3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(14):3079-3086. doi:10.7150/jca.30463 This issue Cite
Research Paper
Prognostic value of site-specific metastases in lung cancer: A population based study
Department of CyberKnife Center, Huashan Hospital, Fudan University, Shanghai, China
Received 2018-10-6; Accepted 2019-4-13; Published 2019-6-2
Abstract
Background: Studies on prognosis of different metastasis sites in patients with lung cancer are limited. The aim of present study was to investigate the prognostic value of metastases sites among patients with metastatic lung cancer.
Methods: Between 2010 and 2014, patients diagnosed with metastatic lung cancer were selected using the Surveillance, Epidemiology, and End Results (SEER) database. Kaplan-Meier methods were adopted and multivariable Cox regression models were built to compare the prognosis of different metastasis sites.
Results: A total of 54,697 eligible patients were identified, including 10,945 (20.0%) patients had isolated bone metastases, 8,294(15.2%) with isolated brain metastases, 5,677(10.4%) with isolated liver metastases, 9,430(17.2%) with isolate lung metastases, and 20,351(37.2%) with multiple organ metastases. The percentage of bone, brain, liver, lung and multisite metastases were 22.3%, 15.4%, 6.1%, 20.1% and 36.1% for non-small cell lung cancer (NSCLC), 12.5%, 14.3%, 24.3%, 7.9%, and 40.9% for small cell lung cancer (SCLC), the difference was statistical(P<0.001). In univariate and multivariable analysis, patients with liver metastases demonstrated a statistically significant disadvantage in cause-specific survival, while those with lung metastases have reduced risk of died of metastases when compared with brain metastases(P<0.001).The difference was consistent when make subgroup analysis in both NSCLC and SCLC(P<0.001).
Conclusions: In patients with distant metastases, those with liver metastases have the poorest survival, whereas those with lung metastases have the best survival. Therefore, we should take into consideration of such discrepancy when making treatment strategies.
Keywords: Lung cancer, Distant metastatic site, Prognosis
Introduction
Lung and bronchus cancer is the leading cause of cancer mortality worldwide [1, 2]. According to the latest update of cancer statistics in the United States in 2018, a total of 234,030 estimated new lung and bronchus cancer cases will be diagnosed; both the incidence of males and females are the second highest among all cancer types [2]. Because of the lack of screening programs in most countries, more than half of lung cancer patients are diagnosed at an advanced stage [3]. Metastatic lung cancer is a debilitating disease that results in a high burden of symptoms and poor quality of life; the estimated prognosis after the diagnosis has been established was less than 1 year until a few years ago [4-6]. At the present, the new targeted therapies are changing the course of this disease, especially for patients having tumors presenting some gene mutations drivers, like mutations activating EGFR [7, 8], ALK translocation [9, 10], ROS rearrangement [11, 12]. However, advanced lung cancer remains an incurable disease, with a poor prognosis for the majority of patients.
In patients diagnosed with advanced lung cancer at initial diagnosis or during follow-up, the most common metastatic sites is lung, followed by the lymph nodes, the brain, the bones, the adrenal glands, and the liver. Oftentimes, lung cancer will spread to more than one area of the body. Due to the differences in biological heterogeneity and treatment strategies, the survival of patients with various metastatic sites is variable[13, 14]. Therefore, knowledge of the patterns of distant metastasis is crucial to personalize the treatment and follow-up strategies. Population-based cancer registries provide an excellent opportunity to investigate the relationship between the patterns of distant metastases and prognosis in metastatic cancer [15, 16]. However, the prognostic value of metastatic sites in lung cancer was only studied in small samples, and the results were controversial [17-21].
In this study, we investigated the relationship between different metastatic sites and cancer-specific survival (CSS) of stage IV lung cancer registered within the surveillance, epidemiology, and end results (SEER) database.
Materials and Methods
Data collection
For the metastatic site code was available from 2010, thus, only patients diagnosed from 2010 to 2014 were included in the study. Other inclusion criteria included: (1) diagnosed with primary lung cancer; (2) with definite lung, liver, brain and bone metastases; (3) histology codes were limited to adenocarcinoma, squamous cell carcinoma, large cell carcinoma, small cell carcinoma, non-small cell carcinoma, and bronchiolo-alveolar adenocarcinoma; (4) lung cancer was the only primary or the first of multiply primaries; (5) information about CSS and survival months were available.
The following data were collected from the SEER database with SEER*Stat 8.3.5: age at diagnosis, gender, tumor grade, tumor size, histology, radiotherapy, chemotherapy, sequence number, surgery of the metastases, CSS status, survival months. CSS was defined as the time from the date of diagnosis to the date of death caused by CRC. T stage was restaged according to the 8th edition TNM stage system [22]. Histology was classified as small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Chemotherapy was denoted as yes or none/unknown, radiotherapy was divided into yes or refused or unknown according to SEER code. This study was approved by the Institutional Review Board of Huashan Hospital, Fudan University.
