3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(13):2991-3005. doi:10.7150/jca.30821 This issue Cite
Research Paper
Liver Metastases in Newly Diagnosed Gastric Cancer: A Population-Based Study from SEER
1. Department of General Surgery, Nanfang Hospital, Southern Medical University, 1838 North Guangzhou Avenue, Guangzhou, China
2. Department of Medical Imaging Center, Nanfang Hospital, Southern Medical University, No. 1838, Guangzhou Avenue North, 510515 Guangzhou, China.
*Zepang Sun, Huan Zhen and Jiang Yu contributed equally to this work.
Received 2018-10-21; Accepted 2019-4-23; Published 2019-6-2
Abstract
Purpose: Population-based data on the proportion and prognosis of liver metastases at diagnosis of gastric cancer are currently lacking. Besides, the treatment of gastric cancer with liver metastases is still controversial now.
Methods: Patients with gastric cancer and liver metastases (GCLM) at the time of diagnosis in advanced gastric cancer were identified using the Surveillance, Epidemiology, and End Result (SEER) database of the National Cancer Institute. Multivariable logistic and Cox regression were performed to identify predictors of the presence of GCLM at diagnosis and factors associated with all-cause mortality.
Results: We identified 3507 patients with gastric cancer and liver metastases at the time of diagnosis, representing 16.89% of the entire cohort and 44.12% of the subset with metastatic disease to any distant site. Among entire cohort, multivariable logistic regression identified thirteen factors (age, race, sex, original, tumor location, pathology grade, Lauren classification, T staging, N staging, tumor size, number of extrahepatic metastatic sites to bone, lung, and brain, insurance situation and smoking) as predictors of the presence of liver metastases at diagnosis. Median survival among the entire cohort with GCLM was 4.0 months (interquartile range: 1.0-10.0 mo). Patients receiving comprehensive therapy had longer median overall survival, of which the median survival was 12.0 months (interquartile range: 6.0-31.0 mo). Multivariable Cox model in SEER cohort confirmed nine factors (age, tumor location, Lauren classification, T staging, number of extrahepatic metastatic sites to bone, lung, and brain, surgery, chemotherapy, RSC and marital status) as independent predictors for overall survival.
Conclusions: The findings of this study provided population-based estimates of the proportion and prognosis for LM at time of GC diagnosis. These findings provide preventive guidelines for screening and treatment of LM in GC patients.
Keywords: gastric cancer, liver metastases, proportion, prognosis, treatment
Introduction
Gastric Cancer (GC) was the fourth malignant tumor in the world, and the second common cause of cancer-related death. It was estimated that a total of 989600 new stomach cancer cases and 738000 deaths had occurred in 2008.[1] Besides, 35% of patients presented with evidence of distant metastases at the time of diagnosis, and 4% to 14% had metastatic disease to the liver, the most common metastatic organ. [2-6] For the complexity of gastric cancer with liver metastases (GCLM), it did not have effective treatment and its treatment strategy was still controversial. [6, 7] The median survival time of GCLM was 2-3 months in unselected patients and the 5-year survival rate was 0 to 10%. [3-6] Liver metastases (LM) were associated with poor survival in patients with advanced gastric cancer because of the impairment of vital organ function and increasing tumor burden to lethal levels. [8-10]
A population-based estimate relating to the proportion and prognosis of liver metastases at diagnosis of gastric cancer was lacking. Previous studies [2-6, 11-15] that described the frequency and clinicopathological features of liver metastases from gastric cancer had yielded varying results, from which the risk factors and prognostic factors about GCLM were not enough clear. Although most research [16-24] showed that patients with GCLM had a survival benefit from chemotherapy, surgery and radiotherapy, there were a few researches [3, 25] which had different sounds. Thus, the treatment strategy of GCLM was yet controversial. Besides, data at present about GCLM were almost from single institution experiences with small sample. [2, 3, 5, 8, 12-14, 18-26] Therefore, a study based on population level with more detail information about GCLM to describe epidemiologic characteristics and prognosis was urgently needed.
It was reported that for the liver metastases, MRI was the optimal diagnostic modality for evaluation of suspected hepatic metastases [27], which was more superior than CT and PET-CT that often could not identify some occupying [27-29], particularly when it was smaller than 1.0cm. However, MRI was not recommended routine assessment in current gastric cancer screening guidelines. We chose it only when patients were allergic to iodine or there was a suspicion of liver occupying on CT. Thus, some GCLM were detected during surgery or were not found at first visit, which might increase unnecessary treatment. Furthermore, MR imaging provided a precise non-radiation based imaging test for detection of liver metastases which could alter patient management and result in significant cost savings by reducing unnecessary laparotomy. [27-29]
In this study, we used data from the Surveillance, Epidemiology, and End Results (SEER) database from 2010-2014 to characterize the incidence proportion of liver metastases at the time of cancer diagnosis among patients with gastric cancer on a population-based level. We also wanted to characterize prognostic factors on the survival of patients with liver metastases at the time of cancer diagnosis. Furthermore, we would like to compare the significance of different treatment on GCLM in order to provide guidelines for treatment of LM in GC patients.
Materials and Methods
Database
Data was obtained from SEER database, which was the largest publicly available cancer dataset and collected cancer data from 18 population-based cancer registries covering about 28 percent of the United States population.[30] This database included information about cancer incidence as well as demographic information: age, sex, race, year at diagnosis, tumor staging, tumor size, treatment, marital status, insurance, education, family income and so on. We used the SEERStat published by SEER to identify eligible patients in this study, which we could get from the official network (https://seer.cancer.gov/). The SEER database provided patients information up to 2014 based on the November 2016 submission, and it started to release metastatic information related to liver metastases from 2010. Thus, we can get information about GCLM between 1 January 2010 and 31 December 2014.
Study population
Within the SEER database, we identified 151909 patients with gastric cancer. Among these patients, we focused on 28559 patients for whom with clear information about liver metastases. Patients with other cancer, less than 18 years old, or with other pathological type were excluded from the analysis, leaving 20761 patients in the final cohort for proportion analysis. Of these, 7948 patients were diagnosed with metastases to any distant site and 3507 patients were diagnosed as GCLM. The percentage of distant metastases was 38.28% and liver metastasis was 16.89%. Data extraction flowchart was showed in Figure S1. The inclusion criteria were as follows: age more than 18 years old at time of diagnosis; gastric cancer as the only one malignant tumor; with identified information about liver metastases; with clear survival time; confirmation of diagnosis based on pathology of a specimen, rather than based on death certificate or autopsy; with active follow-up. And we excluded those patients conformed to one of the following standards: age less than 18 years old at the time of diagnosis; with other cancer except for gastric cancer; without identified information about liver metastases; pathological type confirmed to be neuroendocrine carcinoma, GIST, sarcoma or lymphoma; without clear survival time; confirmation of diagnosis based on death certificate or autopsy; without active follow-up.
We made the descriptive statistics to examine the baseline characteristics of the patient population that were stratified by year at diagnosis, age, sex, race, original, tumor location, pathology grade, Lauren classification, T staging, N staging, tumor size, sequence of radiotherapy and surgery, treatment and other sociodemographic information, such as: marital status, residence type, insurance situation, bachelor education, median household income and smoking status.
Age was divided into 4 intervals (18-40, 41-65, 66-80, 80+ years old). Race contained white, black and others (including Asian and American Indians). Original was divided into Spanish-Hispanic-Latino and Non-Spanish-Hispanic-Latino. Tumor location included the upper, middle, lower, overlapping lesion of stomach and unknown. Pathology grade of cancer was classified into 5 categories: well differentiated (Grade I), moderately differentiated (Grade II), poor differentiated (Grade III), Undifferentiated (Grade IV) and unknown. Lauren classification [31] was divided into intestinal-type, diffuse-type and others. The TNM staging was classified according to the seventh edition of the AJCC Cancer Staging manual of the American Joint Committee on Cancer (AJCC).[32] T staging included Tis, T1, T2, T3, T4 and unknown. N staging included N0, N1, N2, N3 and unknown. Tumor size was divided into 5 intervals (0-1cm, 1-2cm, 2-5cm, 5+cm, unknown). Surgery was defined as gastrectomy (C10-C50) and radical gastrectomy in continuity with the resection of other organs (RGCWROO) (C60-C63). Sequence of radiotherapy and surgery included 4 types: radiotherapy before surgery, radiotherapy after surgery, radiotherapy before and after surgery and others. Treatment was reclassified into 8 categories: patients receiving all these three treatment -- radiotherapy, surgery and chemotherapy (RSC), or patients receiving radiotherapy and surgery (RS), or patients receiving chemotherapy and surgery (SC), or patients receiving radiotherapy and chemotherapy (RC), or patients only receiving radiotherapy (R), or patients only receiving surgery (S), or patients only receiving chemotherapy(C) and patients had not received any treatment above (Others). Residence type included 3 kinds (rural, urban and Metropolitan). Marital Status was divided into married, single, divorced, widowed and unknown. Educational level was defined as an increase of 10% of the bachelor education in the region. Median household income was defined as an increase of every $20000. Smoking status was defined as an increase of every 10%. Residence type, education level, median household income and smoking status were defined by the county attributes from the US Census 2010-2014 American Community Survey 5-year data files, which we could get from the SEER*Stat software.
Statistical analysis
Descriptive statistics was used to calculate the absolute number and percentage among patients with liver metastases at the time of cancer diagnosis. Incidence proportion was defined as the percentage of gastric cancer patients diagnosed with liver metastases among the entire study cohort and the patients with metastatic disease to any distant site. All data were stratified by age, race, sex and so on. Multivariable logistic regression was used to determine predictors of the presence of liver metastases at diagnosis. Survival estimates were obtained according to the Kaplan-Meier method and compared using the log-rank test. Variables that reached significance with P < 0.05 were entered into the multivariable analyses using the Cox regression model to identify covariates associated with increased all-cause mortality.
