 4 doses | 95 47 | 64 (27- 87) 67 (31- 80) | 4 | |
| Georgina V Long 2018NCT02374242 | Full text | Multicentre, open-label randomised, phase 2 trial | Melanoma | Melanoma brain metastases | 1. Nivolumab 1 mg/kg + ipilimumab 3 mg/kg Q3W for four doses; then nivolumab 3 mg/kg Q2W 2. Nivolumab 3 mg/kg Q2W 3. Nivolumab 3 mg/kg Q2W( brain metastases) | 35 25 16 | 59 (53-68) 63 (52-74) 51 (48-56) | 3 | |
| Caroline Robert, 2014KEYNOTE-001 NCT01295827 | Full text | Open-label, international, multicentre phase 1 trial | Melanoma | Progressive, measurable, unresectable melanoma | 1.Pembrolizumab 2 mg/kg Q3W 2.Pembrolizumab 10 mg/kg Q3W | 89 84 | 57 (18-88) 60.7 (27-86) | 3 | |
| Alexander M.M. Eggermont 2018 NCT02362594 | Full text | Randomized, double-blind phase 3 trial | Melanoma | Resected stage III melanoma | 1. Pembrolizumab 200 mg Q3W for 18 doses 2. Placebo | 514 505 | 54 (19-88) 54 (19-83) | 4 | |
| S.J.Antonia 2017 PACIFIC NCT02125461 | Full text | Global, randomized, phase 3 trial | NSCLC | Stage III, locally advanced, unresectableNSCLC | 1. Durvalumab10 mg/kg Q2W for up to 12 months. 2. Placebo | 476 237 | 64(31-84) 64(23-90) | 3 | |
| Martin Reck 2016 KEYNOTE-024 NCT02142738 | Full text | Open-label, randomised , phase 3 trial | NSCLC | Untreated advanced NSCLC with PD-L1 expression on at least 50% of tumor cells kinase gene | 1. Pembrolizumab 200 mg Q3W for 35 cycles 2.Investigator's choice of platinum based chemotherapy for 4 to 6 cycles | 154 151 | 64.5(33-90) 66(38-85) | 3 | |
| D.P. Carbone 2017 CheckMate 026 NCT02041533 | Full text | Open-label randomized phase 3 trial | NSCLC | Untreated stage IV or recurrent NSCLC and a PD-L1 tumor-expression level of 1% or more | 1. Nivolumab 3 mg/kg Q2W 2. Platinum-based chemotherapy Q3W for up to six cycles | 271 270 | 63(32-89) 65(29-87) | 3 | |
| Roy S Herbst 2016 KEYNOTE-010 NCT01905657 | Full text | Randomised, open-label, phase 2/3 study | NSCLC | Previously treated non-small-cell lung cancer with PD-L1 expression on at least 1% of tumour cells | 1. Pembrolizumab 2 mg/kg Q3W 2. Pembrolizumab 10 mg/kg Q3W 3. Docetaxel 75 mg/m² Q3W | 344 346 343 | 63 (56-69) 63 (56-69) 62 (56-69) | 3 | |
| Scott J Antonia 2016 CheckMate 032 NCT01928394 | Full text | Randomised,Cohort of this phase 1/2 multicentre, multi-arm, open-label trial | Recurrent small-cell lung cancer | Limited-stage orextensive-stage SCLC, and had disease progression after at least one previous platinum-containing regimen | 1. Nivolumab 3 mg/kg Q2W 2. Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg Q3W for four cycles, nivolumab3 mg/kg Q2W 3. Nivolumab 3 mg/kg plus ipilimumab 1 mg/kg Q3W for four cycles, nivolumab3 mg/kg Q2W | 98 61 54 | 63 (57-68) 66 (58-71) 61 (56-65) | 3 | |
| M.D. Hellmann 2018 CheckMate 227 NCT02477826 | Full text | Randomised,Multipart, open-label phase 3 trial | stage IV or recurrent NSCLC | stage IV or recurrent NSCLC that was not previously treated with chemotherapy | 1. Nivolumab 3 mg/kg Q2W plus Ipilimumab 1 mg/kg Q6W 2. Nivolumab 240 mg Q2W 3. Chemotherapy | 576 391 570 | - | 3 | |