Statistical analysis
The Chi-square (χ2) test was used to compare the clinicopathological features among different sites of metastases. Survival rate was calculated using Kaplan-Meier curves, and the differences were evaluated using the log-rank test. Multivariate Cox regression analyses were utilized to recognize the specific factors that influence CSS. All statistical analyses were performed with SPSS 22.0 (SPSS Inc., Chicago, IL, USA). All confidence intervals (CIs) were stated at the 95% confidence level. Statistical significance was defined as P<0.05 (two-sided).
Results
Patient characteristics
A total of 54,697 eligible lung cancer patients with definite organ metastases were identified. The flow chart of the study was depicted in Figure 1. Among them, 10,945 (20.0%) patients had isolated bone metastases, 8,294 (15.2%) with isolated brain metastases, 5,677 (10.4%) with isolated liver metastases, 9,430 (17.2%) with isolate lung metastases, and 20,351(37.2%) with multiple organ metastases. Specifically, the percentage of bone, brain, liver, lung and multisite metastases were 22.3%, 15.4%, 6.1%, 20.1% and 36.1% for NSCLC, 12.5%, 14.3%, 24.3%, 7.9%, and 40.9% for SCLC, the difference was statistical (P<0.001) (Fig. 1). Information about adrenal glands and other uncommon metastatic organs was unavailable. Most patients were diagnosed at the age of more than 60-year old (71.2%). The clinical characteristics and pathological features of all the patients were summarized in Table 1.
Interestingly, patients with bone metastases have high percentage of diagnosed with age >60 years (P<0.001) (Fig. 2A), and they are more likely to received surgical resection of metastases or radiotherapy (P<0.001) (Fig. 2B). Patients with liver metastases have high percentage of NSCLC than those with other metastases sites (P<0.001) (Fig. 2C) (Table 1).
Survival analysis
CSS of patients with single or multi organs involvement were compared according to the distant site. In patients with bone, brain, liver, lung and multisite metastases, the median CSS was 7.4, 8.2, 5.5, 9,3 and 5.8 months, respectively (P<0.001). Kaplan-Meier analysis showed that 5-year CSS for patients with bone, brain, liver, lung and multisite metastases were 4.3%, 5.9%, 2.2%, 7.9%, and 1.5%, respectively. Log-rank test indicated that metastatic tumor sites were associated with CSS of stage IV lung cancer patients (P<0.001, Fig. 3).
The univariate Cox regression model indicated that gender, age at diagnosis, race, histology, tumor grade, tumor stage, surgery of metastases, using chemotherapy and radiotherapy, and metastases site were correlated with CSS (P<0.05). Multivariate analysis after adjustment revealed that all above ten factors were independently prognostic factor for CSS for metastatic lung cancer. Using brain metastases as a reference, patients with isolated bone metastases had similar CSS (HR=0.970, 95% CI 0.937-1.004, P=0.082), whereas isolated lung metastases represented better CSS (HR=0.709, 95% CI 0.686-0.733, P<0.001), whereas isolated liver and multisite metastases were associated with worse CSS (liver: HR=1.157, 95% CI 1.114-1.202, P<0.001; multisite: HR=1.299, 95% CI 1.265-1.335, P<0.001).
