3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(13):2857-2867. doi:10.7150/jca.31246 This issue Cite
Research Paper
Resection vs Ablation for Multifocal Hepatocellular Carcinomas meeting the Barcelona-Clinic Liver Cancer A Classification: A Propensity Score Matching Study
1. State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou 510060, P. R. China
2. Department of Hepatobiliary Oncology, Sun Yat-Sen University Cancer Centre, Guangzhou, China
3. Department of Ultrasound, Sun Yat-Sen University Cancer Centre, Guangzhou, China
4. Department of Medical Imaging, Sun Yat-Sen University Cancer Centre, Guangzhou, China
# These authors contributed equally to this work.
* Senior authors contributed equally to this work.
Received 2018-11-5; Accepted 2019-4-14; Published 2019-6-2
Abstract
With development of surgical technology, we aimed to investigate whether resection could challenge the standard treatment, ablation, in treating multifocal hepatocellular carcinomas meeting the Barcelona-Clinic Liver Cancer A stage. From January 2005 to January 2017, the oncological outcomes of patients undergoing resection (n = 72) or ablation (n = 63) were retrospectively analysed using propensity score matching. At baseline, patients in the ablation group had more tri-focal lesions (30.2% vs. 6.9%, P = 0.001) and smaller tumours (2.00 cm vs. 2.50 cm, P = 0.002) than resection group. After matching, the baseline was well-balanced between treatments (n = 46 pairs); resection provided comparable 5-year overall survival (77.0% vs. 83.6, P = 0.790) and superior 5-year recurrence-free survival (40.4% vs. 16.9%, P = 0.022) to ablation. The multivariate Cox model confirmed that ablation was not associated with worse overall survival (HR = 0.89; 95% CI, 0.33 - 2.42, P = 0.819), but identified ablation as an unfavourable predictor of recurrence-free survival (HR = 2.13; 95% CI, 1.27 - 3.57, P <0.001). For subgroup patients with multifocal tumours located in different segments, both treatments offered similar 5-year overall survival (74.3% vs. 95.5%, P = 0.190) and 5-year recurrence-free survival (42.9 vs. 25.9%, P = 0.170). Additionally, ablation resulted in less major complications than resection (3.2% vs 13.9%, P = 0.035). Compared with ablation, resection achieved comparable overall survival and even superior recurrence-free survival for patients with multifocal hepatocellular carcinomas meeting the BCLC A stage.
Keywords: Hepatectomy, Local ablation, Liver cancer, Outcomes
Introduction
Hepatocellular carcinoma (HCC) is one of the most frequent primary malignancies and one of the leading causes of cancer death worldwide [1]. The Barcelona-Clinic Liver Cancer (BCLC) system has been extensively validated and is the most commonly used staging system for HCC. According to the recent clinical practice guidelines from the European Association for the Study of the Liver (EASL), resection is recommended for patients with solitary HCC in the BCLC early stage, while only ablation is suggested for curative treatment in patients with bi- or trifocal tumours ≤3cm when liver transplantation is infeasible [2]. In contrast, the latest clinical practical guideline for HCC from the National Comprehensive Cancer Network (NCCN) suggests that resection can also be considered for multifocal HCCs [3]. Many studies have compared the treatment efficacy of resection and ablation in various situations, especially for single tumour [4-7], but very few studies focused on multiple tumours inside the BCLC A stage [8, 9]. Indeed, along with the development of surgical technique and optimisation of post-operative management, surgical resection has also been adopted as a curative treatment option for multifocal HCCs in daily practice [10], especially when the tumour location is suboptimal for ablation. To clarify this issue, we compared long-term oncological outcomes and post-procedure complications between resection and ablation in treating multifocal HCCs meeting the BCLC A classification; prognostic predictors affecting the outcome were also determined. Propensity score matching (PSM) was employed to reduce potential confounding bias at baseline.
Materials and Methods
Patients
All patients with multifocal HCCs meeting the BCLC A classification [2] who were initially treated with resection (n = 72) or thermal ablation (n = 63) from January 2002 to January 2017 at a single tertiary cancer centre were retrospectively identified. The diagnosis of HCC and the final therapeutic decision was made by a multidisciplinary team consisting of surgeons, physicians and interventional radiologists who were specialized in the management of hepato-pancreato-biliary diseases. For patients undergoing resection, the diagnosis of HCC was confirmed pathologically. For patients treated with ablation, the diagnosis of HCC was confirmed using biopsy (RFA: one patient; MWA, one patient) during the ablation procedure or according to the HCC management guidelines from the EASL or the American Association for the Study of Liver Disease (AASLD) [2, 11]. Patients with any previous anti-cancer treatment or approached with noncurative treatment were excluded. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and the Ethics Committee of Sun Yat-sen University Cancer Centre approved this study. Written informed consent was obtained from patients before treatment.
Treatments
Because radiofrequency ablation (RFA) and microwave ablation (MWA) offer equivalent outcomes for HCC ≤3cm [2, 12, 13], both thermal ablation modalities (RFA, 27 cases; MWA, 36 cases) were included in this study. Ablations were performed by radiologists who had more than five years of experience in interventional therapy at the start of this study. The specific ablation procedures were detailed in our previous study [13]. Briefly, for RFA, before September 2004, a radiofrequency system (RF 2000; Radio-Therapeutics, Mountain View, California, USA) was used and procedure was initiated with 10W of power and increased at a rate of 10 W/min to 90 W. RFA energy was applied until marked increases in impedance were achieved or 15 minutes had elapsed. Since September 2004, a 375-kHz radiofrequency generator (Elektrotom Hitt 106; Berchtold, Medizinelektronik, Germany) has been used with a power output of 60 W for every single energy application. For microwave ablation, a FORSEA MTC-3C microwave system (Qinghai Microwave Electronic Institute, Nanjing, China) with a frequency of 2450 MHz and a power output of 0 - 150 W was always used. The aforementioned procedures were all performed under real-time ultrasound (MyLab 90; Esaote SpA, Firenze, Italia) guidance. Hepatectomy was performed by surgeons who had at least ten years of experience in liver surgery. The surgery procedure was detailed in our previous studies [14, 15]. In brief, preoperative imaging (computed tomography (CT)/magnetic resonance imaging (MRI)) was used to assess the tumour location and size; liver reserve was evaluated comprehensively with Child-Pugh grade and the retention rate of indocyanine green at 15 minutes after injection. The operative plan was comprehensively made based on tumour size and location, liver function and patient performance status. Typically, an anatomical liver resection was prioritized for multifocal tumours located within a segment/sector; a non-anatomical liver resection was preferred for tumours distributed in the nonadjacent liver segments or situated peripherally During the operations, the Cavitron Ultrasonic Surgical Aspirator (CUSA) device (Valleylab, Boulder, CO, USA) and Harmonic scalpel (Ethicon Endo-Surgery, Cincinnati, OH, USA) were mainly used for resection, and intraoperative ultrasonography was routinely used. Additionally, to decrease blood loss, low central venous pressure control (approximately 2 - 4 mm Hg) was routinely performed and the intermittent Pringle manoeuvre was used when necessary.