| Hossein Borghaei 2015 CheckMate 057 NCT01673867 | Full text | Randomised, phase 3 study | Non-squamous NSCLC | stage IIIB/IV or recurrent non-squamous NSCLC | 1. Nivolumab 3 mg/kg Q2W 2. Docetaxel 75 mg/m2 Q3W | 292 290 | 61(37-84) 64(21-85) | 3 | |
| Jeffrey S Weber 2015 CheckMate 037 NCT01721746 | Full text | Randomised, controlled, open-label, phase 3 trial | melanoma | unresectable or metastatic melanoma, and progressed after ipilimumab, or ipilimumab and a BRAF inhibitor if they were BRAFV600 mutation-positive | 1. Nivolumab 3 mg/kg Q2W 2. ICC(dacarbazine 1000 mg/m2 Q3W; carboplatin area under the curve 6+ paclitaxel 175 mg/m2 Q3W) | 272 133 | 59 (23-88) 62 (29-85) | 3 | |
| Caroline Robert 2014 CheckMate 066 NCT01721772 | Full text | Randomised, double-blind, phase 3 trial | Melanoma | unresectable, previously untreated stage III or IV melanoma without a BRAF mutation | 1. Nivolumab(3 mg/kg Q2W)+ placebo Q3W 2. Dacarbazine(1000 mg/ m2 Q3W)+ placebo Q2W | 210 208 | 64 (18-86) 66 (26-87) | 4 | |
| J. Weber 2017 CheckMate 238 NCT02388906 | Full text | Randomized, double-blind, phase 3 trial | Melanoma | complete resection of stage IIIB,IIIC, or IV melanoma | 1. Nivolumab 3 mg/kg Q2W 2. Ipilimumab 10mg/kg Q3W for four doses | 453 453 | 56 (19-83) 54 (18-86) | 4 | |
| Robert J. Motzer 2015 NCT01354431 | Full text | Blinded, randomized, multicenter phase II trial | Renal Cell Carcinoma | Metastatic Renal Cell Carcinoma | 1. Nivolumab 0.3mg/kg Q3W 2. Nivolumab 2mg/kg Q3W 3. Nivolumab 10 mg/kg Q3W | 60 54 54 | 61±9 61±8 61±10 | 3 | |
| Y.-J. Bang 2018 NCT02625623 | Full text | multicentre, international, randomised, open-label, phase III trial | gastric cancer | metastatic gastric cancer/gastrooesophageal junction cancer | 1. Avelumab 10mg/kg Q2W 2. Physician's choice of chemotherapy | 185 186 | 59 (29-86) 61 (18-82) | 3 |
High-dose group versus low-dose group
Three RCTs [7, 26, 47] compared the low-dose and high-dose PD-1/PD-L1 inhibitor monotherapy (n= 1039 patients). Pembrolizumab 2 mg/kg is defined as low dose; 10 mg/kg is high-dose. Nivolumab ≤2 mg/kg is defined as low dose, 10 mg/kg is high-dose. Classification according to tumor type was melanoma (n = 1 study), RCC (n = 1) and NSCLC (n = 1). Results showed no significant risk in the high-dose group (RR 1.15, 95%CI: 0.80-1.67, P=0.446; Figure 2E).
High-grade diarrhea
Figure 3 showed the risk of high-grade (≥ 3) diarrhea according to different treatment regimens: PD-1/PD-L1 inhibitor monotherapy versus PD-1/PD-L1 inhibitor plus ipilimumab, versus chemotherapy, versus placebo, versus ipilimumab, and low-dose versus high-dose.
PD-1/PD-L1 inhibitor monotherapy versus placebo
Inclusion of the study and number of patient's high-grade diarrhea were consistent with previous all-grade diarrhea. As shown in Figure 3A, when compared with placebo, there was not a significant increase in the risk of high-grade diarrhea incidence for PD-1/PD-L1 inhibitor monotherapy (RR 0.85, 95%CI: 0.29-2.44, P=0.756).