Clinical characteristics of patients
| Variables | Metastasis site | |||||
|---|---|---|---|---|---|---|
| Brain | Bone | Liver | Lung | multisite | P Value | |
| Gender | <0.001 | |||||
| Male | 6419(58.6) | 4260(51.4) | 2996(52.8) | 4837(51.3) | 11070(54.4) | |
| Female | 4526(41.4) | 4034(48.6) | 2681(47.2) | 4593(48.7) | 9281(45.6) | |
| Age | <0.001 | |||||
| ≤60 | 2994(27.4) | 3027(36.5) | 1321(23.3) | 1982(21.0) | 6447(31.7) | |
| >60 | 7951(72.6) | 5267(63.5) | 4356(76.7) | 7448(79.0) | 13904(68.3) | |
| Race | ||||||
| Caucasian | 8844(80.8) | 6585(79.4) | 4836(85.2) | 7297(77.4) | 16111(79.2) | |
| Black | 1263(11.5) | 1068(12.9) | 580(10.2) | 1268(13.4) | 2353(11.6) | |
| Others | 838(7.7) | 641(7.7) | 261(4.6) | 865(9.2) | 1887(9.3) | |
| Histology | ||||||
| SCLC | 9332(85.3) | 6453(77.8) | 2551(44.9) | 8409(89.2) | 15095(74.2) | |
| NSCLC | 1613(14.7) | 1841(22.2) | 3126(55.1) | 1021(10.8) | 5256(25.8) | |
| Grade | ||||||
| I | 201(1.8) | 112(1.4) | 33(0.6) | 430(4.6) | 289(1.4) | |
| II | 1144(10.5) | 801(9.7) | 254(4.5) | 1314(13.9) | 1680(8.3) | |
| III | 2333(21.3) | 2146(25.9) | 820(14.4) | 2222(23.6) | 4087(20.1) | |
| IV | 320(2.9) | 363(4.4) | 397(7.0) | 228(2.4) | 774(3.8) | |
| Unknown | 6947(63.5) | 4872(58.7) | 4173(73.5) | 5236(55.5) | 13521(66.4) | |
| T stage | ||||||
| T1 | 2696(24.6) | 2110(25.4) | 1118(19.7) | 2003(21.2) | 4088(20.1) | |
| T2 | 2625(24.0) | 2067(24.9) | 1097(19.3) | 2092(22.2) | 4845(23.8) | |
| T3 | 1623(14.8) | 1366(16.5) | 793(14.0) | 1431(15.2) | 3304(16.2) | |
| T4 | 1359(12.4) | 1230(14.8) | 825(14.5) | 1415(15.0) | 2994(14.7) | |
| Tx | 2642(24.1) | 1521(18.3) | 1844(32.5) | 2489(26.4) | 5120(25.2) | |
| Surgery of Metastases | ||||||
| No | 10349(94.6) | 6596(79.5) | 5573(98.2) | 9199(97.6) | 19122(94.0) | |
| Yes | 577(5.3) | 1682(20.3) | 79(1.4) | 216(2.3) | 1199(5.9) | |
| Unknown | 19(0.2) | 16(0.2) | 25(0.4) | 15(0.2) | 30(0.1) | |
| Radiotherapy | ||||||
| Yes | 5362(49.0) | 6399(77.2) | 880(15.5) | 2102(22.3) | 10473(51.5) | |
| No | 147(1.3) | 106(1.3) | 92(1.6) | 194(2.1) | 288(1.4) | |
| Unknown | 5436(49.7) | 1789(21.6) | 4705(82.9) | 7134(75.7) | 9590(47.1) | |
| Chemotherapy | ||||||
| No | 4665(42.6) | 3522(42.5) | 2627(46.3) | 4419(46.9) | 8593(42.2) | |
| Yes | 6280(57.4) | 4772(57.5) | 3050(53.7) | 5011(53.1) | 11758(57.8) | |
Subgroup analysis of metastatic site with different histology
The survival and recurrence patterns are different for SCLC and NSCLC. Therefore, we made subgroup survival analysis for SCLC and NSCLC, respectively. For NSCLC, the median CSS for patients with bone, brain, liver, lung and multisite metastases were 7.5, 8.4, 6.2, 9.5 and 6.0 months, respectively, the difference was statistical significance (P<0.001) (Fig. 4A). In the multivariate analysis, patients with bone (HR=0.959, 95% CI 0.922-0.996, P=0.032) and lung metastases (HR=0.697, 95% CI 0.672-0.722, P<0.001) have better survival than those with brain metastases, while patients diagnosed with liver (HR=1.087, 95% CI 1.033-1.143, P=0.001) and multisite metastases (HR=1.282, 95% CI 1.244-1.321, P<0.001) have worse survival outcome than those with brain metastases.
The flow chart of eligible patients' selection in present study.
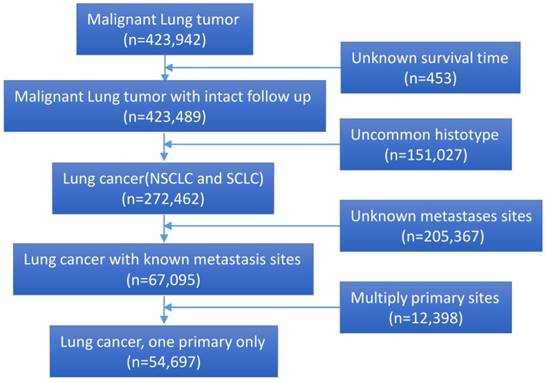
The percentage of distant metastasis sites. (A) The percentage of distant metastasis sites according to age group (P<0.001). B. The rate of surgery for metastases on different distant metastasis sites (P<0.001). (C) The percentage of histotype based on different distant metastasis sites (P<0.001).