Follow-up
The first follow-up visit was performed approximately one month after treatment, and then the patients were followed up every three months during the first two years and every three to six months thereafter until death or dropout. The follow-up protocol consisted of a physical examination, liver function, serum alpha-fetoprotein (AFP) test and at least one imaging examination (abdominal enhanced CT or MRI). Salvage treatment was given to patients with recurrence, whenever possible. Repeated ablation or resection was the first choice for patients with recurrent tumours meeting the BCLC 0/A stage, while transarterial chemoembolization (TACE) and other nonradical treatments were appropriately offered for more advanced HCC.
Endpoints
The primary endpoint of the study was overall survival (OS), and secondary endpoints were recurrence-free survival (RFS) and procedure related complications. Relapse pattern was also recorded and defined as follows: (a) local tumour progression (LTP): any new tumour foci inside or at the margin of the treatment site after eradication of all tumours; (b) intrahepatic distant recurrence (IDR): new tumour foci within the liver separate from the treatment area; or (c) extrahepatic distant recurrence (EDR): any recurrence outside the liver [13, 16]. The complications were classified based on the adverse event classification [17]. Minor complications were adverse events requiring no therapy or nominal therapy, while major complications were defined as those that led to major therapy, unplanned increase in the level of care, prolonged hospitalization >48 hours or permanent adverse sequelae. Complications were observed and classified separately by two authors (WWL and ZWY), with disagreements settled by discussion. Notably, the anatomic distribution of tumours was identified as an independent predictor of RFS in the following multivariate Cox analysis. Hence, we conducted subgroup analysis comparing resection and ablation in treating multiple tumours located in different segments because en bloc resection was prioritized for multifocal disease located in the same segment due to its more radical removal of lesions and moderate damage of liver parenchyma.
Propensity score matching (PSM)
The clinically important factors and variables associated with survival as indicated in univariate Cox models (P <0.10) were used for calculating propensity scores [18]. Notably, most of the patients had liver disease of Child-Pugh A (resection: 97.2%, ablation: 96.8%) in the present study. Since the newly developed Albumin-Bilirubin (ALBI) grade can reveal two classes with clearly different prognoses in patients with Child-Pugh A disease [19], we used the new evidence-based ALBI to evaluate the liver function. Thus, the covariables used to build the propensity score were tumour number, tumour size, age, sex, white blood cell count (WBC), platelet counts (PLT) and ALBI grade, portal hypertension (PHT) and anatomic distribution of multifocal HCCs (within a segment or not). The propensity was then estimated by logistic regression model, with the response variable being ablation (yes/no). A one to one nearest-neighbour matching algorithm with an optimal calliper of 0.2 and without replacement was used [20]. The MatchIt package (version 3.0.2) was used in the PSM analyses [21].
Statistical analysis
Both continuous and ordinal variables were assessed by the Mann-Whitney test because the distribution of continuous variables tested by onesample KolmogorovSmirnov test was usually non-normal; categorical variables were compared using the chi-squared test (Fisher's exact test when appropriate). Treatment procedure, tumour number, tumour size and factors with P value less than 0.10 in univariate Cox analyses were introduced into the multivariate Cox proportional hazards model to infer on the effect of using resection vs. ablation, and the prognostic factors of OS and RFS were identified. The survival curves were depicted using the Kaplan-Meier method and compared by log-rank test before and after PSM. All tests were two-tailed, and P values less than 0.05 were considered statistically significant. All Statistical analyses were performed using the R version 3.5.0 software.
Results
Patients
A total of 135 patients with 294 tumours meeting the BCLC A classification were enrolled in this study. Among them, 63 patients with 145 tumours underwent thermal ablation and 72 patients with 149 tumours received resection as initial anticancer treatment. There were significantly more patients with tri-multifocal lesions (30.2% vs. 6.9%, P = 0.001) and smaller tumours (2.00 cm vs. 2.50 cm, P = 0.002) in the ablation group than those in the resection group. Liver function of ALBI grade 1 (77.8% vs. 61.9%, P = 0.068), incidence of clinical portal hypertension (20.8 vs. 31.7%, P = 0.213) and anatomic distribution of tumours (in a same segment: 31.9% vs. 28.6%, P = 0.812) were not statistically different between both groups (Table 1). In the 46 matched pairs of patients generated by the PSM procedure, all variables were well balanced between groups (Table 1, all P values >0.05). Median follow-up was 26 months in the ablation group (range 1-115 months) and 32 months in the resection group (range 4-113 months) (P = 0.179).