PD-1/PD-L1 inhibitor monotherapy versus chemotherapy
Inclusion of the study and number of patient's high-grade diarrhea were consistent with previous all-grade diarrhea. Results showed a significant decreased in the risk of high-grade diarrhea after monotherapy (RR 0.50, 95%CI: 0.26-0.95, P = 0.035; Figure 3B). Of the 7 RCTs on non-small cell lung cancer, 5 were treated with nivolumab and 2 with pembrolizumab. The use of nivolumab or pembrolizumab seems to reduce the risk of diarrhea compared to chemotherapy, but the results are not significant (RR 0.58, 95%CI: 0.25-1.31, P = 0.190; RR 0.61, 95%CI: 0.03-13.01, P = 0.754, respectively).
PD-1/PD-L1 inhibitor monotherapy versus ipilimumab
Inclusion of the study and number of patients of high-grade diarrhea were consistent with previous grades of diarrhea. Results showed significantly decreased in the risk of high-grade diarrhea after PD-1/PD-L1 inhibitor monotherapy (RR 0.38, 95%CI: 0.18-0.79, P=0.009; Figure 3C). Our meta-analysis reveals that PD-1 antibodies (pembrolizumab or nivolumab) reduce the risk of severe diarrhea compared to ipilimumab.
Nivolumab plus ipilimumab compared to nivolumab monotherapy
Inclusion of the study and number of patients of high-grade diarrhea were consistent with previous grades of diarrhea. The tumor type was melanoma in all cases. Results showed no significant increase in the risk of high-grade diarrhea after nivolumab plus ipilimumab treatment (RR 3.29, 95%CI: 1.80-6.03, P=0.000; Figure 3E).
The risk of high-grade (≥ 3) diarrhea according to different treatment regimens.
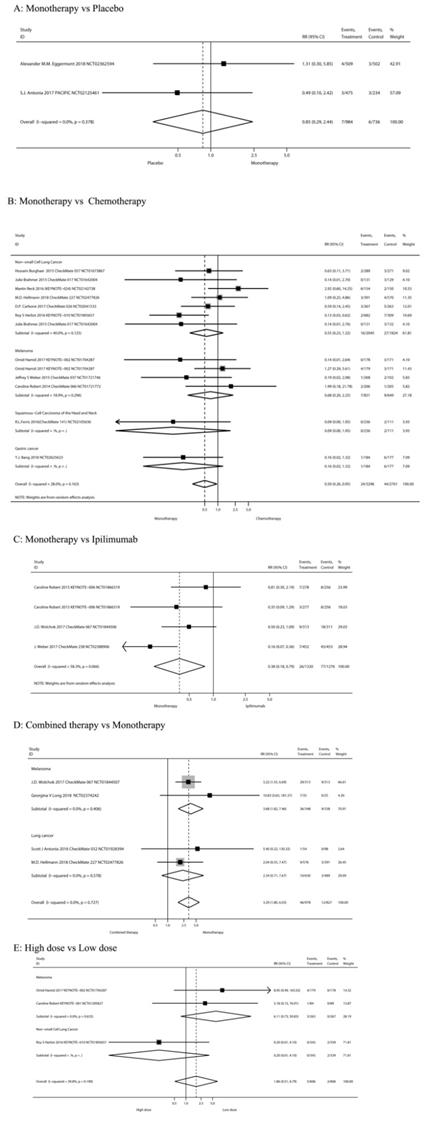
High-dose group versus low-dose group
Three RCTs [7, 12, 47] compared the High-dose and Low-dose treatments (n=1,212 patients). Classification of tumor type was melanoma (n=2 studies) and NSCLC (n=1). Results showed no significant increase in the risk of high-grade diarrhea after high-dose treatment (RR 1.86, 95%CI: 0.51-6.79, P=0.345; Figure 3F).
Study quality and publication bias
Fifteen trials were open label, whereas five trials were double blind controlled. The Jadad score ranged from 3 to 4. For RR of all-grade between PD-1/PD-L1 inhibitor monotherapy and chemotherapy or high-grade diarrhea between the monotherapy and ipilimumab, the Egger test suggested some evidence of publication bias. No evidence of bias was found in other comparisons of Egger tests (all P>0.05), or in all Begg tests (all P>0.05).