In the Cox regression model, we used the model 1 and 2 for analysis, separately. The model 1 contained the following variables: year at diagnosis, age, sex, tumor location, pathology grade, Lauren classification, T staging, N staging, tumor size, number of extrahepatic metastatic sites to bone, lung and brain, surgery, chemotherapy, radiotherapy, sequence of radiotherapy and surgery, marital status, insurance situation, residence type, median household income, bachelor education and smoking status. In the model 2, we used treatment (RSC, RS, SC, RC, R, S, C, Others) to replace three variables (surgery, chemotherapy and radiotherapy), and other variables were same to the model 1.
All statistical analyses were performed using SPSS statistical software (version 18.0). Statistical significance was set at two-sided (P < 0.05).
Results
Patient characteristics
A total of 20761 patients in the U.S. were diagnosed with gastric cancer between 2010 and 2014, including 3507 patients diagnosed with GCLM whose median age was 66 years old, consisted of 2493 men (71.08%) and 1014 women (28.91%). Their demographic and clinical characteristics were shown in Table 1.
Proportion
Among the 20761 patients in the United States diagnosed with gastric cancer between 2010 and 2014, 7948 (38.28%) presented with synchronous metastases, and 3507 (16.89%) presented with synchronous liver metastases identified at the time of diagnosis.
Clinical characteristics of Patients With Gastric Cancer With Identified Liver Metastases at Diagnosis.
| Variable | Patients, No. | Proportion of Liver Metastases, % | Survival Among Patients With Liver Metastases,Median (IQR), mo | |||
|---|---|---|---|---|---|---|
| With Gastric Cancer (n=20761) | With Metastatic Disease (n=7948) | With Liver Metastases (n=3507) | Among Entire Cohort | Among Subset With Metastatic Disease | ||
| Year at diagnosis | ||||||
| 2010 | 4005 | 1570 | 696 | 17.38 | 44.33 | 3.0 (1.0-9.0) |
| 2011 | 3982 | 1475 | 700 | 17.58 | 47.46 | 4.0 (1.0-10.0) |
| 2012 | 4280 | 1591 | 671 | 15.68 | 42.17 | 3.0 (1.0-9.0) |
| 2013 | 4233 | 1628 | 745 | 17.60 | 45.76 | 4.0 (1.0-11.0) |
| 2014 | 4333 | 1684 | 695 | 16.04 | 41.27 | 5.0 (1.0-NA) |
| Age at diagnosis, Y | ||||||
| 18-40 | 929 | 523 | 118 | 12.70 | 22.56 | 5.0 (2.0-12.0) |
| 41-65 | 8818 | 3875 | 1588 | 18.01 | 40.98 | 5.0 (1.0-12.0) |
| 66-80 | 7399 | 2557 | 1296 | 17.52 | 50.68 | 3.0 (1.0-9.0) |
| 80+ | 3615 | 993 | 505 | 13.97 | 50.86 | 2.0 (0.0-4.0) |
| Race | ||||||
| White | 14490 | 5692 | 2521 | 17.40 | 44.29 | 4.0 (1.0-11.0) |
| Black | 2696 | 1062 | 534 | 19.81 | 50.28 | 4.0 (1.0-10.0) |
| Othersa | 3419 | 1153 | 432 | 12.64 | 37.47 | 3.0 (1.0-9.0) |
| Unknown | 156 | 41 | 20 | 12.82 | 48.78 | 5.0 (1.0-NA) |
| Sex | ||||||
| Male | 13093 | 5126 | 2493 | 19.04 | 48.63 | 4.0 (1.0-10.0) |
| Female | 7668 | 2821 | 1014 | 13.22 | 35.94 | 3.0 (1.0-9.0) |
| Original | ||||||
| Hispanic | 4234 | 1790 | 598 | 14.12 | 33.41 | 4.0 (1.0-10.0) |
| Non-Hispanic | 16527 | 6156 | 2909 | 17.60 | 47.25 | 4.0 (1.0-10.0) |
| Tumor location | ||||||
| Upper | 7617 | 2895 | 1628 | 21.37 | 56.23 | 5.0 (1.0-11.0) |
| Middle | 1978 | 752 | 274 | 13.85 | 36.44 | 3.0 (1.0-10.0) |
| Lower | 4397 | 1316 | 534 | 12.14 | 40.58 | 3.0 (1.0-11.0) |
| Overlapping lesion | 1585 | 733 | 234 | 14.76 | 31.92 | 2.0 (1.0-7.0) |
| Unknown | 5184 | 2252 | 837 | 16.15 | 37.17 | 2.0 (1.0-7.0) |
| Pathology grade | ||||||
| I | 949 | 127 | 68 | 7.17 | 53.54 | 4.0 (1.0-14.0) |
| II | 4537 | 1415 | 897 | 19.77 | 63.39 | 5.0 (2.0-13.0) |
| III | 11177 | 4446 | 1705 | 15.25 | 38.35 | 4.0 (1.0-9.0) |
| IV | 317 | 100 | 36 | 11.36 | 36.00 | 2.0 (1.0-11.0) |
| Unknown | 3781 | 1860 | 801 | 21.18 | 43.06 | 2.0 (1.0-9.0) |
| Lauren classification | ||||||
| Intestinal-type | 14264 | 5381 | 2973 | 20.84 | 55.25 | 4.0 (1.0-11.0) |
| Diffuse-type | 5957 | 2383 | 444 | 7.45 | 18.63 | 3.0 (1.0-8.0) |
| Othersb | 540 | 184 | 90 | 16.67 | 48.91 | 1.0 (0.0-3.0) |
| Tumor stagingc | ||||||
| I | 3846 | 0 | 0 | 0.00 | NA | NA |
| II | 2814 | 0 | 0 | 0.00 | NA | NA |
| III | 4243 | 0 | 0 | 0.00 | NA | NA |
| IV | 7948 | 7948 | 3507 | 44.12 | 44.12 | 4.0 (1.0-10.0) |
| Unknown | 1910 | 0 | 0 | 0.00 | NA | NA |
| T stagingc | ||||||
| Tis | 129 | 41 | 16 | 12.40 | 39.02 | 1.0 (0.0-4.0) |
| T1 | 4977 | 1419 | 728 | 14.63 | 51.30 | 3.0 (1.0-10.0) |
| T2 | 1785 | 360 | 97 | 5.43 | 26.94 | 6.0 (2.0-13.0) |
| T3 | 5038 | 1106 | 400 | 7.94 | 36.17 | 6.0 (3.0-16.0) |
| T4 | 3883 | 1750 | 641 | 16.51 | 36.63 | 4.0 (1.0-10.0) |
| Unknown | 4949 | 3272 | 1625 | 32.83 | 49.66 | 3.0 (1.0-9.0) |
| N stagingc | ||||||
| N0 | 9355 | 2813 | 1183 | 12.65 | 42.05 | 3.0 (1.0-9.0) |
| N1 | 5541 | 2749 | 1314 | 23.71 | 47.80 | 5.0 (1.0-11.0) |
| N2 | 1852 | 439 | 172 | 9.29 | 39.18 | 7.0 (2.0-15.0) |
| N3 | 1953 | 501 | 149 | 7.63 | 29.74 | 6.0 (2.0-14.0) |
| Unknown | 2060 | 1446 | 689 | 33.45 | 47.65 | 2.0 (0.0-7.0) |
| M stagingc | ||||||
| M0 | 12813 | 0 | 0 | 0.00 | NA | NA |
| M1 | 7948 | 7948 | 3067 | 38.59 | 38.59 | 4.0 (1.0-10.0) |
| Surgery | ||||||
| Gastrectomy | 7029 | 718 | 223 | 3.17 | 31.06 | 8.0 (2.0-19.0) |
| RGCWROOd | 952 | 169 | 29 | 3.05 | 17.16 | 12.0 (6.0-NA) |
| No | 11273 | 7008 | 3234 | 28.69 | 46.15 | 3.0 (1.0-10.0) |
| Refuse | 555 | 53 | 21 | 3.78 | 39.62 | 1.0 (0.0-5.0) |
| Radiotherapy | ||||||
| Yes | 5484 | 1285 | 542 | 9.88 | 42.18 | 6.0 (2.0-11.0) |
| No | 15277 | 6663 | 2965 | 19.41 | 44.50 | 3.0 (1.0-10.0) |
| Chemotherapy | ||||||
| Yes | 107882 | 4549 | 1869 | 1.73 | 41.09 | 8.0 (4.0-15.0) |
| No | 9979 | 3399 | 1638 | 16.41 | 48.19 | 1.0 (0.0-3.0) |
| Sequence of radiotherapy and surgerye | ||||||
| RBS | 1127 | 56 | 14 | 1.24 | 25.00 | 12.0 (9.0-NA) |
| RAS | 1952 | 183 | 45 | 2.31 | 24.59 | 9.0 (2.0-16.0) |
| RBAS | 45 | 2 | 1 | 2.22 | 50.00 | NA |
| Others | 17637 | 7707 | 3447 | 19.54 | 44.73 | 4.0 (1.0-10.0) |
| Treatmentf | ||||||
| RSC | 2781 | 130 | 30 | 1.08 | 23.08 | 12.0 (6.0-17.0) |
| RS | 130 | 15 | 5 | 3.85 | 33.33 | 1.0 (1.0-4.0) |
| SC | 1969 | 412 | 96 | 4.88 | 23.30 | 12.0 (6.0-31.0) |
| RC | 1975 | 816 | 359 | 18.18 | 44.00 | 7.0 (4.0-13.0) |
| R | 598 | 324 | 148 | 24.75 | 45.68 | 2.0 (1.0-3.0) |
| S | 4001 | 330 | 121 | 3.02 | 36.67 | 4.0 (1.0-12.0) |
| C | 4065 | 3191 | 1384 | 34.05 | 43.37 | 8.0 (4.0-15.0) |
| Others | 5242 | 2730 | 1364 | 26.02 | 49.96 | 1.0 (0.0-3.0) |
| Tumor size, cm | ||||||
| 0-1 | 955 | 137 | 51 | 5.34 | 37.23 | 2.0 (0.0-7.0) |
| 1-2 | 1502 | 243 | 100 | 6.66 | 41.15 | 5.0 (1.0-13.0) |
| 2-5 | 5167 | 1370 | 666 | 12.89 | 48.61 | 5.0 (1.0-12.0) |
| 5+ | 4041 | 1310 | 617 | 15.27 | 47.10 | 4.0 (1.0-11.0) |
| Unknown | 9096 | 4888 | 2073 | 22.79 | 42.41 | 3.0 (1.0-9.0) |
| Extrahepatic metastatic sites to bone, lung, and brain, No. | ||||||
| 0 | 18406 | 5628 | 2432 | 13.21 | 43.21 | 4.0 (1.0-11.0) |