Baseline characteristics by treatment cohort
| Variable | Overall population | Matched cohorts | |||||
|---|---|---|---|---|---|---|---|
| Resection (n = 72) | Ablation (n = 63) | P | Resection (n = 46) | Ablation (n = 46) | P | ||
| Age (years) | 53.5 (17.5) | 55.0 (14.5) | 0.217 | 53.0 (21.0) | 54.0 (12.5) | 0.699 | |
| Male (%) | 64 (88.9) | 60 (95.2) | 0.303 | 42 (91.3) | 43 (93.5) | 1.000 | |
| Tumour number (%) | 0.001 | 1.000 | |||||
| 2 | 67 (93.1) | 44 (69.8) | 41 (89.1) | 41 (89.1) | |||
| 3 | 5 (6.9) | 19 (30.2) | 5 (10.9) | 5 (10.9) | |||
| Tumour size (cm) | 2.5 (0.7) | 2.0 (0.7) | 0.002 | 2.0 (0.5) | 2.2 (0.6) | 0.738 | |
| ALBI (%) | 0.068 | 0.824 | |||||
| Garde 1 | 56 (77.8) | 39 (61.9) | 32 (69.6) | 30 (65.2) | |||
| Grade 2 | 16 (22.2) | 24 (38.1) | 14 (30.4) | 16 (34.8) | |||
| Child-Pugh | 1.000 | 1.000 | |||||
| A | 70 (97.2) | 61 (96.8) | 44 (95.7) | 45 (97.8) | |||
| B | 2 (2.8) | 2 (3.2) | 2 (4.3) | 1 (2.2) | |||
| WBC (×109) | 5.7 (2.4) | 5.2 (2.0) | 0.126 | 5.6 (3.0) | 5.3 (1.7) | 0.139 | |
| RBC (×109) | 4.7 (0.8) | 4.6 (0.8) | 0.522 | 4.7 (0.7) | 4.7 (0.9) | 0.705 | |
| Hb (g/L) | 148.0 (31.3) | 145.0 (23.4) | 0.163 | 148.0 (19.3) | 145.0 (22.9) | 0.226 | |
| PLT (×109) | 137.1 (77.3) | 109.0 (73.1) | 0.016 | 130.3 (76.4) | 122.0 (74.6) | 0.173 | |
| ALT (U/L) | 36.4 (21.2) | 39.90 (25.1) | 0.485 | 38.4 (22.0) | 38.2 (23.1) | 0.894 | |
| AST (U/L) | 32.7 (18.1) | 35.5 (19.4) | 0.262 | 33.6 (18.8) | 33.2 (22.1) | 0.845 | |
| ALB (g/L) | 42.6 (6.0) | 41.6 (5.3) | 0.259 | 42.1 (5.8) | 42.3 (5.9) | 0.585 | |
| TBIL (μmol/L) | 12.9 (8.3) | 14.4 (9.3) | 0.015 | 13.7 (8.6) | 15.7 (12.5) | 0.095 | |
| PT (second) | 11.9 (1.4) | 12.4 [(1.7) | 0.089 | 12.2 (1.3) | 12.5 (1.6) | 0.238 | |
| AFP (ng/ml) | 74.7 (259.9) | 53.5 (228.8) | 0.286 | 70.6 (232.2) | 55.8 (255.6) | 0.637 | |
| Etiology (%) | 0.241 | 0.235 | |||||
| Other | 1 (1.4) | 2 (3.2) | 0 (0.0) | 2 (4.3) | |||
| HBV | 70 (97.2) | 57 (90.5) | 45 (97.8) | 41 (89.1) | |||
| HCV | 1 (1.4) | 4 (6.3) | 1 (2.2) | 3 (6.5) | |||
| Cirrhosis (%) | 63 (87.5) | 51 (81.0) | 0.418 | 41 (89.1) | 36 (78.3) | 0.259 | |
| Esophageal varices (%) | 3 (4.2) | 5 (7.9) | 0.472 | 1 (2.2) | 4 (8.7) | 0.361 | |
| Splenomegaly (%) | 36 (50.0) | 27 (42.9) | 0.49 | 23 (50.0) | 18 (39.1) | 0.402 | |
| Portal Hypertension (%) | 15 (20.8) | 20 (31.7) | 0.213 | 10 (21.7) | 13 (28.3) | 0.630 | |
| Anatomic distribution (%) | 0.812 | 0.639 | |||||
| Same segment | 23 (31.9) | 18 (28.6) | 14 (30.4) | 11 (23.9) | |||
| Different segments | 49 (68.1) | 45 (71.4) | 32 (69.6) | 35 (76.1) | |||
Continuous variable was reported as median (interquartile range) and compared using the Mann-Whitney test. Categorical variables were expressed in percentages and compared using Pearson's Chi-square or Fisher's exact test, as appropriate. Abbreviations: ALBI, Albumin-Bilirubin; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; TBIL, total bilirubin; PT, prothrombin time; AFP, alpha-fetoprotein; HBV, Hepatitis B virus; HCV, Hepatitis C virus.
Overall survival and recurrence-free survival
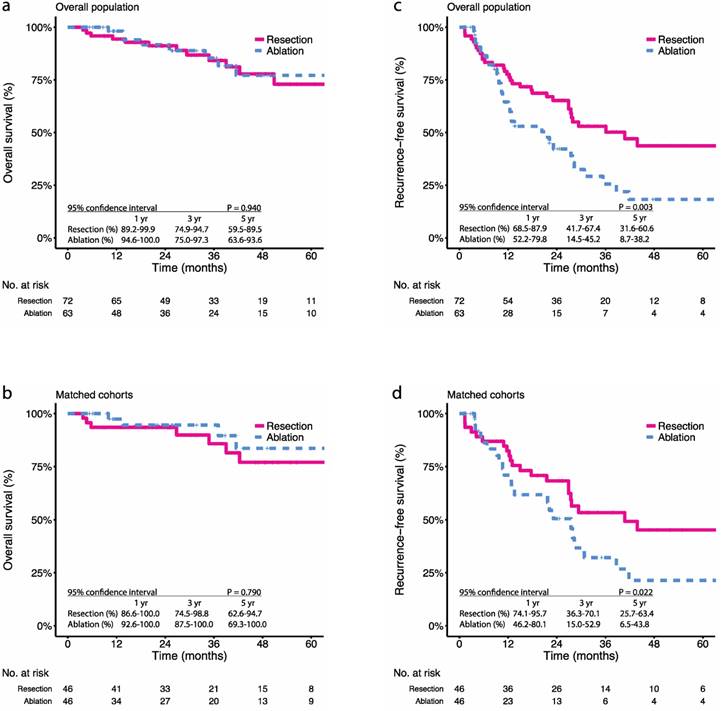
During the study period, there were 16.7% (12 in 72) all-cause deaths in the resection group and 15.9% (10 in 63) all-cause deaths in the ablation group (P = 1.000). Before PSM, the 1-, 3-, and 5-year OS were 94.4%, 84.2%, and 72.9% in the resection group and 98.1%, 85.4%, and 77.2% in the ablation group, respectively (P = 0.940) (Figure 1a). After PSM, the 1-, 3-, and 5-year OS were 93.5%, 85.8%, and 77.0% in the resection group and 97.4%, 94.6%, and 83.6% in the ablation group, respectively (P = 0.790) (Figure 1b). In terms of tumour recurrence, 48.6% (35 in 72) patients in the resection group and 58.7% (37 in 63) patients in the ablation group had been observed with relapse (P = 0.316). The 1-, 3-, and 5-year RFS were 77.6%, 53.0%, and 43.7% in the resection group and 64.5%, 25.6%, and 18.3% in the ablation group, respectively (P = 0.003) (Figure 1c). After PSM, the 1-, 3-, and 5-year RFS were 84.2%, 50.4%, and 40.4% in the resection group and 60.8%, 28.1%, and 16.9% in the ablation group, respectively (P = 0.022) (Figure 1d).