3. Discussion
Although an increasing number of clinical studies have confirmed the overall survival benefit of PD-1/PD-L1 inhibitors treatment, PD-1/PD-L1 inhibitors therapy increases the toxicity of the drug remains controversial, especially diarrhea. Our meta-analysis is the first large-scale analysis of different immunologic treatment regimens compared with chemotherapy or ipilimumab for the toxic side effects of diarrhea. In our research, the risk of all-grade diarrhea after the PD-1/PD-L1 inhibitors plus CTLA-4 inhibitor combination was 1.90 times higher than that of PD-1/PD-L1 inhibitors monotherapy (P<0.05), and the risk was 0.72 and 0.60 times higher than that of chemotherapy and ipilimumab compared with monotherapy (P<0.05). Chemotherapy is the most prone to diarrhea, followed by PD-1/PD-L1 inhibitors plus CTLA-4 inhibitor combination, and finally PD-1/PD-L1 inhibitors monotherapy. When compared with placebo, we did not observe a significant increase in the risk of all-grade or severe diarrhea incidence, and our meta-analysis also reveals that high-dose PD-1/PD-L1 inhibitor monotherapy did not increase the risk of all-grade or severe diarrhea when compared with low-dose(all P>0.05), which suggested that PD-1/PD-L1 inhibitor monotherapy is relatively safe. The risk of grade ≥ 3 diarrheas for PD-1/PD-L1 inhibitors alone significantly decreased than chemotherapy or ipilimumab, while the PD-1/PD-L1 inhibitors plus CTLA-4 inhibitor combination significantly increase than monotherapy.
The basic principle of binding PD-1 / PD-L1 inhibitors and CTLA-4 inhibitors is that they have different mechanisms of action. Anti-CTLA-4 mainly acts on the lymph node area, restores the induction and proliferation of activated T cells, and resists PD-1 acts mainly on the periphery of the tumor site, preventing the tumor-infiltrating tumor-infiltrating PD-L1-expressing tumor and plasma-like dendritic cells from neutralizing cytotoxic T cells [53]. Our result found that patients taking CTLA-4 inhibitors ipilimumab had a significantly higher risk of developing diarrhea than those using PD-1 / PD-L1 inhibitors. This is likely to be related to the different mechanisms of action of the two drugs. Although the combination of PD-1/PD-L1 inhibitors plus CTLA-4 inhibitor has achieved good efficacy [25, 50, 52], the corresponding toxic side effects of combination therapy, especially diarrhea, are significantly higher than those of PD-1/PD-L1 inhibitors alone. Combination therapy with both CTLA-4 and PD-1 blockers raised the risk of GI toxicities to about 45% which is much higher than monotherapy [54]. The risk of diarrhea was significantly different compared to the use of PD-1/PD-L1 inhibitors alone and chemotherapy in different tumor types. In patients with NSCLC patients, the risk of diarrhea using PD-1 / PD-L1 inhibitors monotherapy is significantly lower than that of patients receiving chemotherapy. However, this result did not find in melanoma patients. Our results show that patients taking pembrolizumab or nivolumab have a slightly different risk of all-grade diarrhea compared with chemotherapy [11, 12, 47]. Furthermore, our study demonstrates no significant difference in the incidence of diarrhea between low-dose versus high-dose PD-1/PD-L1 inhibitors, which are consistent with another study [12]. This provides reliable evidence for further exploration of adjusting drug doses in future clinical trial design and clinical practice.
As far as we know, this is the systematic review including the largest number of RCTs for analysis of immune-related diarrhea. The present study has some limitations. Firstly, these relevant studies have applied PD-1/PD-L1 inhibitors to different treatment lines, and there may be inconsistencies in the underlying characteristics of the patients. Secondly, since the present study is based on a secondary analysis of the final results of each report, we were unable to obtain patient-level disease characteristics and variables, or to determine the specific risk factors associated with the development of immune-related diarrhea. Thirdly, our results were influenced by the limitations of individual clinical trial design. Some of the clinical trials included in the meta- analysis were open label, which may lead to subjective bias. Finally, clinical RCTs included in the meta-analysis had strict inclusion and exclusion criteria. The patients selected in the study were with good PS, but in clinical practice, a large number of patients suffered impaired organ dysfunction and/or functional status and may have a higher incidence of actual toxicity. In the future, large-sample RCTs are needed to compare the incidence and severity of PD-1/PD-L1 inhibitor associated diarrhea among more tumor types and among more combination regimens.