| 1 | 1657 | 1657 | 669 | 40.37 | 40.37 | 3.0 (1.0-7.0) |
| 2 | 265 | 265 | 136 | 51.32 | 51.32 | 2.0 (1.0-7.0) |
| 3 | 17 | 17 | 11 | 64.71 | 64.71 | 3.0 (1.0-7.0) |
| Unknown | 416 | 381 | 259 | 62.26 | 67.98 | 2.0 (0.0-7.0) |
| Insurance situation | ||||||
| Yes | 19271 | 7299 | 3229 | 16.76 | 44.24 | 4.0 (1.0-10.0) |
| No | 960 | 502 | 197 | 20.52 | 39.24 | 3.0 (1.0-7.0) |
| Unknown | 530 | 147 | 81 | 15.28 | 55.10 | 3.0 (0.0-13.0) |
| Marital status | ||||||
| Married | 11782 | 4594 | 2032 | 17.25 | 44.23 | 5.0 (1.0-11.0) |
| Single | 3257 | 1405 | 584 | 17.93 | 41.57 | 3.0 (1.0-8.0) |
| Divorced | 1737 | 708 | 308 | 17.73 | 43.50 | 3.0 (1.0-9.0) |
| Widowed | 2888 | 896 | 432 | 14.96 | 48.21 | 2.0 (1.0-6.0) |
| Unknown | 1097 | 345 | 151 | 13.76 | 43.77 | 4.0 (1.0-12.0) |
| Residence type | ||||||
| Rural | 1546 | 610 | 290 | 18.76 | 47.54 | 2.0 (0.0-7.0) |
| Urban | 346 | 90 | 48 | 13.87 | 53.33 | 2.0 (1.0-8.0) |
| Metropolitan | 18869 | 7248 | 3169 | 16.79 | 43.72 | 4.0 (1.0-10.0) |
| Bachelor education (per 10% increase) | ||||||
| 0-10% | 151 | 59 | 31 | 20.53 | 52.54 | 2.0 (1.0-5.0) |
| 10-20% | 3366 | 1332 | 610 | 18.12 | 45.80 | 3.0 (1.0-9.0) |
| 20-30% | 4559 | 1748 | 830 | 18.21 | 47.48 | 3.0 (1.0-10.0) |
| 30-40% | 8800 | 3307 | 1356 | 15.41 | 41.00 | 4.0 (1.0-11.0) |
| 40-50% | 3302 | 1280 | 579 | 17.53 | 45.23 | 5.0 (1.0-11.0) |
| 50-60% | 583 | 222 | 101 | 17.32 | 45.50 | 4.0 (1.0-10.0) |
| Median household income (per $20,000 increase) | ||||||
| 20,000-40,000 | 249 | 88 | 46 | 18.47 | 52.27 | 2.0 (0.0-7.0) |
| 40,000-60,000 | 4521 | 1776 | 823 | 18.20 | 46.34 | 3.0 (1.0-9.0) |
| 60,000-80,000 | 9465 | 3596 | 1546 | 16.33 | 42.99 | 4.0 (1.0-10.0) |
| 80,000-100,000 | 4751 | 1820 | 791 | 16.65 | 43.46 | 4.0 (1.0-11.0) |
| 100,000-120,000 | 1775 | 668 | 301 | 16.96 | 45.06 | 5.0 (1.0-12.0) |
| Current smoking status (per 10% increase) | ||||||
| 0-10% | 894 | 320 | 130 | 14.54 | 40.63 | 5.0 (2.0-13.0) |
| 10-20% | 13846 | 5296 | 2255 | 16.29 | 42.58 | 4.0 (1.0-10.0) |
| 20-30% | 5585 | 2152 | 1036 | 18.55 | 48.14 | 3.0 (1.0-9.0) |
| 40-50% | 436 | 180 | 86 | 19.72 | 47.78 | 3.0 (1.0-7.0) |
| Ever smoking status (per 10% increase) | ||||||
| 20-30% | 1514 | 603 | 255 | 16.84 | 42.29 | 4.0 (1.0-9.0) |
| 30-40% | 9971 | 3821 | 1583 | 15.88 | 41.43 | 4.0 (1.0-11.0) |
| 40-50% | 8008 | 3019 | 1430 | 17.86 | 47.37 | 4.0 (1.0-10.0) |
| 50-60% | 1268 | 505 | 239 | 18.85 | 47.33 | 3.0 (1.0-9.0) |
Abbreviations:
IQR: interquartilerange, CI: confidence interval, GCLM: gastric cancer with liver metastases;
aincluding Asian and American Indians;
b including linitis plastica, hepatoid adenocarcinoma, adenosquamous carcinoma;
c according to the seventh edition of the AJCC Cancer Staging manual;
d RGCWROO: radical gastrectomy in continuity with the resection of other organs;
e including RBS: radiotherapy before surgery, RAS: radiotherapy after surgery, RBAS: radiotherapy before and after surgery, others: without radiotherapy or surgery or unknown sequence;
f including RSC: radiotherapy, surgeryandchemotherapy, RS: radiotherapy and surgery, SC: chemotherapy and surgery, RC: radiotherapy and chemotherapy, R: only radiotherapy, S: only surgery, C: only chemotherapy, others: other treatmentexcept for radiotherapy, surgery and chemotherapy.
On univariable logistic regression (Table S1) among the entire cohort, there were sixteen factors that showed significance (P value <0.05). They were age, race, sex, original, tumor location, pathology grade, Lauren classification, T staging, N staging, tumor size, number of extrahepatic metastatic sites to bone, lung, and brain, marital status, insurance situation, bachelor education, current smoking status and ever smoking status. We put them on multivariable logistic regression which showed that age, race, sex, original, tumor location, pathology grade, Lauren classification, T staging, N staging, tumor size, number of extrahepatic metastatic sites to bone, lung, and brain, insurance situation and current smoking status had significance among the entire cohort and age, race, sex, original, tumor location, pathology grade, Lauren classification, T staging, N staging, number of extrahepatic metastatic sites to bone, lung, and brain had significance among the subset with metastatic disease to any distant site.
On the multivariable logistic regression (Table 2) among the entire cohort, male (vs female, OR, 1.301; 95%CI, 1.186-1.428; P<0.001), age 41-65 years (vs age 18-40 years; OR, 1.364; 95%CI, 1.088-1.710; P=0.007), age 66-80 years (vs age 18-40 years; OR, 1.418; 95%CI, 1.125-1.787; P=0.003) and age 80+ years (vs age 18-40 years; OR, 1.015; 95%CI, 0.791-1.304; P>0.05), black (vs others; OR, 1.562; 95%CI, 1.335-1.828; P<0.001) and white (vs others; OR, 1.261; 95%CI, 1.107-1.436; P<0.001), Hispanic (vs Non-Hispanic; OR, 1.200; 95% CI, 1.067-1.350; P= 0.002), grade II (vs grade I; OR, 3.058; 95%CI, 2.328-4.018; P<0.001), grade III (vs grade I; OR, 2.544; 95%CI, 1.946-3.327; P<0.001) and grade IV (vs grade I; OR, 2.160; 95%CI, 1.364-3.420; P=0.001), intestinal-type (vs diffuse-type; OR, 3.234; 95%CI, 2.876-3.637; P<0.001) and others (vs diffuse-type; OR, 2.172; 95%CI, 1.644-2.870; P<0.001), N1 (vs N0; OR, 1.977; 95%CI, 1.786-2.187; P<0.001), N2 (vs N0; OR, 1.015; 95%CI, 0.839-1.226; P>0.05), tumor size 1-2cm (vs tumor size 0-1cm; OR, 1.332; 95%CI, 0.915-1.938; P >0.05), tumor size 2-5cm (vs tumor size 0-1cm; OR, 2.628; 95%CI, 1.900-3.633; P<0.001) and tumor size 5+cm (vs tumor size 0-1cm; OR, 3.419; 95%CI, 2.462-4.748; P<0.001), 1 extrahepatic metastatic site (vs 0 extrahepatic metastatic site; OR, 2.842; 95%CI, 2.522-3.202; P<0.001), 2 extrahepatic metastatic sites (vs 0 extrahepatic metastatic site; OR, 4.416; 95%CI, 3.371-5.785; P<0.001), 3 extrahepatic metastatic sites (vs 0 extrahepatic metastatic site; OR, 5.323; 95%CI, 1.773-15.980; P=0.003), without insurance (vs with insurance; OR, 1.680; 95%CI, 1.215-2.325; P=0.002), current smoking per 10% increased (OR, 1.161;95%CI, 1.046-1.288; P=0.005) were associated with significantly greater odds of having liver metastases at diagnosis. While, marital status, bachelor education and ever smoking status was not associated with a risk of liver metastasis at diagnosis in the multivariable model. And middle of stomach (vs upper of stomach; OR, 0.761;95%CI, 0.650-0.890; P= 0.001), lower of stomach (vs upper of stomach; OR, 0.703; 95%CI, 0.621-0.795; P<0.001) and overlapping lesion (vs upper of stomach; OR, 0.769; 95%CI, 0.649-0.912; P=0.003), T2 (vs T1; OR, 0.311; 95%CI, 0.247-0.391; P<0.001), T3 (vs T1; OR, 0.388; 95%CI, 0.335-0.448; P<0.001) were associated with marginally lower odds of liver metastasis at diagnosis. The multivariable logistic regression of subset with metastatic disease was also showed in Table 2.