Local tumour progression and intrahepatic distant recurrence
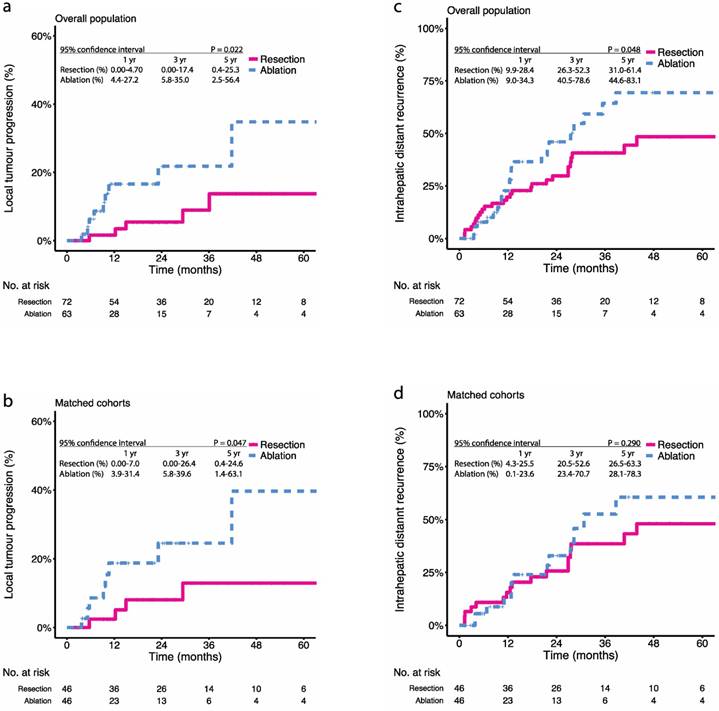
We also explored the relapse patterns after treatment with resection or ablation. During the study period, 6.9% (5 in 72) patients in the resection group and 14.3% (9 in 63) patients in the ablation group had been observed with LTP (P = 0.266) (Table 2). Before PSM, the 1-, 3-, and 5-year LTP rates were 1.6%, 8.9%, and 13.7% in the resection group and 16.4%, 21.8%, and 34.8% in the ablation group, respectively (P = 0.022) (Figure 2a). After PSM, the 1-, 3-, and 5-year LTP rates were 2.4%, 12.9%, and 12.9% in the resection group and 18.8%, 24.6%, and 39.7% in the ablation group, respectively (P = 0.047) (Figure 2b). Besides LTP, 40.3% (29 in 72) patients in the resection group and 41.3% (26 in 63) patients in the ablation group had been observed with IDR (P = 1.000) (Table 2). The 1-, 3-, and 5-year IDR rate were 19.7%, 40.7%, and 48.4% in the resection group and 12.8%, 64.3%, and 69.4% in the ablation group, respectively (P = 0.048) (Figure 2c). After PSM, the 1-, 3-, and 5-year IDR rate were 15.6%, 38.4%, and 48.1% in the resection group and 12.6%, 52.6%, and 60.5% in the ablation group, respectively (P = 0.290) (Figure 2d).
Procedure related complications and management for tumour recurrence
Pain and fever (>38.5 Celsius degree) were the most commonly seen minor complications after treatment. In the resection group, fever was observed in 13 cases, analgesic drugs were given in 29 cases, blood transfusion was needed in 15 cases, lung infection was observed in two cases and drainage of pleural effusion was performed in two cases. In the ablation group, fever was observed in five cases, analgesic drugs were given in 19 cases and drainage of pleural effusion was performed in two cases. In summary, 51.4% (37 in 72) patients after resection and 36.5% (23 in 63) patients after ablation had minor complications, respectively (P = 0.118); while 13.9% (10 in 72) patients after resection and 3.2% (2 in 63) patients after ablation suffered from major complications, respectively (P = 0.035). Treatments for recurrence in the resection group included repeated ablation (21 cases), repeated resection (7 cases), TACE (38 cases), biotherapy (2 cases), chemotherapy (1 case) and sorafenib (1 case), while treatments for recurrence in the ablation group included repeated ablation (27 cases), repeated resection (1 case) and TACE (29 cases).
Kaplan-Meier survival curves comparing 5-year overall survival (OS) and recurrence-free survival (RFS) among patients with multifocal HCCs meeting the BCLC A classification undergoing resection or ablation. OS in the (a) overall population, (b) propensity score-matched cohorts; RFS in the (c) overall population, (d) propensity score-matched cohorts. Numbers at bottom indicate patients at risk.