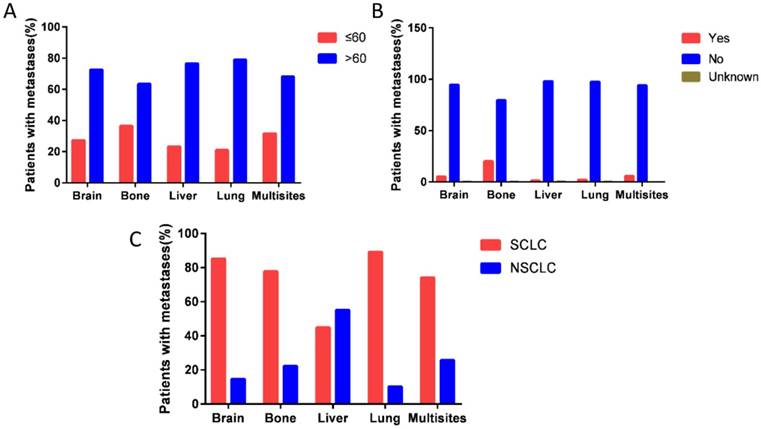
Kaplan-Meier curve of cancer-specific survival based on the site of metastases. 5-year cancer-specific survival for patients with bone, brain, liver, lung and multisite metastases were 4.3%, 5.9%, 2.2%, 7.9%, and 1.5%, respectively. The difference was statistical (P<0.001).
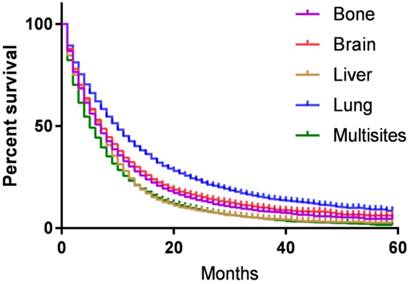
Kaplan-Meier curve of cancer-specific survival based on different histotype. Metastases sites were associated with survival in both (A) NSCLC and (B) SCLC (P<0.001).
Univariate and multivariate Cox proportional hazards analysis of CSS for patients with metastatic lung cancer in the SEER database
| Variables | Univariate analysis | P value | Multivariate analysis | P value |
|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | |||
| Gender | <0.001 | <0.001 | ||
| Male | 1.000 | 1.000 | ||
| Female | 0.826 (0.810-0.842) | 0.842 (0.826-0.859) | ||
| Age | <0.001 | <0.001 | ||
| ≤60 | 1.000 | 1.000 | ||
| >60 | 1.275(1.248-1.302) | 1.142(1.142-1.167) | ||
| Race | <0.001 | |||
| Caucasian | 1.000 | 1.000 | ||
| Black | 0.969(0.941-0.998) | 0.039 | 0.975(0.946-1.004) | 0.095 |
| Others | 0.680(0.655-0.706) | <0.001 | 0.694(0.668-0.721) | <0.001 |
| Histology | <0.001 | <0.001 | ||
| SCLC | 1.000 | 1.000 | ||
| NSCLC | 1.238(1.210-1.266) | 1.234(1.203-1.266) | ||
| Grade | <0.001 | <0.001 | ||
| I | 1.000 | 1.000 | ||
| II | 1.301(1.198-1.412) | 0.042 | 1.268(1.168-1.376) | <0.001 |
| III | 1.703(1.575-1.841) | <0.001 | 1.624(1.501-1.757) | <0.001 |
| IV | 1.941 (1.776-2.122) | <0.001 | 1.690 (1.542-1.852) | <0.001 |
| Unknown | 1.710 (1.584-1.845) | <0.001 | 1.602 (1.483-1.730) | <0.001 |
| T stage | <0.001 | <0.001 | ||
| T1 | 1.000 | 1.000 | ||
| T2 | 1.110 (1.078-1.143) | <0.001 | 1.126 (1.093-1.159) | <0.001 |
| T3 | 1.251 (1.211-1.292) | <0.001 | 1.263 (1.223-1.305) | <0.001 |
| T4 | 1.416(1.370-1.464) | <0.001 | 1.402(1.356-1.450) | <0.001 |
| Tx | 1.484(1.442-1.527) | <0.001 | 1.392(1.352-1.433) | <0.001 |
| Surgery of Metastases | <0.001 | <0.001 | ||
| No | 1.000 | 1.000 | ||
| Yes | 0.732(0.703-0.762) | <0.001 | 0.762(0.731-0.794) | <0.001 |
| Unknown | 1.201(0.976-1.477) | 0.083 | 0.828(0.673-1.019) | 0.075 |
| Radiotherapy | <0.001 | <0.001 | ||
| Yes | 1.000 | 1.000 | ||
| No | 1.971(1.828-2.125) | <0.001 | 1.227(1.137-1.325) | <0.001 |
| Unknown | 1.245(1.221-1.270) | <0.001 | 1.140(1.116-1.164) | <0.001 |
| Chemotherapy | <0.001 | <0.001 | ||
| No | 1.000 | 1.000 | ||
| Yes | 0.379(0.371-0.386) | 0.352(0.345-0.360) | ||
| Metastasis site | <0.001 | <0.001 | ||
| Brain | 1.000 | 1.000 | ||
| Bone | 0.915(0.885-0.946) | <0.001 | 0.970(0.937-1.004) | 0.082 |
| Liver | 1.329(1.281-1.378) | <0.001 | 1.157(1.114-1.202) | <0.001 |
| Lung | 0.786(0.761-0.812) | <0.001 | 0.709(0.686-0.733) | <0.001 |
| multisite | 1.245(1.212-1.278) | <0.001 | 1.299(1.265-1.335) | <0.001 |
Univariate and multivariate Cox proportional hazards analysis of CSS for patients with metastatic small cell lung cancer in the SEER database
| Variables | Univariate analysis | P value | Multivariate analysis | P value |