From the finding above, it seemed that GC patients with factors like higher age, male, the black and white race, Hispanic, intestinal-type, later N staging, poor tumor grade, upper of stomach, presence of more extrahepatic metastatic sites, larger tumor, absence of insurance and heavy smoking had higher risk to develop liver metastases.
Survival
On univariate analysis for all-cause mortality among the subset with liver metastases, there were eighteen factors that were significantly associated with overall survival (P value <0.05). Table S2 showed univariate analysis for all-cause mortality among GCLM. They were year at diagnosis, age, tumor location, Lauren classification, T staging, N staging, tumor size, number of extrahepatic metastatic sites to bone, lung, and brain, surgery, chemotherapy, radiotherapy, sequence of radiotherapy and surgery, treatment, marital status, residence type, median household income, bachelor education and current smoking status. We put them on Cox regression model which showed that age, tumor location, Lauren classification, T staging, number of extrahepatic metastatic sites to bone, lung, and brain, surgery, chemotherapy and marital status were significantly associated with overall survival in the model 1 (Table 3) and age, tumor location, Lauren classification, T staging, number of extrahepatic metastatic sites to bone, lung, and brain, treatment, marital status and residence type were significantly associated with overall survival in the model 2 (Table 3). We put RSC, C and Others as comparison standard in the model 2 separately in order to explain the significance of different treatments, which was showed in Table S3.
Multivariable Logistic Regression for the Presence of Liver Metastases at Diagnosis of Gastric Cancer.
| Variable | Patients, No. | Among Entire Cohort | Among Subset With Metastatic Disease | |||||
|---|---|---|---|---|---|---|---|---|
| Patients (n=20761) | With Liver Metastases (n =3507) | OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| Age at diagnosis, Y | ||||||||
| 18-40 | 929 | 118 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| 41-65 | 8818 | 1588 | 1.364 (1.088-1.710) | 0.007 | 1.577 (1.243-2.001) | <0.001 | ||
| 66-80 | 7399 | 1296 | 1.418 (1.125-1.787) | 0.003 | 2.155 (1.683-2.760) | <0.001 | ||
| 80+ | 3615 | 505 | 1.015 (0.791-1.304) | 0.905 | 2.005 (1.520-2.645) | <0.001 | ||
| Race | ||||||||
| Othersa | 3419 | 432 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| White | 14490 | 2521 | 1.261 (1.107-1.436) | <0.001 | 1.197 (1.024-1.398) | 0.024 | ||
| Black | 2696 | 534 | 1.562 (1.335-1.828) | <0.001 | 1.630 (1.346-1.974) | <0.001 | ||
| Unknown | 156 | 20 | NA | NA | 2.143 (1.058-4.343) | 0.034 | ||
| Sex | ||||||||
| Female | 7668 | 1014 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| Male | 13093 | 2493 | 1.301 (1.186-1.428) | <0.001 | 1.347 (1.205-1.505) | <0.001 | ||
| Original | ||||||||
| Hispanic | 4234 | 598 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| Non-Hispanic | 16527 | 2909 | 1.200 (1.067-1.350) | 0.002 | 1.302 (1.132-1.498) | <0.001 | ||
| Tumor location | ||||||||
| Upper | 7617 | 1628 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| Middle | 1978 | 274 | 0.761 (0.650-0.890) | 0.001 | 0.655 (0.542-0.791) | <0.001 | ||
| Lower | 4397 | 534 | 0.703 (0.621-0.795) | <0.001 | 0.675 (0.578-0.787) | <0.001 | ||
| Overlapping lesion | 1585 | 234 | 0.769 (0.649-0.912) | 0.003 | 0.534 (0.439-0.650) | <0.001 | ||
| Unknown | 5184 | 837 | 0.700 (0.627-0.782) | <0.001 | 0.578 (0.506-0.660) | <0.001 | ||
| Pathology grade | ||||||||
| I | 949 | 68 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| II | 4537 | 897 | 3.058 (2.328-4.018) | <0.001 | 1.522 (1.035-2.237) | 0.033 | ||
| III | 11177 | 1705 | 2.544 (1.946-3.327) | <0.001 | 0.901 (0.619-1.311) | 0.586 | ||
| IV | 317 | 36 | 2.160 (1.364-3.420) | 0.001 | 0.817 (0.458-1.455) | 0.492 | ||
| Unknown | 3781 | 801 | 2.624 (1.992-3.456) | <0.001 | 0.988 (0.673-1.451) | 0.951 | ||
| Lauren classification | ||||||||
| Diffuse-type | 5957 | 444 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| Intestinal-type | 14264 | 2973 | 3.234 (2.876-3.637) | <0.001 | 3.847 (3.395-4.358) | <0.001 | ||
| Othersb | 540 | 90 | 2.172 (1.644-2.870) | <0.001 | 3.442 (2.458-4.819) | <0.001 | ||
| T stagingc | ||||||||
| T1 | 4977 | 728 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| Tis | 129 | 16 | 1.357 (0.764-2.410) | 0.297 | 0.686 (0.300-1.570) | 0.373 | ||
| T2 | 1785 | 97 | 0.311 (0.247-0.391) | <0.001 | 0.448 (0.338-0.592) | <0.001 | ||
| T3 | 5038 | 400 | 0.388 (0.335-0.448) | <0.001 | 0.555 (0.463-0.666) | <0.001 | ||
| T4 | 3883 | 641 | 1.121 (0.977-1.286) | 0.103 | 0.742 (0.629-0.874) | <0.001 | ||
| Unknown | 4949 | 1625 | 1.884 (1.678-2.115) | <0.001 | NA | NA | ||
| N stagingc | ||||||||
| N0 | 9355 | 1183 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| N1 | 5541 | 1314 | 1.977 (1.786-2.187) | <0.001 | 1.133 (1.005-1.278) | 0.042 | ||
| N2 | 1852 | 172 | 1.015 (0.839-1.226) | 0.881 | 0.866 (0.685-1.095) | 0.229 | ||
| N3 | 1953 | 149 | 0.886 (0.723-1.085) | 0.241 | 0.762 (0.599-0.970) | 0.027 | ||
| Unknown | 2060 | 689 | 1.769 (1.555-2.014) | <0.001 | 1.126 (0.972-1.305) | 0.114 | ||
| Tumor size, cm | ||||||||
| 0-1 | 955 | 51 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| 1-2 | 1502 | 100 | 1.332 (0.915-1.938) | 0.135 | NA | NA | ||
| 2-5 | 5167 | 666 | 2.628 (1.900-3.633) | <0.001 | NA | NA | ||
| 5+ | 4041 | 617 | 3.419 (2.462-4.748) | <0.001 | NA | NA | ||
| Unknown | 9096 | 2073 | 3.351 (2.442-4.598) | <0.001 | NA | NA | ||
| Extrahepatic metastatic sites to bone, lung, and brain, No. | ||||||||
| 0 | 18406 | 2432 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| 1 | 1657 | 669 | 2.842 (2.522-3.202) | <0.001 | 0.728 (0.644-0.824) | <0.001 | ||
| 2 | 265 | 136 | 4.416 (3.371-5.785) | <0.001 | 1.205 (0.918-1.581) | 0.179 | ||
| 3 | 17 | 11 | 5.323 (1.773-15.980) | 0.003 | 1.495 (0.502-4.452) | 0.470 | ||
| Unknown | 416 | 259 | 6.889 (5.497-8.634) | <0.001 | 2.654 (2.075-3.394) | <0.001 | ||
| Marital status | ||||||||
| Married | 11782 | 2032 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| Single | 3257 | 584 | NA | NA | NA | NA | ||
| Divorced | 1737 | 308 | NA | NA | NA | NA | ||
| Widowed | 2888 | 432 | NA | NA | NA | NA | ||
| Unknown | 1097 | 151 | NA | NA | NA | NA | ||
| Residence type | ||||||||
| Rural | 1546 | 290 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| Urban | 346 | 48 | NA | NA | NA | NA | ||
| Metropolitan | 18869 | 3169 | NA | NA | NA | NA | ||
| Insurance situation | ||||||||
| Yes | 19271 | 3229 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| No | 960 | 197 | 1.680 (1.215-2.325) | 0.002 | NA | NA | ||
| Unknown | 530 | 81 | 1.438 (1.094-1.891) | 0.009 | NA | NA | ||
| Bachelor education (per 10% increase) | 20761 | 3057 | 1 (Reference) | NA | 1 (Reference) | NA | ||
| Current smoking status (per 10% increase) | 20761 | 3057 | 1.161 (1.046-1.288) | 0.005 | 1 (Reference) | NA | ||
| Ever smoking status (per 10% increase) | 20761 | 3057 | 1 (Reference) | NA | 1 (Reference) | NA | ||
Abbreviations:
CI: confidence interval, OR: odds ratio, GCLM: gastric cancer with liver metastases;
a including Asian and American Indians;
b including linitis plastica, hepatoid adenocarcinoma, adenosquamous carcinoma;
c according to the seventh edition of the AJCC Cancer Staging manual.