Kaplan-Meier survival curves comparing 5-year local tumour progression (LTP) and intrahepatic distant recurrence (IDR) among patients with multifocal HCCs meeting the BCLC A classification undergoing resection or ablation. LTP in the (a) overall population, (b) propensity score-matched cohorts; IDR in the (c) overall population, (d) propensity score-matched cohorts. Numbers at bottom indicate patients at risk.
Prognostic factors associated with OS and RFS
Multivariable Cox regression analysis confirmed again that resection procedure was not an independent risk factor for OS (ablation vs resection, hazard ratio [HR] = 0.89; 95% confidence interval [CI], 0.33 - 2.42, P = 0.819), but indicated that ablation was still associated with worse RFS (ablation vs. resection, HR = 2.13; 95% CI, 1.27 - 3.57, P < 0.001) (Table 3). After PSM, similar results were yielded in multivariable Cox models for OS (ablation vs. resection, HR = 0.67; 95% CI, 0.20 - 2.31, P = 0.529) and for RFS (ablation vs. resection, HR = 2.02; 95% CI, 1.12 - 3.64, P = 0.019). Notably, multifocal tumours located in the same segment was a favourable predictor for RFS (HR = 0.549; 95% CI, 0.33 - 0.92, P = 0.020) (Table 3), and was validated again after PSM (HR = 0.49; 95% CI, 0.25 - 0.96, P = 0.038).
Characteristics of recurrent tumours and subsequent treatments
| Variable | Overall population | Matched Cohorts | |||||
|---|---|---|---|---|---|---|---|
| resection (72) | ablation (63) | P | resection (46) | ablation (46) | P | ||
| Relapse pattern (%) | |||||||
| LTP | 5 (6.9) | 9 (14.3) | 0.266 | 4 (8.7) | 8 (17.4) | 0.249 | |
| IDR | 29 (40.3) | 26 (41.3) | 1.000 | 18 (39.1) | 16 (34.8) | 1.000 | |
| EDR | 1 (1.4) | 1 (1.6) | 0.074 | 0 (0.0) | 1 (2.2) | 0.467 | |
| Recurrent HCC No. (%) | |||||||
| Solitary | 21 (29.2) | 14 (22.2) | 0.471 | 12 (26.1) | 12 (26.1) | 0.892 | |
| Multiple | 13 (18.1) | 20 (31.7) | 0.100 | 9 (19.6) | 12 (26.1) | 0.418 | |
| Recurrent HCC size (cm) | 1.5 (1.4) | 1.7 (1.1) | 0.900 | 1.5 (1.4) | 1.6 (1.2) | 0.689 | |
Continuous variables were reported as median (interquartile range) and compared using the Mann-Whitney test. Categorical variables were expressed in percentages and compared using Pearson's Chi-square or Fisher's exact test, as appropriate.
Abbreviations: HCC, hepatocellular carcinoma; LTP, local tumor progression; IDR, intrahepatic distant recurrence; EDR, extrahepatic distant recurrence.
Prognostic factors of overall survival and recurrence-free survival
| Variable | Overall survival | Recurrence-free survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||||
| Ablation (yes/no) | 1.03 (0.44-2.40) | 0.942 | 0.89 (0.33-2.42) | 0.819 | 2.01 (1.30-3.20) | 0.003 | 2.13 (1.27-3.57) | 0.004 | |||
| Tumour number | 0.93 (0.31-2.80) | 0.895 | 0.77 (0.22-2.76) | 0.691 | 1.56 (0.89-2.70) | 0.119 | 1.14 (0.62-2.09) | 0.666 | |||
| Tumour size (cm) | 1.83 (0.69-4.80) | 0.222 | 3.11 (1.02-9.41) | 0.045 | 1.24 (0.74-2.10) | 0.416 | 1.41 (0.82-2.41) | 0.212 | |||
| Age (≥60 years) | 1.06 (0.44-2.60) | 0.899 | 1.10 (0.67-1.80) | 0.705 | |||||||
| Male | - | - | 1.69 (0.68-4.2) | 0.261 | |||||||
| WBC (>4.0×109/L) | 2.51 (1.00-6.20) | 0.047 | 1.86 (0.70-4.95) | 0.211 | 1.47 (0.82-2.70) | 0.200 | |||||
| RBC (>4.3×109/L) | 1.91 (0.82-4.40) | 0.132 | 0.88 (0.52-1.50) | 0.623 | |||||||
| PLT (>100×109/L) | 2.28 (0.96-5.40) | 0.061 | 1.22 (0.33-7.84) | 0.765 | 1.00 (0.61-1.60) | 0.993 | |||||
| ALT (>50 U/L) | 1.68 (0.72-3.90) | 0.229 | 1.62 (1.00-2.60) | 0.044 | 1.57 (0.98-2.52) | 0.062 | |||||
| AST (>40 U/L) | 0.93 (0.36-2.40) | 0.883 | 1.16 (0.69-2.00) | 0.578 | |||||||
| ALB (>35 g/L) | 4.50 (1.30-16) | 0.018 | 1.75 (0.39-7.84) | 0.464 | 0.51 (0.13-2.10) | 0.353 | |||||
| TBIL (>17.1 μmol/L) | 1.02 (0.42-2.50) | 0.965 | 1.36 (0.84-2.20) | 0.211 | |||||||
| PT (prolongation >3 seconds) | - | - | 0.42 (0.06-3.00) | 0.391 | |||||||
| AFP (>200 ng/mL) | 0.47 (0.16-1.40) | 0.173 | 0.99 (0.60-1.60) | 0.969 | |||||||
| Cirrhosis | 0.47 (0.18-1.20) | 0.118 | 1.57 (0.77-3.20) | 0.215 | |||||||
| Oesophageal varices | 2.53 (0.71-9.00) | 0.152 | 1.01 (0.40-2.50) | 0.981 | |||||||
| Splenomegaly | 1.26 (0.54-2.90) | 0.594 | 0.79 (0.49-1.30) | 0.322 | |||||||
| Portal hypertension | 2.27 (0.98-5.30) | 0.057 | 1.53 (0.44-5.36) | 0.503 | 0.77 (0.45-1.3) | 0.353 | |||||
| ALBI grade | 3.38 (1.50-7.90) | 0.005 | 2.70 (0.88-8.28) | 0.082 | 1.28 (0.78-2.10) | 0.335 | |||||
| Same segment | 0.40 (0.17-0.95) | 0.038 | 0.47 (0.19-1.15) | 0.098 | 0.64 (0.39-1.00) | 0.077 | 0.55 (0.33-0.91) | 0.020 | |||
Treatment option, tumour number, tumour size and variables with P value <0.10 at univariate Cox analysis were retained for multivariate Cox analysis. Abbreviations: WBC, white blood cell; RBC, red blood cell; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; TBIL, total bilirubin; PT, prothrombin time; AFP, alpha-fetoprotein; ALBI, Albumin-Bilirubin.