|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | |||
| Gender | <0.001 | <0.001 | ||
| Male | 1.000 | 1.000 | ||
| Female | 0.901 (0.867-0.937) | 0.896 (0.862-0.932) | ||
| Age | <0.001 | <0.001 | ||
| ≤60 | 1.000 | 1.000 | ||
| >60 | 1.296(1.240-1.353) | 1.127(1.078-1.178) | ||
| Race | <0.001 | |||
| Caucasian | 1.000 | 1.000 | ||
| Black | 0.928(0.866-0.994) | 0.033 | 0.930(0.867-0.996) | 0.039 |
| Others | 0.831(0.749-0.922) | <0.001 | 0.812(0.730-0.902) | <0.001 |
| Grade | <0.001 | 0.037 | ||
| I | 1.000 | 1.000 | ||
| II | 2.507(1.025-6.132) | 0.044 | 3.261(1.331-7.988) | 0.010 |
| III | 2.297(1.145-4.609) | 0.019 | 2.549(1.269-5.122) | 0.009 |
| IV | 2.368 (1.182-4.744) | 0.015 | 2.721 (1.357-5.458) | 0.005 |
| Unknown | 2.433 (1.217-4.867) | 0.012 | 2.639 (1.318-5.285) | 0.006 |
| T stage | <0.001 | <0.001 | ||
| T1 | 1.000 | 1.000 | ||
| T2 | 1.127 (1.057-1.202) | <0.001 | 1.109 (1.040-1.182) | 0.002 |
| T3 | 1.095 (1.022-1.172) | 0.010 | 1.135 (1.059-1.216) | <0.001 |
| T4 | 1.127(1.057-1.203) | <0.001 | 1.185(1.110-1.265) | <0.001 |
| Tx | 1.316(1.243-1.394) | <0.001 | 1.233(1.164-1.306) | <0.001 |
| Surgery of Metastases | <0.001 | <0.001 | ||
| No | 1.000 | 1.000 | ||
| Yes | 0.727(0.655-0.907) | <0.001 | 0.772(0.692-0.860) | <0.001 |
| Unknown | 1.271(0.912-1.771) | 0.157 | 0.822(0.588-1.148) | 0.250 |
| Radiotherapy | <0.001 | <0.001 | ||
| Yes | 1.000 | 1.000 | ||
| No | 2.884(2.496-3.333) | <0.001 | 1.576(1.361-1.826) | <0.001 |
| Unknown | 1.672(1.606-1.742) | <0.001 | 1.321(1.264-1.381) | <0.001 |
| Chemotherapy | <0.001 | <0.001 | ||
| No | 1.000 | 1.000 | ||
| Yes | 0.281(0.269-0.293) | 0.295(0.282-0.309) | ||
| Metastasis site | <0.001 | <0.001 | ||
| Brain | 1.000 | 1.000 | ||
| Bone | 0.956(0.886-1.032) | 0.247 | 1.032(0.954-1.117) | 0.432 |
| Liver | 1.417(1.324-1.517) | <0.001 | 1.259(1.175-1.348) | <0.001 |
| Lung | 0.966(0.883-1.057) | 0.450 | 0.880(0.804-0.963) | 0.006 |
| multisite | 1.378(1.293-1.468) | <0.001 | 1.405(1.319-1.498) | <0.001 |
Univariate and multivariate Cox proportional hazards analysis of CSS for patients with metastatic non-small cell lung cancer in the SEER database
| Variables | Univariate analysis | P value | Multivariate analysis | P value |
|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | |||
| Gender | <0.001 | <0.001 | ||
| Male | 1.000 | 1.000 | ||
| Female | 0.802 (0.784-0.820) | 0.826 (0.807-0.845) | ||
| Age | <0.001 | <0.001 | ||
| ≤60 | 1.000 | 1.000 | ||
| >60 | 1.264(1.233-1.296) | 1.144(1.115-1.173) | ||
| Race | <0.001 | <0.001 | ||
| Caucasian | 1.000 | 1.000 | ||
| Black | 0.996(0.963-1.029) | 0.791 | 0.983(0.951-1.017) | 0.319 |
| Others | 0.689(0.661-0.717) | <0.001 | 0.686(0.658-0.715) | <0.001 |
| Grade | <0.001 | <0.001 | ||
| I | 1.000 | 1.000 | ||
| II | 2.507(1.025-6.132) | 0.044 | 1.238(1.140-1.345) | <0.001 |
| III | 2.297(1.145-4.609) | 0.019 | 1.577(1.457-1.707) | <0.001 |
| IV | 2.368 (1.182-4.744) | 0.015 | 1.827 (1.604-2.081) | <0.001 |
| Unknown | 2.433 (1.217-4.867) | 0.012 | 1.549 (1.434-1.674) | <0.001 |
| T stage | <0.001 | <0.001 | ||
| T1 | 1.000 | 1.000 | ||