Multivariable Cox Regression for All-Cause Mortality Among Patients With Liver Metastases.
| Variable | Patients, No. | All-Cause Mortality (Model 1) | All-Cause Mortality (Model 2) | |||
|---|---|---|---|---|---|---|
| All Patients (n = 20761) | With Liver Metastases (n = 3507) | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Year at diagnosis | ||||||
| 2010 | 4005 | 696 | 1 (Reference) | NA | 1 (Reference) | NA |
| 2011 | 3982 | 700 | NA | NA | NA | NA |
| 2012 | 4280 | 671 | NA | NA | NA | NA |
| 2013 | 4233 | 745 | NA | NA | NA | NA |
| 2014 | 4333 | 695 | NA | NA | NA | NA |
| Age at diagnosis, Y | ||||||
| 80+ | 3615 | 505 | 1 (Reference) | NA | 1 (Reference) | NA |
| 18-40 | 929 | 118 | 0.798 (0.630-0.997) | 0.047 | 0.808 (0.638-1.022) | 0.076 |
| 41-65 | 8818 | 1588 | 0.746 (0.660-0.843) | <0.001 | 0.748 (0.662-0.845) | <0.001 |
| 66-80 | 7399 | 1296 | 0.834 (0.742-0.937) | 0.002 | 0.838 (0.746-0.941) | 0.003 |
| Tumor location | ||||||
| Upper | 7617 | 1628 | 1 (Reference) | NA | 1 (Reference) | NA |
| Middle | 1978 | 274 | 1.011 (0.876-1.167) | 0.881 | 1.027 (0.890-1.186) | 0.714 |
| Lower | 4397 | 534 | 0.948 (0.845-1.064) | 0.364 | 0.963 (0.859-1.080) | 0.520 |
| Overlapping lesion | 1585 | 234 | 1.292 (1.111-1.503) | 0.001 | 1.289 (1.108-1.499) | 0.001 |
| Unknown | 5184 | 837 | 0.999 (0.908-1.099) | 0.989 | 1.009 (0.917-1.110) | 0.862 |
| Lauren classification | ||||||
| Intestinal-type | 14264 | 2973 | 1 (Reference) | NA | 1 (Reference) | NA |
| Diffuse-type | 5957 | 444 | 1.204 (1.076-1.348) | 0.001 | 1.200 (1.072-1.343) | 0.001 |
| Othersa | 540 | 90 | 1.476 (1.178-1.850) | 0.001 | 1.440 (1.149-1.805) | 0.002 |
| T stagingb | ||||||
| T1 | 4977 | 728 | 1 (Reference) | NA | 1 (Reference) | NA |
| Tis | 129 | 16 | 1.213 (0.613-2.397) | 0.579 | 1.234 (0.624-2.439) | 0.546 |
| T2 | 1785 | 97 | 0.712 (0.559-0.906) | 0.006 | 0.708 (0.556-0.902) | 0.005 |
| T3 | 5038 | 400 | 0.906 (0.781-1.051) | 0.194 | 0.902 (0.777-1.047) | 0.174 |
| T4 | 3883 | 641 | 1.075 (0.952-1.214) | 0.244 | 1.073 (0.950-1.211) | 0.257 |
| Unknown | 4949 | 1625 | 0.999 (0.904-1.104) | 0.989 | 1.001 (0.906-1.107) | 0.979 |
| N stagingb | ||||||
| N0 | 9355 | 1183 | 1 (Reference) | NA | 1 (Reference) | NA |
| N1 | 5541 | 1314 | NA | NA | NA | NA |
| N2 | 1852 | 172 | NA | NA | NA | NA |
| N3 | 1953 | 149 | NA | NA | NA | NA |
| Unknown | 2060 | 689 | NA | NA | NA | NA |
| Surgery | ||||||
| Gastrectomy | 7981 | 223 | 1 (Reference) | NA | NA | NA |
| RGCWROOc | 952 | 29 | 0.654 (0.384-1.114) | 0.118 | NA | NA |
| No | 11273 | 3234 | 1.899 (1.574-2.293) | <0.001 | NA | NA |
| Refuse | 555 | 21 | 1.813 (1.109-2.965) | 0.018 | NA | NA |
| Radiotherapy | ||||||
| Yes | 5484 | 542 | 1 (Reference) | NA | NA | NA |
| No | 15277 | 2965 | 1.037 (0.930-1.156) | 0.512 | NA | NA |
| Chemotherapy | ||||||
| Yes | 10782 | 1869 | 1 (Reference) | NA | NA | NA |
| No | 9979 | 1638 | 3.064 (2.818-3.332) | <0.001 | NA | NA |
| Sequence of radiotherapy and surgeryd | ||||||
| RBS | 1127 | 14 | 1 (Reference) | NA | 1 (Reference) | NA |
| RAS | 1952 | 45 | NA | NA | NA | NA |
| RBAS | 45 | 1 | NA | NA | NA | NA |
| Others | 17637 | 3447 | NA | NA | NA | NA |
| Treatmente | ||||||
| Others | 5242 | 1364 | NA | NA | 1 (Reference) | NA |
| RSC | 2781 | 30 | NA | NA | 0.217 (0.138-0.340) | <0.001 |
| RS | 130 | 5 | NA | NA | 0.419 (0.155-1.136) | 0.087 |
| SC | 1969 | 96 | NA | NA | 0.192 (0.147-0.256) | <0.001 |
| RC | 1975 | 359 | NA | NA | 0.339 (0.294-0.390) | <0.001 |
| R | 598 | 148 | NA | NA | 0.753 (0.631-0.899) | 0.002 |
| S | 4001 | 121 | NA | NA | 0.419 (0.332-0.528) | <0.001 |
| C | 4065 | 1384 | NA | NA | 0.299 (0.272-0.327) | <0.001 |
| Tumor size, cm | ||||||
| 0-1 | 955 | 51 | 1 (Reference) | NA | 1 (Reference) | NA |
| 1-2 | 1502 | 100 | NA | NA | NA | NA |
| 2-5 | 5167 | 666 | NA | NA | NA | NA |
| 5+ | 4041 | 617 | NA | NA | NA | NA |
| Unknown | 9096 | 2073 | NA | NA | NA | NA |
| Extrahepatic metastatic sites to bone, lung, and brain, No. | ||||||
| 0 | 18406 | 2432 | 1 (Reference) | NA | 1 (Reference) | NA |
| 1 | 1657 | 669 | 1.362 (1.240-1.497) | <0.001 | 1.370 (1.247-1.505) | <0.001 |
| 2 | 265 | 136 | 1.476 (1.212-1.798) | <0.001 | 1.495 (1.227-1.821) | <0.001 |
| 3 | 17 | 11 | 0.798 (0.423-1.505) | 0.485 | 0.763 (0.405-1.441) | 0.405 |
| Unknown | 416 | 259 | 1.151 (1.001-1.324) | 0.048 | 1.152 (1.002-1.324) | 0.047 |
| Marital status | ||||||
| Married | 11782 | 2032 | 1 (Reference) | NA | 1 (Reference) | NA |
| Single | 3257 | 584 | 1.150 (1.038-1.274) | 0.008 | 1.144 (1.033-1.267) | 0.010 |
| Divorced | 1737 | 308 | 1.153 (1.012-1.314) | 0.033 | 1.152 (1.011-1.312) | 0.034 |
| Widowed | 2888 | 432 | 0.962 (0.851-1.087) | 0.535 | 0.961 (0.850-1.086) | 0.525 |
| Unknown | 1097 | 151 | 0.858 (0.711-1.036) | 0.112 | 0.847 (0.701-1.022) | 0.083 |
| Residence type | ||||||
| Rural | 1546 | 290 | 1 (Reference) | NA | 1 (Reference) | NA |
| Urban | 346 | 48 | NA | NA | NA | NA |
| Metropolitan | 18869 | 3169 | NA | NA | 0.858 (0.743-0.990) | 0.036 |
| Bachelor education (per 10% increase) | 20761 | 3057 | 1 | NA | 1 | NA |
| Median household income (per $ 20,000 increase) | 20761 | 3057 | 1 | NA | 1 | NA |
| Current smoking status (per 10% increase) | 20761 | 3057 | 1 | NA | 1 | NA |
Abbreviations:
CI: confidence interval, GCLM: gastric cancer with liver metastases;
a including linitis plastica, hepatoid adenocarcinoma, adenosquamous carcinoma;
baccording to the seventh edition of the AJCC Cancer Staging manual;
c RGCWROO: radical gastrectomy in continuity with the resection of other organs;
d including RBS: radiotherapy before surgery, RAS: radiotherapy after surgery, RBAS: radiotherapy before and after surgery, others: without radiotherapy or surgery or unknown sequence;
e including RSC: radiotherapy, surgery and chemotherapy, RS: radiotherapy and surgery, SC: chemotherapy and surgery, RC: radiotherapy and chemotherapy, R: only radiotherapy, S: only surgery, C: only chemotherapy, others: other treatment except for radiotherapy, surgery and chemotherapy.