Subgroup analyses of multifocal tumours distributed in different segments
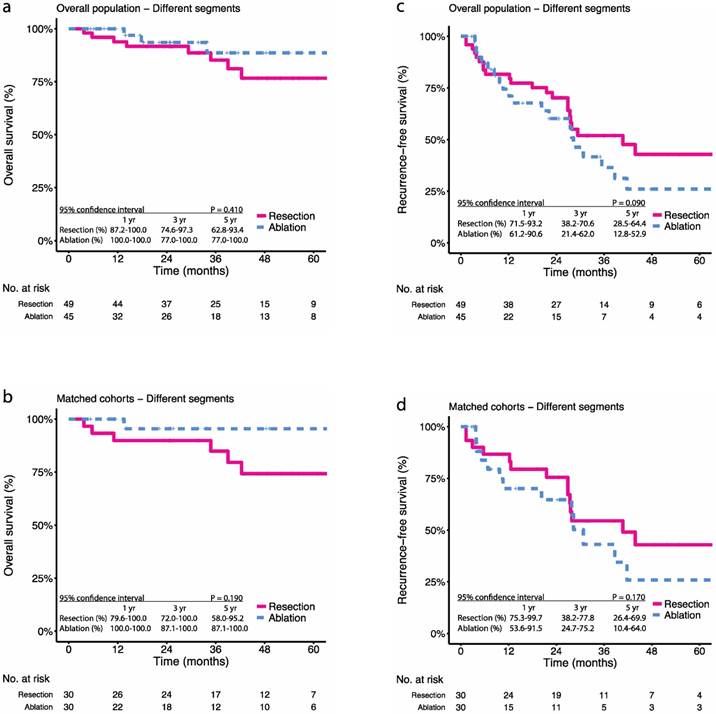
For patients with multifocal tumours distributed in different segments, the 1-, 3-, and 5-year OS were 93.8%, 85.2%, and 76.6% in the resection group and 100.0%, 88.6%, and 88.6% in the ablation group, respectively (P = 0.410) (Figure 3a). After PSM, the 1-, 3-, and 5-year OS were 89.9%, 84.9%, and 74.3% in the resection group and 100.0%, 95.5%, and 95.5% in the ablation group, respectively (P = 0.090) (Figure 3b). The 1-, 3-, and 5-year RFS were 81.6%, 51.9%, and 42.8% in the resection group and 74.5%, 36.5%, and 26.0% in the ablation group, respectively (P = 0.190) (Figure 3c). After PSM, the 1-, 3-, and 5-year RFS were 86.7%, 54.5%, and 42.9% in the resection group and 70.1%, 43.1%, and 25.9% in the ablation group, respectively (P = 0.170) (Figure 3d).
Discussion
The EASL suggests local ablation over resection for multiple HCCs meeting the BCLC A classification not amenable to liver transplantation as tumour multiplicity is correlated with high recurrence and unsatisfactory survival [22-24]. Recently, several studies have supported that tumour multiplicity was not a contraindication per se for surgical intervention [6, 10, 25, 26]. However, few studies focused on the perioperative and long-term outcomes of patients with multifocal HCCs meeting the BCLC A stage undergoing resection or ablation [8, 9]. To address this issue, we only included patients with bi- and tri-focal HCCs ≤3cm and then applied PSM analysis to reduce the baseline confounding bias between treatments. We included both RFA and MWA treatments in this study since previous studies and our recent study demonstrated that both modalities were equally effective in treating HCCs ≤3 cm[13, 27, 28].The present study indicated that resection could provide comparable long-term OS to ablation and even better tumour recurrence control, whereas ablation resulted in less major complications.
In 2008, Ishizawa et al. studied the impact of multiplicity on HCC patients treated with resection and found that although tumour multiplicity was a poor predictor of recurrence after resection (HR = 1.64, P = 0.001), the 5-year OS was still competitive (58%) in patients with Child-Pugh A disease, whereas patients with Child-Pugh B disease had much inferior 5-year OS (19%) [25]. In 2013, a large multicentre survey (including the Eastern and Western) investigated the results of patients with HCC across all BCLC stages undergoing surgery and demonstrated that resection could reach an acceptable 5-year OS of 61% in BCLC 0/A stage [10]. In 2018, Ohkubo et al. classified patients undergoing resection into two groups: patients with single HCC without portal hypertension and those with at least one factors of portal hypertension and bi- and tri-focal HCCs ≤3cm. In that study, incidence of morbidities (3.3% vs 5.5%, P = 0.143) and mortalities (0.4% vs 1.0%, P = 0.305) was not significantly different between those two groups. Indeed, surgical resection has been adopted as an effective and safe treatment option for treating multifocal HCCs in daily practice, especially in the Eastern. When compared with the proposed standard role of ablation in treating multifocal HCCs, a recent systematic review included five randomized controlled trials examining 742 patients and indicated that resection could offer similar 3-year OS (RR = 1.40, P = 0.290) but superior 5-year OS (RR = 1.91, P = 0.001) to RFA for HCC meeting the BCLC 0 and A classification [29]. However, that study called out more well-designed randomized controlled trials to reduce random errors. Wang et al. retrospectively compared outcomes of 462 HCC patients within BCLC A stage treated with resection or RFA and demonstrated that 5-year OS was similar between both treatments (P = 0.088), but RFA was related to higher risk of recurrence (HR = 2.09, P <0.001) [6]. Notably, the results of those above-mentioned studies were informative but might not be directly transferred to patients with multifocal HCCs meeting the BCLC A classification due to the following reasons: (a) the inclusion criteria in those studies was broad because the BCLC A stage includes not only bi- and tri-focal HCCs ≤3cm but also solitary HCC ≥2cm, and (b) for solitary HCC within the BCLC A stage, resection is generally regarded as superior to ablation since the efficacy of ablation is compromised with increasing tumour size [30, 31].