| T2 | 1.127 (1.057-1.202) | <0.001 | 1.129 (1.092-1.167) | <0.001 |
| T3 | 1.095 (1.022-1.172) | 0.010 | 1.293 (1.246-1.342) | <0.001 |
| T4 | 1.127(1.057-1.203) | <0.001 | 1.485(1.428-1.544) | <0.001 |
| Tx | 1.316(1.243-1.394) | <0.001 | 1.441(1.394-1.490) | <0.001 |
| Surgery of Metastases | <0.001 | <0.001 | ||
| No | 1.000 | 1.000 | ||
| Yes | 0.727(0.655-0.907) | <0.001 | 0.757(0.724-0.792) | <0.001 |
| Unknown | 1.271(0.912-1.771) | 0.157 | 0.811(0.622-1.056) | 0.120 |
| Radiotherapy | <0.001 | <0.001 | ||
| Yes | 1.000 | 1.000 | ||
| No | 2.884(2.496-3.333) | <0.001 | 1.133 (1.036-1.240) | 0.006 |
| Unknown | 1.672(1.606-1.742) | <0.001 | 1.095(1.068-1.122) | <0.001 |
| Chemotherapy | <0.001 | <0.001 | ||
| No | 1.000 | 1.000 | ||
| Yes | 0.382(0.373-0.391) | 0.377(0.368-0.386) | ||
| Metastasis site | <0.001 | <0.001 | ||
| Brain | 1.000 | 1.000 | ||
| Bone | 0.900(0.867-0.934) | <0.001 | 0.959(0.922-0.996) | 0.032 |
| Liver | 1.210(1.151-1.272) | <0.001 | 1.087(1.033-1.143) | 0.001 |
| Lung | 0.771(0.744-0.798) | <0.001 | 0.697 (0.672-0.722) | <0.001 |
| multisite | 1.207(1.172-1.243) | <0.001 | 1.282(1.244-1.321) | <0.001 |
For SCLC, 3-year CSS for patients with bone, brain, liver, lung and multisite metastases were 3.0%, 6.4%, 1.7%, 8.4%, and 1.2%, respectively, the difference was statistical significance (P<0.001) (Fig. 4B). In the multivariate analysis, patient with brain metastases have similar survival outcome with those having bone metastases (HR=1.032, 95% CI 0.954-1.117, P=0.432), but better than those with liver (HR=1.259, 95% CI 1.175-1.348, P<0.001) and multisite metastases (HR=1.405, 95% CI 1.319-1.498, P<0.001). Patients diagnosed with lung metastases have 12% decreased in the risk of died of metastases when compared with those with brain metastases (HR=0.880, 95% CI 0.804-0.963, P<0.001).
Discussion
Distant metastases at the time of presentation of lung cancer are a frequent clinical problem. It is reported that approximately 30-40% of NSCLC patients and 60% SCLC present with metastatic disease at the time of diagnosis [23-25]. Previous population-based study indicates that the prognosis of several metastatic cancers differs according to the site of metastases [15, 16, 26]. However, the prognostic value of metastatic sites in metastatic lung cancer remains controversial. Some studies indicated that the site of involvement did not associate with survival [18-20], while other researchers reported that metastasis to specific organs appear to affect prognosis [21, 27, 28]. Finkelstein et al demonstrated that patients with bone and liver metastases exhibited shorter survival compared to those with other metastatic sites [21]. Sorensen et al reported that patients with brain metastasis were identified as independent prognostic factors in NSCLC patients [27]. Hoang et al uncovered that liver metastases were unfavorable prognostic factors in metastatic NSCLC [28], while Bauml et al reported that those with bone metastasis had a poor prognosis treated with target therapy [29]. Hence, it is important to clarify the prognostic value of different metastatic sites in lung cancer.