Overall, PD-1/PD-L1 inhibitors have a lower risk of developing diarrhea than chemotherapy and CTLA-4 inhibitor. There is no direct relationship between the dose of PD-1/PD-L1 inhibitors and the risk of developing diarrhea. This study provides reliable evidence for further exploring the combination of PD-1/PD-L1 inhibitors with other drugs in clinical trial design and clinical practice.
Acknowledgements
Funding & Support
This work was supported by grants from the Beijing Natural Science Foundation (grant number 7184200 to Lei Zhao); Beijing Municipal Administration of Hospitals' Youth Programme (grant number QML20170102 to Lei Zhao); Beijing Municipal Administration of Hospitals' Digestive Medical Coordinated Development Center Funding Suppor (grant number XXT01 to Bangwei Cao); Beijing Natural Science Foundation (grant number 7172061 to Bangwei Cao); the Capital Health Research and Development of Special (grant number 2018-2-2022 to Bangwei Cao); China Population Publicity and Education Center Research and Promotion Project (grant number 2017-A001 to Bangwei Cao).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167-3175
2. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144
3. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454
4. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030
5. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-135
6. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837-1846
7. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-1550
8. Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M. et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35:3924-3933
9. Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C. et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918
10. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330
11. Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B. et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384
12. Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R. et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117
13. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L. et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521-2532
14. Zimmer L, Apuri S, Eroglu Z, Kottschade LA, Forschner A, Gutzmer R. et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47-55
15. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018-2028
16. Hui R, Garon EB, Goldman JW, Leighl NB, Hellmann MD, Patnaik A. et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: a phase 1 trial. Ann Oncol. 2017;28:874-881
17. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF. et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497-1508
18. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A. et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-1833
19. Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA Approval Summary: Pembrolizumab for the Treatment of Patients With Metastatic Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. Oncologist. 2016;21:643-650
20. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP. et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956-965
21. Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P. et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol. 2017;35:2125-2132
22. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67-76
23. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L. et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376:1015-1026
24. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M. et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013
25. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017
26. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S. et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803-1813
27. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311-319
28. Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S. et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283-1294
29. Sharma P, Retz M, Siefker-Radtke A, A B, Necchi A, Bedke J. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312-322
30. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191
31. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502
32. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909-1920
33. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265
34. Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K. et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J Clin Oncol. 2017;35:2117-2124
35. Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J. et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34:3119-3125
36. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S. et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148
37. Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-Related Pneumonitis during Cancer Immunotherapy. N Engl J Med. 2015;373:288-290
38. Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS. et al. Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab. Cancer Immunol Res. 2015;3:1185-1192
39. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697
40. Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F. et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559-574
41. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12
42. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919-1929
43. Eggermont A, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S. et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378:1789-1801
44. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639
45. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M. et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376:2415-2426
46. Ferris RL, Blumenschein GJ, Fayette J, Guigay J, Colevas AD, Licitra L. et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856-1867
47. Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D. et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45
48. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C. et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med. 2018;378:2093-2104
49. Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M. et al. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052-2060
50. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL. et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345-1356
51. Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL. et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377:1824-1835
52. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP. et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19:672-681
53. Eggermont AMM, Crittenden M, Wargo J. Combination Immunotherapy Development in Melanoma. Am Soc Clin Oncol Educ Book. 2018:197-207
54. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD. et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23-34
Author contact
![]() Corresponding author: Dr. Bangwei Cao, Cancer center, Beijing Friendship Hospital, Capital Medical University, 95 Yong An Road, Xicheng District, Beijing 100050, China.TEL: +86-010-63139321,Fax: +86-010-63139321, E-mail address: oncologyedu.cn.
Corresponding author: Dr. Bangwei Cao, Cancer center, Beijing Friendship Hospital, Capital Medical University, 95 Yong An Road, Xicheng District, Beijing 100050, China.TEL: +86-010-63139321,Fax: +86-010-63139321, E-mail address: oncologyedu.cn.

 Global reach, higher impact
Global reach, higher impact