On multivariable Cox regression for all-cause mortality among patients with GCLM at diagnosis, overlapping lesion of stomach (vs upper of stomach; HR, 1.292; 95%CI, 1.111-1.503; P=0.001), diffuse-type (vs intestinal-type; HR,1.204; 95%CI, 1.076-1.348; P=0.001) and others (vs intestinal-type; HR,1.476; 95%CI, 1.178-1.850; P=0.001), 1 extrahepatic metastatic site (vs 0 extrahepatic metastatic site; HR, 1.362; 95%CI, 1.240-1.497; P<0.001), 2 extrahepatic metastatic sites (vs 0 extrahepatic metastatic site; HR, 1.476; 95%CI, 1.212-1.798; P<0.001), single (vs married; HR, 1.150; 95%CI, 1.038-1.274;P=0.008) and divorced (vs married; HR, 1.153; 95%CI, 1.012-1.314;P=0.033), without chemotherapy (vs chemotherapy; HR, 3.064; 95%CI, 2.818-3.332; P<0.001), without surgery (vs with gastrectomy only; HR, 1.899; 95% CI, 1.574-2.293; P<0.001) and refuse surgery (vs with gastrectomy only; HR, 1.813; 95%CI, 1.109-2.965; P=0.018), C (vs RSC; HR, 1.375; 95%CI, 0.879-2.153; P=0.163) S (vs SC; HR, 1.929; 95%CI, 1.184-3.145; P=0.008) and others (vs RSC; HR,4.607; 95%CI, 2.938-7.224; P<0.001) were significantly associated with an increased all-cause mortality. And year at diagnosis, N staging, radiotherapy, sequence of radiotherapy and surgery, tumor size, residence type, bachelor education, median house income and current smoking status were not associated with all-cause mortality. However, age 18-40 years (vs age 80+ years; HR, 0.798; 95%CI, 0.630-0.997; P=0.047), age 41-65 years (vs age 80+ years; HR, 0.746; 95%CI, 0.660-0.843; P<0.001), age 66-80 years (vs age 80+ years; HR, 0.834; 95%CI, 0.742-0.937; P=0.002), T2 (vs T1; HR, 0.712; 95%CI, 0.559-0.906; P=0.006) and SC (vs C; HR, 0.650; 95%CI, 0.492-0.857; P=0.002) were significantly associated with an decreased all-cause mortality. All data except for treatment came from the model 1.
In general, it seemed that higher age, overlapping lesion, diffuse-type, absence of surgery, absence of chemotherapy, and presence of more extrahepatic metastatic sites, unmarried (single and divorced) were associated with poor prognosis in GCLM.
Patient management
Among GCLM, number of patients with and without radiotherapy were 542 (15.45%) and 2965 (84.55%), with and without chemotherapy were 1869 (53.29%) and 1638 (46.71%), with, without and refuse surgery were 252 (7.19%), 3234 (92.22%) and 21 (0.60%), separately. Based on these three, we had reclassified different treatment. The patients with RSC, RS, SC, RC, R, S, C and others were 30 (0.86%), 5 (0.14%), 96 (2.74%), 359 (10.24%), 148 (4.22%), 121 (3.45%), 1384 (39.46%) and 1364 (38.89%). Venn diagram was made to visualize these data (Figure S2). (Venny's on-line reference: http://bioinfogp.cnb.csic.es/tools/venny/index.html)
Besides, among the entire cohort, 2781 (13.40%), 130 (0.63%), 7969 (9.48%), 1975 (9.51%), 598 (2.88%), 4001 (19.27%), 4065 (19.58%) and 5242 (25.25%) of GC had been treated with RSC, RS, SC, RC, R, S, C and others, respectively. Among the cohort with metastatic disease, 130 (1.64%), 15 (0.19%), 412 (5.18%), 816 (10.27%), 324 (4.08%), 330 (4.15%), 3191 (40.15%) and 2730 (34.35%) of GC had been treated with RSC, RS, SC, RC, R, S, C and others, respectively. Among the cohort with metastatic disease except liver, 100 (2.25%), 10 (0.23%), 316 (7.12%), 457 (10.29%), 176 (3.96%), 209 (4.71%), 1807 (40.69%) and 1366 (30.76%) of GC had been treated with RSC, RS, SC, RC, R, S, C and others, respectively. The proportion of patients with GCLM receiving RSC, SC, S was significantly lower than patients of entire cohort. The treatment among patients with distant metastasis, with distant metastatic disease except for the liver or with liver metastasis was similar (Figure S3 and Figure S4).
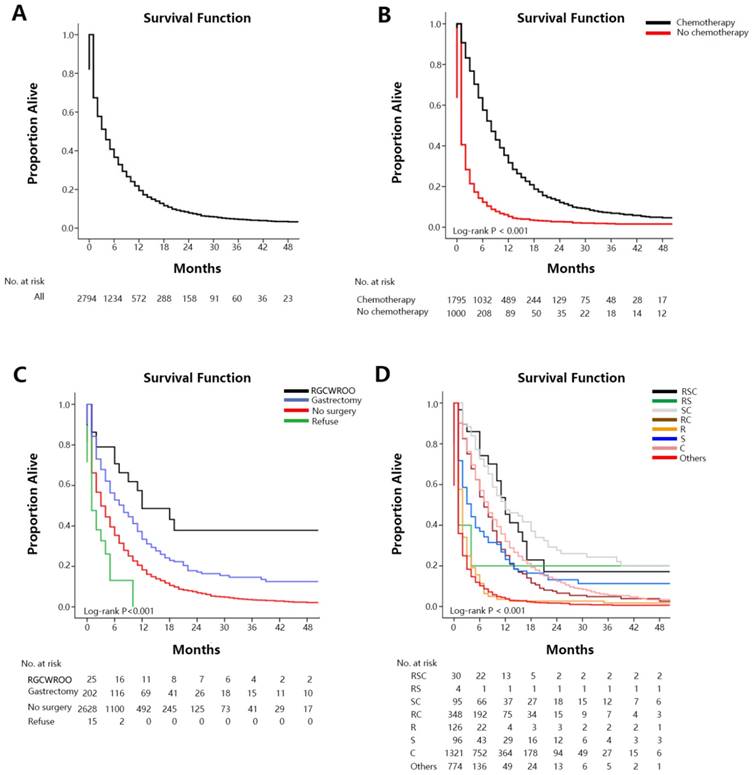
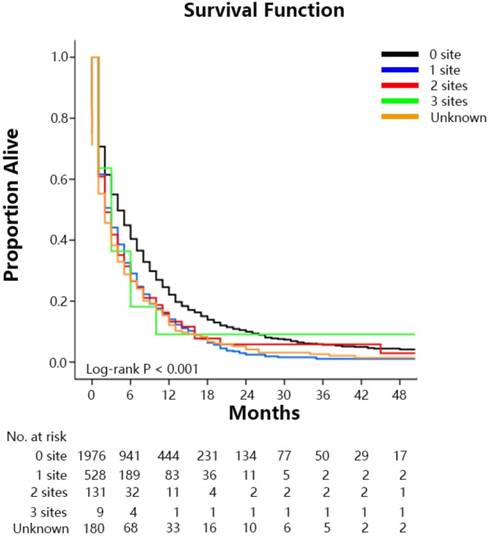
The median survival time of the SEER cohort included in the survival analysis was 4.0 months (IQR: 1.0-10.0 mo) (Figure 1A). Median survival time among GCLM patients treated with chemotherapy was 8.0 months (IQR: 4.0-15.0 mo), among those without chemotherapy was 1.0 month (IQR: 0.0-3.0 mo) (Figure 1B). Median survival time with radical gastrectomy in continuity with the resection of other organs was 12.0 months (6.0-NA mo), with gastrectomy only was 8.0 months (IQR: 2.0-19.0 mo), without surgery was 3.0 months (IQR: 1.0-10.0 mo) and among those who refused surgery although they were recommended was 1.0 month (IQR: 0.0-5.0 mo) (Figure 1C). Median survival time with radiation therapy was 6.0 months (IQR: 2.0-11.0 mo), and without radiation therapy was 3.0 months (IQR: 1.0-10.0 mo). Median survival time among patients treated with RSC was 12.0 months (IQR: 6.0-17.0mo), with RS was 1.0 months (IQR: 1.0-4.0mo), with SC was 12.0 months (IQR:6.0-31.0mo), with RC was 7.0 months (IQR: 4.0-13.0mo), with R was 2.0 months (IQR: 1.0-3.0mo), with S was 4.0 months (IQR: 1.0-12.0mo), with C was 8.0 months (IQR: 4.0-15.0mo), with others was 1.0 month (IQR:0.0-3.0mo) (Figure 1D). Survival estimates stratified by extent of extrahepatic metastases were displayed in the Figure 2 as supplementary.
Our study also found that the 6 month, 1, 2, and 3-year survival rate (Table S4 and Figure S5) of GCLM treated with RSC were 74.2%, 45.3%, 17.2% and 17.2% respectively, with RS were 17.9%, 17.9%, 17.9% and 17.9% respectively, with SC were 72.5%, 47.5%, 27.7% and 22.1% respectively, with RC were 53.9%, 25.1%, 6.6% and 4.9% respectively, with R were 11.3%, 3.5%, 2.7% and 1.8% respectively, with S were 37.0%, 23.1%, 13.2% and 11.3% respectively, with C were 57.1%, 32.0%, 12.4% and 5.6% respectively, with others were 10.2%, 3.8%, 1.7% and 1.0% respectively.
The result showed that patients receiving positive treatment had a significantly benefit on the first 3-year accumulate survival rate. The prognosis of patients treated with RSC or SC was best, while patients who received other treatments had the worst prognosis.
Discussion
In this study, we described the proportion and survival of gastric cancer patients who had liver metastases at their initial diagnosis, based on available data from the SEER database. Because early detection and comprehensive therapy of liver metastases may alter the natural progression of gastric cancer, and improve overall survival, quality of life and cost savings, it was important for us to study patients who presented with de novo GCLM in a large independent cohort.
We found that 16.89% of patients with gastric cancer had liver metastases at diagnosis, and 44.12% of those with any metastases at diagnosis had liver metastases. This result was a little higher than that of previously published study [2-6], and was similar to that of a previous study using SEER database.[11]
We identified predictors of the presence of liver metastases at diagnosis using multivariate logistic regression to distinguish patients at increased risk of liver metastases. This study found that male, the black and white race, intestinal-type, poor tumor grade, upper of stomach, more extrahepatic metastatic sites and absence of insurance increased risk to be GCLM among the entire cohort, which was same to the study published before.[4, 6, 33] Furthermore, we also found that patients with higher age, Hispanic, larger primary tumor and heavy smoking were easier to be GCLM, which had not been reported before as we known. However, our study showed that only N1 had higher risk to be GCLM and T2 or T3 had lower risk to be GCLM, which were different to the research published before.[6, 33] These research [6, 33]showed that the later T staging and later N staging based on pathological staging were the risk factors to be GCLM, but our study had not showed the same phenomenon. We thought that it might be because most T staging and N staging of our study were based on clinical staging, which was not accurate enough.[34]
Overall survival among patients with GCLM at diagnosis (A. overall), stratified by chemotherapy (B), surgery (C), treatment (D).