Kaplan-Meier survival curves comparing 5-year overall survival (OS) and recurrence-free survival (RFS) among patients with multifocal HCCs meeting the BCLC A classification undergoing resection or ablation of subgroups (according to anatomic distribution of tumours). OS in the (a) overall population, (b) propensity score-matched cohorts; RFS in the (c) overall population, (d) propensity score-matched cohorts. Numbers at bottom indicate patients at risk.
To answer this specific question, we systematically searched the Pubmed and only found two retrospective studies comparing treatment efficacy of resection and ablation for multiple HCCs inside the BCLC A stage. The first one was conducted by Min et al. and included 62 patients undergoing RFA and 26 patients undergoing resection [8]. In that study, resection resulted in similar 5-year OS (100% vs 63.3%, P = 0.061) and 5-year RFS (30% vs. 60%, P = 0.054) to RFA. However, resection was found associated with better RFS (HR = 0.51, P = 0.043) in the multivariate analysis, and the discrepancy between the two statistical methods might be caused by the loss of sample size to 20 pairs of patients after PSM. Li et al. [9] conducted a larger retrospective study analysing the outcomes of 140 matched pairs of patients with bi- and tri-focal HCCs ≤3cm following RFA or resection, and found that both therapies reached equivalent 5-year OS (resection vs RFA, 36.3% vs. 37.8%, P = 0.609), but resection provided better 5-year RFS (20.1% vs. 9.7%, P = 0.001), which was consistent with our findings. Notably, the recent clinical practice guidelines from the EASL demonstrated that median survival of patients with the BCLC early HCC could reach 50% to 70% at five years after resection or local ablation [24], whereas the 5-year OS was less than 40% in that study which was also much lower than the above-mentioned studies[10, 25]. We speculated that the unsatisfactory long-term OS might due to the less strict inclusion criteria for operation. In the study of Li et al., the indication for resection was only the presence of appropriate residual liver volume evaluated by CT or MRI. As mentioned in the study conducted by Ishizawa et al. [25], patients with Child-Pugh B disease treated with resection had a significantly inferior 19% OS at five years. Conversely, most of the patients enrolled in our study had good liver function of Child Pugh A class (resection: 97.2%, ablation: 96.8%), which contributed to the satisfactory 5-year OS of 72.9% after resection and of 77.2% after ablation. In terms of ablation procedures, Li et al. used laparoscopic (19 cases) or open (60 cases) approach instead of percutaneous (81 cases) methods to treat tumours adjacent to large vessels or gastrointestinal tract [9]. Several studies demonstrated that tumours adjacent to the high-risk areas (gastrointestinal tract, large vessels, central bile ducts, diaphragm and subcapsule) could increase primary procedure failure and result in more morbidities and higher local tumour progression rate after ablation, finally resulting in decreased long-term OS[32, 33]. Therefore, we reviewed all cases in the present study and identified 62 patients presenting with tumours located in risk areas, 53 (85.5%) of which were treated with resection and only nine (14.5%) received ablation. Therefore, the 5-year OS after ablation could reach 77.2%, which was in line with the clinical practice guidelines from the EASL [24].
Additionally, we explored the relapse patterns after both treatments and found that ablation resulted in higher local tumour progression rate. Previous studies found that even for solitary HCC ≤3 cm, approximately 28% patients were observed with microvascular invasion and the incidence of microvascular invasion increased with number of tumours [34-36], and this situation could be handled more radically by resection which resulted in better local tumour recurrence control. As regards to similar OS in both treatments, it could be explained by that most patients with intrahepatic recurrence remained eligible for rescue therapy. Rossi et al. explored the role of repeated RFA for the management of HCC in a prospective series of 706 patients with 859 HCCs ≤3.5 cm initially treated with RFA and found that 69.4% (323 in 465) of patients with initial recurrence were restored to disease-free status by repeated RFA [37].
Last, a randomized controlled trial comparing RFA and resection in treating small HCCs found that patient subset with tumours located in different segments has inferior 3-year OS (47.5% vs 77.0%, P = 0.023) than those with tumours located in the same segment [5]. The multivariate analysis in the present study also identified tumours located in the same segment as a favourable predictor for RFS. Indeed, for multiple tumours located in the same segment, en bloc resection might be preferred due to its more radical removal of lesions and moderate damage of liver parenchyma. We then conducted subgroup analysis for patients with tumours distributed in different segments and found that the advantage of resection to provide superior 5-year RFS lost when compared with ablation. It might be explained by that multiple tumours in different segments reflected potential intrahepatic metastasis or multicentre occurrence. In this situation, both resection and ablation could not effectively deal with those potential cancerous focal [38].
This study was limited by its small sample size and retrospective nature which was prone to cause selection bias, even though PSM was applied to reduce the baseline imbalance. The single-centre study in a highly endemic area of hepatitis B virus infection could be an additional source of bias. Furthermore, some adverse events could be missed as complications were recorded only when patients were admitted to clinics. Prospective studies are warranted to determine the optimal treatment of multiple tumours inside the BCLC A stage.
Conclusions
In summary, for patients with multifocal HCCs meeting the BCLC A classification, resection could achieve competitive survival rates and acceptable procedure-related complications when not only remnant liver volume-preserving principle was met, but also liver function, patient performance status and co-morbidities were taken into consideration[24]. However, ablation might be prioritized when tumour location was appropriate due to its minimally-invasive advantage, especially for lesions distributed in deep liver parenchymal or different segments. Notably, for tumours located in risk areas, resection might be a better option due to its more confidence to guarantee safe margin.