In present large population based study, we reported that multiple organ metastases are very common in both NSCLC and SCLC. For patients with isolated metastasis, bone was the most common metastases site, followed by lung, brain, and liver in NSCLC, while for SCLC, the most common site was liver, followed by brain, bone, and lung. In the survival analysis, we found patients with lung metastases had the best survival outcome, followed by bone metastases and brain metastases, patients with liver metastases had the worst survival in those diagnosed with isolated metastases. This trend keeps consist, not matter in NSCLC or in SCLC.
The results of present study have some important implications in clinical practice. First, we systematical described the survival characters of metastatic lung cancer with different metastatic sites, which may help us to make accurate assessment of metastatic lung cancer. Second, brain and bone metastases were not unfavorable prognostic factors in present study. Several previous studies suggested that patients diagnosed with bone and brain metastases have unfavorable survival outcomes [27, 30, 31]. Most of the studies were published in early years. The survival of patients with brain and bone metastasis has improved steadily these years, and may be partly attributed to the early diagnoses of metastatic lesions and new treatment strategies [4, 32, 33], such as increasing use of stereotactic radiosurgery alone for patients with limited brain metastases instead of whole-brain radiotherapy [32]. Third, patients diagnosed with liver metastasis had the worst survival in those with isolated organ metastasis in our study, which was consistent with previously studies [21, 28]. Liver metastatic diseases are always multiple lesions [34], and the majority of NSCLC patients with liver metastasis do not respond well to chemotherapy [35, 36]. Moreover, some patients with liver metastases cannot tolerance to chemotherapy due to liver dysfunction [37]. We should acknowledge that there are some limitations in our study. First, the SEER database only included four specific sites of distant metastases, and we could not compare the survival with other metastatic sites, such as adrenal metastasis. Second, there was a lack of details about chemotherapy, targeted therapy, which may cause bias. Third, the gene expression status, such as EGFR, is not available; we cannot adjust it in survival analysis. Fourth, some other information, such as family history, follow up protocol were also missing in SEER database, which may cause bias in present study. However, this is a large population based study, and the design of the present study is more like real world study, such limitation will not impair the power of our conclusion.
Taken together, our large population-based study suggests that in patients with distant metastases, those with liver metastases have the poorest survival, whereas those with lung metastases have the best survival. Therefore, we should take into consideration of such discrepancy when making treatment strategies.
Abbreviations
NSCLC: Non-small cell lung cancer.
SCLC: small cell lung cancer.
SEER: Surveillance, Epidemiology, and End Results.
CSS: cancer-specific survival.
CIs: confidence intervals.
Acknowledgements
This study used the linked SEER database. The interpretation and reporting of these data are the sole responsibilities of the authors. The authors acknowledge the efforts of the SEER Program tumor registries in the creation of the SEER database.
Funding support
This research was supported by the National Science Foundation of China (No. 81802374). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors' contributions
JL and XW conceived this study. XXL, XW and WQX improved the study design and contributed to the interpretation of results. JL and XW performed the study. JL and XXL performed data processing and statistical analysis. JL wrote the manuscript. XW revised the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Global Burden of Disease Cancer C, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA. et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-48
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30
3. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86
4. Ambroggi M, Biasini C, Toscani I, Orlandi E, Berte R, Mazzari M. et al. Can early palliative care with anticancer treatment improve overall survival and patient-related outcomes in advanced lung cancer patients? A review of the literature. Support Care Cancer. 2018
5. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA. et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733-42
6. Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18:893-903
7. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-57
8. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239-46
9. Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ. et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385-94
10. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T. et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167-77
11. Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R. et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963-71
12. Mazieres J, Zalcman G, Crino L, Biondani P, Barlesi F, Filleron T. et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33:992-9
13. Riihimaki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J. et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78-84
14. Rami-Porta R, Call S, Dooms C, Obiols C, Sanchez M, Travis WD. et al. Lung cancer staging: a concise update. Eur Respir J. 2018:51
15. Wu SG, Zhang WW, He ZY, Sun JY, Chen YX, Guo L. Sites of metastasis and overall survival in esophageal cancer: a population-based study. Cancer Manag Res. 2017;9:781-8
16. Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A. et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A Surveillance Epidemiology and End Results database analysis. World J Gastroenterol. 2017;23:1872-80
17. Tamura T, Kurishima K, Nakazawa K, Kagohashi K, Ishikawa H, Satoh H. et al. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. 2015;3:217-21
18. Sakurai M, Shinkai T, Eguchi K, Sasaki Y, Tamura T, Miura K. et al. Prognostic factors in non-small cell lung cancer: multiregression analysis in the National Cancer Center Hospital (Japan). J Cancer Res Clin Oncol. 1987;113:563-6
19. Shinkai T, Sakurai M, Eguchi K, Sasaki Y, Tamura T, Fujiwara Y. et al. Prognostic factors in small cell lung cancer: multivariate analysis in the National Cancer Center Hospital (Japan). Jpn J Clin Oncol. 1989;19:135-41
20. Paralkar VR, Li T, Langer CJ. Population characteristics and prognostic factors in metastatic non-small-cell lung cancer: a Fox Chase Cancer Center retrospective. Clin Lung Cancer. 2008;9:116-21
21. Finkelstein DM, Ettinger DS, Ruckdeschel JC. Long-term survivors in metastatic non-small-cell lung cancer: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1986;4:702-9
22. Buyyounouski MK, Choyke PL, Kattan M. Prostate Amin MB, editors. AJCC Cancer Staging Manual, 8th ed. Chicago: Springer. 2017 pp. 715-726
23. Matsuda A, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H. et al. Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2014;44:388-96
24. Little AG, Gay EG, Gaspar LE, Stewart AK. National survey of non-small cell lung cancer in the United States: epidemiology, pathology and patterns of care. Lung Cancer. 2007;57:253-60
25. Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H. et al. Specific organ metastases and survival in small cell lung cancer. Oncol Lett. 2012;4:617-20
26. Dong F, Shen Y, Gao F, Xu T, Wang X, Zhang X. et al. Prognostic value of site-specific metastases and therapeutic roles of surgery for patients with metastatic bladder cancer: a population-based study. Cancer Manag Res. 2017;9:611-26
27. Sorensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474-80
28. Hoang T, Xu R, Schiller JH, Bonomi P, Johnson DH. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with third-generation chemotherapy regimens based on eastern cooperative oncology group data. J Clin Oncol. 2005;23:175-83
29. Bauml J, Mick R, Zhang Y, Watt CD, Vachani A, Aggarwal C. et al. Determinants of survival in advanced non-small-cell lung cancer in the era of targeted therapies. Clin Lung Cancer. 2013;14:581-91
30. Sanchez de Cos J, Sojo Gonzalez MA, Montero MV, Perez Calvo MC, Vicente MJ, Valle MH. Non-small cell lung cancer and silent brain metastasis. Survival and prognostic factors. Lung Cancer. 2009;63:140-5
31. Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860-7
32. Churilla TM, Weiss SE. Emerging Trends in the Management of Brain Metastases from Non-small Cell Lung Cancer. Curr Oncol Rep. 2018;20:54
33. Confavreux CB, Pialat JB, Belliere A, Brevet M, Decroisette C, Tescaru A. et al. Bone metastases from lung cancer: a paradigm for multidisciplinary onco-rheumatology management. Joint Bone Spine. 2018
34. Kagohashi K, Satoh H, Ishikawa H, Ohtsuka M, Sekizawa K. Liver metastasis at the time of initial diagnosis of lung cancer. Med Oncol. 2003;20:25-8
35. Gorg C, Schwerk WB, Wolf M, Havemann K. Prognostic value of response to chemotherapy using ultrasound in lung cancer with metastatic liver involvement. Bildgebung. 1990;57:70-3
36. Yamamoto N, Tamura T, Fukuoka M, Saijo N. Survival and prognostic factors in lung cancer patients treated in phase I trials: Japanese experience. Int J Oncol. 1999;15:737-41
37. Nakagawa T, Okumura N, Ohata K, Igai H, Matsuoka T, Kameyama K. Postrecurrence survival in patients with stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;34:499-504
Author contact
![]() Corresponding author: Xin Wang, MD. Department of CyberKnife Center, Huashan Hospital, Fudan University. No.525, Hongfeng Road, Pudong District, Shanghai 200041, China. Tel: +86-021-38719999. Fax: +86-021-38719999. Email: wangxinckedu.cn.
Corresponding author: Xin Wang, MD. Department of CyberKnife Center, Huashan Hospital, Fudan University. No.525, Hongfeng Road, Pudong District, Shanghai 200041, China. Tel: +86-021-38719999. Fax: +86-021-38719999. Email: wangxinckedu.cn.

 Global reach, higher impact
Global reach, higher impact