The percentage of male with GCLM in the entire cohort and subset with metastatic disease were 19.04% and 48.63%, respectively, female were 13.22% and 35.94%, respectively. The proportion of male was 1.44 times to female (P<0.001), which might owe to the bad living habit and alcoholism [35]. The black (19.81% and 50.28%) and white (17.40% and 44.29%) race had a significantly greater likelihood of presenting liver metastases than others (12.64% and 37.47%) (P<0.001). The reason was unknown which need further study. For tumor pathology grade, grade II (19.77% and 63.39%), grade III (15.25% and 38.35%) and grade IV (11.36% and 36.00%) had higher proportion of liver metastasis than grade I (7.17% and 53.54%) tumors (P<0.001). In the Lauren classification, intestinal-type (20.84% and 55.45%) had a significantly greater likelihood to be liver metastasis than diffuse-type (7.45% and 18.63%) (P<0.001). Takahashi et al thought it might due to higher expression of extracellular matrix metalloproteinase inducer in intestinal-type cell, which may stimulate matrix metalloproteinase and vascular endothelial growth factor expression of surrounding stromal cells, then promoted tumor growth and metastasis.[36] Primary tumor located at the upper of stomach (21.37% and 56.23%) had significantly higher percentage of liver metastasis, while middle (13.85% and 36.44%), lower (12.14% and 40.58%) and overlapping lesion (14.76% and 31.92%) had lower percentage of liver metastasis (P<0.001). According to the present results [37-39], cardia cancer was actually more likely to metastasize to the liver compared with non-cardia cancer, which indicated a difference in biology, according to the “seed and soil” hypothesis (“seed-and-soil” hypothesis implies organ specific tropism of circulating tumor cells). Patients with lager tumor (P<0.001) and more extrahepatic metastatic sites (P<0.001) had significantly higher rate of liver metastasis, too. Because gastric cancer spread to the liver primarily through hematogenous dissemination, lymphatic dissemination, and serosal invasion from the tumor tissue, large tumor had more chance to occur vessel, lymphatic system and serosal invasion, then to develop liver metastases.[6] Besides, the liver metastatic rate in uninsured patients (20.52%) was higher than insured patients (16.76%) (P<0.001). Patients with insurance might receive more early intervention and had a lower risk to develop metastatic diseases.[11] And patients with heavy smoking currently was easier to be GCLM (P=0.007), which might owe to these patients lack of screening according to a global research. [40]
Overall survival among patients with GCLM at diagnosis stratified by the extent of extrahepatic metastastic disease.
This study provided a basis for future studies to evaluate the utility of MRI among these high-risk patients. From the finding above, we thought that we should pay more attention to those patients with factors like higher age, male, the black and white race, Hispanic, intestinal-type, later N staging, poor tumor grade, upper of stomach, presence of more extrahepatic metastatic sites and larger tumor, who might have higher risk of liver metastases. These patients need further examination at first diagnosis or during the patients' disease course. And we need to encourage patients without insurance and with heavy smoking to get screening.
Our study found that higher age, overlapping lesion, diffuse-type, absence of surgery, absence of chemotherapy, and presence of more extrahepatic metastatic sites, unmarried (single and divorced) had a significant negative impact on overall survival, however T2 staging showed an opposite result. And N staging, pathology grade, tumor size, radiotherapy, sequence of radiotherapy and surgery, residence type, median household income, bachelor education, insurance status and smoking status were not associated with prognosis (Table 3). The prognosis of single and divorced with similar HR were poorer compared with the married, but widowed showed no significant difference to the married. It was similar to the article [41, 42] published before, which thought that unmarried (single, divorced, widowed) patients may accept less treatment support because of lack of spousal support, leading to the poor survival. The phenomenon of patients with more extrahepatic metastasis sites associating with poor survival also had been reported.[12-14] However, patients with 3 extrahepatic metastatic sites showed not significance with prognosis in our article, which might owe to this subset with 11 patients only. The study showed that the elderly had a poorer prognosis, because the elder might be often treated with more conservative treatment for the poor basic conditions or had short natural life. It was interesting that intestinal-type GC had higher incidence of LM, but showed better survival. And we did not know why T2 showed better prognosis among patients with GCLM. Inaccuracy of clinical staging may explain it.
NCCN clinical practice guidelines in oncology (NCCN Guideline) and Japanese gastric cancer treatment guidelines 2014 (version4) recommended systemic chemotherapy based on fluorouracil or paclitaxel, supplemented by targeted therapy and best supportive therapy as the main treatment methods for advanced gastric cancer.[16, 17] However, there was still great controversial in the treatment of GCLM, because the effect of above treatment was limited. Most studies [16-24] showed that chemotherapy and surgery by selected patients had a positive prognosis to GCLM, which could improve the median survival time from 2-3months to 7-15months, and 40-55% patients selected might benefit from combined liver resection. What's more, GCLM got better benefit from combination of surgery and chemotherapy.[22-24] However, there were yet some research [3, 25] hold the opposite conclusion: no improvement in median survival for patients increased use of chemotherapy or surgery. And a study [18] published before showed that radiotherapy had a survival benefit to metastatic gastric cancer, however, we did not get the same result of our study. Furthermore, our study provided a supplement that the prognosis of GCLM was not influenced by the sequence of radiotherapy and surgery with limited data. We then thought that radiotherapy should be carefully selected if the aim was to improve median survival, although it might provide some help to the treatment of bleeding, obstruction and so on. Further investigation about the function of radiotherapy was necessary.
Among the whole study cohort, we can see that most patients received surgery (54.30%) and chemotherapy (48.07%). However, the rate of surgery was only 11.16% among the subset of metastatic disease to any distant site and 7.18% among the subset of liver metastases. Moreover, number of patients who received radical gastrectomy in continuity with the resection of other organs was only 29 (0.83%), among which 27 patients had liver metastases only and the other two concurrent with pulmonary metastases. None of them had bone or brain metastases. While the chemotherapy rate among the subset of metastatic disease (57.23%) and the subset of liver metastases (53.29%) was a little higher than the whole cohort (48.07%). Among the subset of liver metastases, 0.86%, 0.14%, 2.74%,10.24%, 4.22%, 3.45%, 39.46% and 38.89% had been treated with RSC, RS, SC, RC,R, S, C and others, separately, which was similar to the subset of metastatic disease and subset of metastatic disease to any distant site except of liver. We could see that patients with GCLM receiving RC, C and others were nearly 90%, and the surgery rate of GCLM was lower. Few patients received comprehensive therapy.
In model 1, Median survival time among GCLM increased 7 months from absence of chemotherapy to chemotherapy (P<0.001). And median survival time increased 5 months from absence of surgery to gastrectomy, 7 months from refusing surgery to gastrectomy, 9 months from absence of surgery to RGCWROO and 11 months from refusing surgery to RGCWROO (P<0.001). Median survival time increased 3 months from absence of radiotherapy to radiotherapy (P>0.05). Besides, patients who received radical gastrectomy in continuity with the resection of other organs seemed to had a better median survival (12 mo) compared with gastrectomy only (8 mo), although it showed no significance (P=0.118), which need further investigation including more cases.
In model 2, median survival time among GCLM increased 11 months from others to RSC, 11 months from others to RC, 3 months from others to S, 7 months from others to C, and it had not significantly increased from others to RS or R. Moreover, the median survival time of RSC and SC was same (P=0.675). Although it showed no significant difference (P=0.162), the median survival time increased 4 months from C to RSC. And the median survival time of RSC had significant increased from RS, RC, R, S or others (P<0.05). Besides, the median survival time increased 4 months from C to SC with significance (P=0.002). On the other hand, an aggregate 6-month survival rate estimates and 1, 2, 3-year survival rate estimates showed an absolute increase of 64%, 41.5%, 15.5% and 16.2% from others to RSC, 62.3%, 43.7%, 26% and 21.1% from others to SC, 43.7%, 21.3%, 4.9% and 3.9% from others to RC, 26.8%, 19.3%, 11.5% and 10.3% from others to S, and 46.9%, 28.2%, 10.7% and 4.6% from others to C. Furthermore, an aggregate 6-month survival rate estimates and 1, 2, 3-year survival rate estimates showed an absolute increase of 17.1%, 13.3%, 4.8% and 11.6% from C to RSC, and 15.4%, 15.5%, 15.3% and 16.5% from C to SC. The result showed that patients receiving positive treatment had a significantly benefit on the first 3-year accumulate survival rate. From the result above, it showed that patients with GCLM can get benefit from chemotherapy and surgery, especially a combination of two treatments, but radiotherapy showed no significant effect for overall survival. The median survival of patients with RSC or SC was longest, while patients with other treatments had the worst prognosis.
Although there may have some limitations of our study, we yet could make a conclusion that GCLM might get benefit from the comprehensive therapy. Chemotherapy might make the biggest survival benefit as the prime treatment. And surgery might make some help to those highly selected patients, believed to be the only radical cure. However, the importance of radiotherapy needed to be reconsidered because it showed no significant effect in this study. Aggressive treatment might make significant benefit to GCLM patients, so we need to screen more patients who were available to comprehensive therapy, based on comprehensive therapy seldom receiving by GCLM at present.