Abbreviations
BCLC: Barcelona-Clinic Liver Cancer; HCC: hepatocellular carcinoma; EASL: European Association for the Study of the Liver; PSM: propensity score matching; AASLD: American Association for the Study of Liver Disease; AFP: serum alpha-fetoprotein; CT: computed tomography; MRI: magnetic resonance imaging; TACE: transarterial chemoembolization; OS: overall survival; RFS: recurrence-free survival; LTP: local tumour progression; IDR: intrahepatic distant recurrence; EDR: extrahepatic distant recurrence; ALBI: Albumin-Bilirubin; WBC: white blood cell count; PLT: platelet counts; PHT: portal hypertension.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81772598, and 81772625), the Guangdong Provincial Natural Science Foundation of China (No. 2017A030311006) and the Guangzhou Science and Technology Program of China (No. 201804020093).
Author contributions
Yun Zheng, Binkui Li and Yunfei Yuan: designed the study; Wenwu Liu Zhiwen Yang and Ruhai Zou: performed research; Wenwu Liu, Zhiwen Yang, Ruhai Zou, Chenwei Wang, Jiliang Qiu, Jingxian Shen, Yadi Liao, Yuanping Zhang, Yongjin Wang, Yichuan Yuan, Kai Li, Dinglan Zuo and Wei He: collected the data; Wenwu Liu Zhiwen Yang and Ruhai Zou: analyzed the data; All authors: interpreted the results; Wenwu Liu Zhiwen Yang and Ruhai Zou: wrote the paper; Yun Zheng, Binkui Li and Yunfei Yuan: gave critical comments and revised the manuscript; All authors discussed the results and revised the manuscript. All authors approved the final version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
2. Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L. et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236
3. Benson AB 3rd, D'Angelica MI, Abbott DE. et al. NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15:563-73
4. Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B. et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma </=3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59:89-97
5. Feng K, Yan J, Li X, Xia F, Ma K, Wang S. et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794-802
6. Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412-8
7. Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G. et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol. 2015;62:617-24
8. Min JH, Kang TW, Cha DI, Song KD, Lee MW, Rhim H. et al. Radiofrequency ablation versus surgical resection for multiple HCCs meeting the Milan criteria: propensity score analyses of 10-year therapeutic outcomes. Clin Radiol. 2018;73:676.e15-e24
9. Jiang L, Yan L, Wen T, Li B, Zeng Y, Yang J. et al. Comparison of Outcomes of Hepatic Resection and Radiofrequency Ablation for Hepatocellular Carcinoma Patients with Multifocal Tumors Meeting the Barcelona-Clinic Liver Cancer Stage A Classification. J Am Coll Surg. 2015;221:951-61
10. Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G. et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929-37
11. Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-2
12. Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nature reviews Gastroenterology & hepatology. 2017;14:527-39
13. Liu W, Zheng Y, He W, Zou R, Qiu J, Shen J. et al. Microwave vs radiofrequency ablation for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Aliment Pharmacol Ther. 2018;48:671-81
14. Li B, Yuan Y, Chen G, He L, Zhang Y, Li J. et al. Application of tumor-node-metastasis staging 2002 version in locally advanced hepatocellular carcinoma: is it predictive of surgical outcome? BMC Cancer. 2010;10:535
15. Zeng QA, Qiu J, Hong J, Li Y, Li S, Zou R. et al. Hepatectomy for hepatocellular carcinoma patients with macronodular cirrhosis. Eur J Gastroenterol Hepatol. 2012;24:575-82
16. Ng KK, Poon RT, Lo CM, Yuen J, Tso WK, Fan ST. Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. J Gastrointest Surg. 2008;12:183-91
17. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW. et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. J Vasc Interv Radiol. 2014;25:1691-705 e4
18. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242-58
19. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL. et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-8
20. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150-61
21. Stuart DEHaKIaGKaEA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw. 2011;42:1-28
22. Lau H, Fan ST, Ng IO, Wong J. Long term prognosis after hepatectomy for hepatocellular carcinoma: a survival analysis of 204 consecutive patients. Cancer. 1998;83:2302-11
23. Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M. et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536-43
24. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018
25. Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K. et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-16
26. Ohkubo T, Midorikawa Y, Nakayama H, Moriguchi M, Aramaki O, Yamazaki S. et al. Liver resection of hepatocellular carcinoma in patients with portal hypertension and multiple tumors. Hepatol Res. 2018;48:433-41
27. Yu J, Yu XL, Han ZY, Cheng ZG, Liu FY, Zhai HY. et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66:1172-3
28. Roberts SK, Fazli O. Microwave Ablation versus Radiofrequency Ablation for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Journal of Hepatology. 2016;64:S701-S2
29. Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency Ablation versus Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology. 2018;287:461-72
30. Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L. et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59:300-7
31. Cucchetti A, Piscaglia F, Cescon M, Serra C, Colecchia A, Maroni L. et al. An explorative data-analysis to support the choice between hepatic resection and radiofrequency ablation in the treatment of hepatocellular carcinoma. Dig Liver Dis. 2014;46:257-63
32. Wells SA, Hinshaw JL, Lubner MG, Ziemlewicz TJ, Brace CL, Lee FT Jr. Liver Ablation: Best Practice. Radiol Clin North Am. 2015;53:933-71
33. Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-51
34. Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A. et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016;151:356-63
35. Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I. et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086-92
36. Shen J, Wen T, Chen W, Lu C, Yan L, Yang J. Model predicting the microvascular invasion and satellite lesions of hepatocelluar carcinoma after hepatectomy. Anz J Surg. 2018 (in press)
37. Rossi S, Ravetta V, Rosa L, Ghittoni G, Viera FT, Garbagnati F. et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53:136-47
38. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. The Lancet. 2018;391:1301-14
Author contact
![]() Corresponding author: Prof. Yuan Yunfei. Email: yuanyfsysu.edu.cn
Corresponding author: Prof. Yuan Yunfei. Email: yuanyfsysu.edu.cn

 Global reach, higher impact
Global reach, higher impact