In conclusions, the findings of this study provided population-based estimates of the proportion and prognosis for GCLM at time of diagnosis. Chemotherapy and surgery made benefit to GCLM on overall survival, especially a combination of both, but radiotherapy showed not significant benefit to overall survival. And we might need to screen more patients who were available to comprehensive therapy, because comprehensive therapy was seldom received by GCLM at present.
Although our study was based on population-level, containing large of case, we should not ignore its limitations.
Firstly, we could know those patients with metastatic disease of the liver, bone, lung and brain, but the SEER database did not provide information about other metastatic sites, like peritoneal metastases. Moreover, we only had information on synchronous metastasis to liver, lack of a relative minority compared to those patients who may develop metachronous metastasis; Secondly, we could only know the patient undergo radical gastrectomy in continuity with the resection of other organs, but we did not know the clear type of organ; Thirdly, information relating to comorbidities, performance status was not available in the SEER database; Fourth, residence type, education level, and median household income were defined at a county level, not a patient level, possibly affecting the results of the logistic and Cox regressions; Fifth, the morbidity and mortality after treatment were not recorded in the SEER database; Sixth, the SEER did not record the information about the types and grading (H1,H2 and H3) of liver metastases, and the size of tumor metastases.
To the best of our knowledge, this study was the first population-based analysis of patients with liver metastases at initial diagnosis of gastric cancer. It provided important suggestion for clinicians to consider designing studies that evaluate the utility of MRI among patients with higher risk of liver metastases. The prognostic factors on GCLM were analyzed in this study too. Besides, we compared the significance of different treatment on GCLM, which might provide some help to clinical practice.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
We would like to thank the staff members of the National Cancer Institute and their colleagues across the United States and at Information management Services, Inc., who have been involved with the Surveillance, Epidemiology and End Results (SEER) Program.
Ethics approval and consent to participate
The SEER was public-use data: informed consent was waived. And our study was deemed exempt from institutional review board approval by NanFang Hospital, Southern Medical University.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87-108
2. Martella L, Bertozzi S, Londero AP, Steffan A, De Paoli P, Bertola G. Surgery for Liver Metastases From Gastric Cancer: A Meta-Analysis of Observational Studies. Medicine. 2015;94:e1113
3. Bernards N, Creemers GJ, Nieuwenhuijzen GA, Bosscha K, Pruijt JF, Lemmens VE. No improvement in median survival for patients with metastatic gastric cancer despite increased use of chemotherapy. Annals of oncology: official journal of the European Society for Medical Oncology. 2013;24:3056-60
4. Riihimaki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7:52307-16
5. Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW. et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. International journal of cancer. 2014;134:622-8
6. Liu J, Chen L. Current status and progress in gastric cancer with liver metastasis. Chinese medical journal. 2011;124:445-56
7. Kataoka K, Kinoshita T, Moehler M, Mauer M, Shitara K, Wagner AD. et al. Current management of liver metastases from gastric cancer: what is common practice? New challenge of EORTC and JCOG. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017;20:904-12
8. Fidler IJ, Balch CM. The biology of cancer metastasis and implications for therapy. Current problems in surgery. 1987;24:129-209
9. Sporn MB. The war on cancer. Lancet. 1996;347:1377-81
10. Sleeman J, Steeg PS. Cancer metastasis as a therapeutic target. European journal of cancer. 2010;46:1177-80
11. Qiu MZ, Shi SM, Chen ZH, Yu HE, Sheng H, Jin Y. et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Medicine. 2018;7:3662-3672
12. Ueda K, Iwahashi M, Nakamori M, Nakamura M, Naka T, Ishida K. et al. Analysis of the prognostic factors and evaluation of surgical treatment for synchronous liver metastases from gastric cancer. Langenbecks Archives of Surgery. 2009;394:647-53
13. Hwang SE, Yang DH, Kim CY. Prognostic Factors for Survival in Patients with Hepatic Recurrence After Curative Resection of Gastric Cancer. World Journal of Surgery. 2009;33:1468-1472
14. Norio S, Koichi S, Kazuhiro Y, Masafumi I, Seigo K. Multivariate prognostic study on large gastric cancer. Journal of Surgical Oncology. 2010;96:14-8
15. Montagnani F, Crivelli F, Aprile G, Vivaldi C, Pecora I, De Vivo R. et al. Long-term survival after liver metastasectomy in gastric cancer: Systematic review and meta-analysis of prognostic factors. Cancer treatment reviews. 2018;69:11-20
16. Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017;20:1-19
17. Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P. et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network Jnccn. 2016;14:1286-1312
18. Shridhar R, Meredith KL, Weber J, Chuong MD, Almhanna K, Hoffe SE. Increased Survival Associated With Surgery and Radiation Therapy in Metastatic Gastric Cancer: A SEER Database Analysis. Cancer. 2013;119:1636-42
19. Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. British journal of cancer. 1995;71:587-91
20. Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37-41
21. Leong T. Chemotherapy and radiotherapy in the management of gastric cancer. Curr Opin Gastroenterol. 2005;21:673-8
22. Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C. et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. Journal of Clinical Oncology. 2007;25:3205-9
23. Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK. et al. Webb, A. et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin and methotrexate in advanced esophagogastric cancer. J. Clin. Oncol. 15, 261-267. Journal of Clinical Oncology. 1997;15:261-7
24. Rudloff U, Langan RC, Mullinax JE, Beane JD, Steinberg SM, Beresnev T. et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. Journal of Surgical Oncology. 2014;110:275-84
25. Tiberio GA, Roviello F, Donini A, De MG. Hepatic metastases from gastric cancer: A surgical perspective. World Journal of Gastroenterology. 2015;21:11489-92
26. Bernards N, Haj Mohammad N, Creemers GJ, Rozema T, Roukema JA, Nieuwenhuijzen GA. et al. Improvement in survival for patients with synchronous metastatic esophageal cancer in the south of the Netherlands from 1994 to 2013. Acta oncologica. 2016;55:1161-7
27. Hussain SM, Semelka RC. Hepatic imaging: comparison of modalities. Radiologic Clinics of North America. 2005;43:929-47
28. Donati OF, Hany TF, Reiner CS, von Schulthess GK, Marincek B, Seifert B. et al. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. Journal of Nuclear Medicine. 2010;51:692-9
29. Liu PS, Francis IR. Hepatic imaging for metastatic disease. Cancer Journal. 2010;16:93-102
30. Horner M, Ries L, Krapcho M, Neyman N, Aminou R. Surveillance Epidemiology and End Results (SEER) Program. wwwseercancergov. 2011
31. Lauren P. The two histological main type gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histological classification. Acta Pathol Microbiol Scand. 1965:64-67
32. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Annals of Surgical Oncology. 2010;17:1471-4
33. Kerkar SP, Kemp CD, Duffy A, Kammula US, Schrump DS, Kwong KF. et al. The GYMSSA trial: a prospective randomized trial comparing gastrectomy, metastasectomy plus systemic therapy versus systemic therapy alone. Trials. 2009;10:121-128
34. Makino T, Fujiwara Y, Takiguchi S, Tsuboyama T, Kim T, Nushijima Y. et al. Preoperative T staging of gastric cancer by multi-detector row computed tomography. Surgery. 2011;149:672-9
35. Viadana E, Bross IDJ, Pickren JW. The Metastatic Spread of Cancers of the Digestive System in Man. Oncology. 1978;35:114-26
36. Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Miwa S. et al. Pathobiological characteristics of intestinal and diffuse-type gastric carcinoma in Japan: an immunostaining study on the tissue microarray. Journal of Clinical Pathology. 2007;60:273-7
37. Kim MA, Lee HS, Yang HK, Kim WH. Clinicopathologic and protein expression differences between cardia carcinoma and noncardia carcinoma of the stomach. Cancer. 2005;103:1439-46
38. Inoue M. Epidemiology of gastric cancer. International journal of cancer. 2002:38-42
39. Hu B, Hajj NE, Sittler S, Lammert N, Barnes R, Meloniehrig A. Gastric cancer: Classification, histology and application of molecular pathology. Journal of Gastrointestinal Oncology. 2012;3:251-61
40. Peleteiro B, Castro C, Morais S, Ferro A, Lunet N. Worldwide Burden of Gastric Cancer Attributable to Tobacco Smoking in 2012 and Predictions for 2020. Digestive Diseases & Sciences. 2015;60:2470-2476
41. Jin JJ, Wang W, Dai FX, Long ZW, Cai H, Liu XW. et al. Marital status and survival in patients with gastric cancer. Cancer Medicine. 2016;5:1821-9
42. Zhang J, Gan L, Wu Z, Yan S, Liu X, Guo W. The influence of marital status on the stage at diagnosis, treatment, and survival of adult patients with gastric cancer: a population-based study. Oncotarget. 2017;8:22385-405
Author contact
![]() Corresponding authors: Guoxin Li, Department of General Surgery, Nanfang Hospital, Southern Medical University, 1838 North Guangzhou Avenue, Guangzhou, 510515, China; Tel and Fax: +86-20-6278 7170; E-mail: gzliguoxincom;Yuming Jiang, Department of General Surgery, Nanfang Hospital, Southern Medical University, 1838 North Guangzhou Avenue, Guangzhou, 510515, China; Tel and Fax: +86-20-6278 7170; E-mail: jiangymbestcom.
Corresponding authors: Guoxin Li, Department of General Surgery, Nanfang Hospital, Southern Medical University, 1838 North Guangzhou Avenue, Guangzhou, 510515, China; Tel and Fax: +86-20-6278 7170; E-mail: gzliguoxincom;Yuming Jiang, Department of General Surgery, Nanfang Hospital, Southern Medical University, 1838 North Guangzhou Avenue, Guangzhou, 510515, China; Tel and Fax: +86-20-6278 7170; E-mail: jiangymbestcom.

 Global reach, higher impact
Global reach, higher impact